Abstract
Passerine species have been increasingly used as bioindicators of metal bioaccumulation especially by taking benefit of non-invasive procedures, such as collecting feathers and excrements. In 2009, metal (As, Cd, Cu, Hg, Ni, Pb, Se and Zn) concentrations were determined in feathers and excrements of nestling and adult female great tits (Parus major) in industrial (a paper mill) and rural sites in maritime pine forests on the west coast of Portugal. The aim of this study was to compare the levels of metals between the areas but also between sampling methods (feather vs. excrement) and age classes (nestling vs. adult). Although excrements and feathers of nestling great tits showed different concentrations, similar patterns of accumulation were detected in both study areas. There was a significantly higher concentration of mercury in the industrial area and significantly higher concentrations of arsenic in the rural area in both sample types. Metal levels in adult females had quite different results when compared to nestlings, and only nickel presented significantly higher levels near the paper mill. Since metal levels showed a consistent pattern in feathers and excrements of nestling great tits, we conclude that both represent good and non-invasive methods for the evaluation of these elements in polluted areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollutants have continuously been introduced into ecosystems as a consequence of urbanisation and industrial processes. Metals are globally distributed, and persistent pollutants with bioaccumulation potential being important to monitor their possible effects on wildlife. Non-invasive procedures, such as collecting feathers and excrements, have been successfully used in biomonitoring studies focusing on heavy metal pollution (Denneman and Douben 1993; Hahn et al. 1993). Birds excrete elements into growing feathers (Burger 1993) and can also eliminate metals through excrements or by depositing them in the uropygial gland and salt gland (Burger and Gochfeld 1985). Females can also excrete metals in their eggs and eggshells (Burger 1994). However, each matrix has potential problems as regards to biomonitoring, such as external contamination in feathers or the tissue-specific mechanisms that regulate excretion (Dauwe et al. 2004).
The pulp and paper industry is known for the emission of malodorous sulphurous air pollutants such as hydrogen sulphide (H2S), methyl mercaptan (CH3SH), and methyl sulphides, but the available information concerning the effects of these pollutants on wildlife is still sparse (Haahtela et al. 1992; Soimasuoa et al. 1995; Harris and Elliott 2000; Isaksson et al. 2005).
Insectivorous Great Tits, Parus major, are potentially good biomonitors of heavy metal pollution because they are ubiquitous and abundant and, sometimes, the only forest passerine species available in reasonable densities in polluted areas. They readily nest in man-made nest boxes, and so breeding populations can easily be monitored. In 2009, we measured the concentrations of heavy metals and calcium levels in feathers and excrements of P. major adult females and nestlings in an industrial environment around a pulp and paper mill and in a rural control environment, both located in a coastal area of Portugal.
The objective of this study was to compare heavy metal levels between the two study areas, industrial and rural, while comparing the performance of the two non-invasive procedures (feathers and excrement sampling) to assess heavy metal contamination. Although previous studies showed low correlations between both methods (Morrissey et al. 2005; Berglund et al. 2011), these comparisons may be important for understanding the excretion processes and toxicity of metals.
Material and methods
Study area
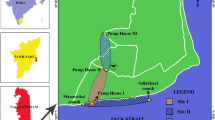
The present work is part of a long-term monitoring study on the effects of air pollution on wild birds, which has been ongoing since 2003 (Costa et al. 2011a). The study has been carried out at multiple study plots in two maritime pine (Pinus pinaster) forest areas located in Figueira da Foz (Portugal). Both areas share the same altitude (±50 m asl) and average temperature, both presenting a sandy soil and including 70–80-year-old pine plantations dominated by P. pinaster interspersed by some Pinus pinea patches, with a tree density varying from 666 to 1,066 individuals/ha. The shrub layer is dominated by Myrica faya, Halimium halimifolium, Cytisus scoparius, Ulex spp., Cistus spp. and Acacia spp.
One area is located in the National Pine Forest of Quiaios (MQ), a 6,000-ha forested area bordered by agriculture fields without a direct influence of industrial pollution, included in the Natura 2000 site “Dunas de Mira” (40°14′ N, 8°47′ W). Because of the north–northwest prevalent winds in this area, MQ is not exposed to any emissions from the pulp mill complexes. The second area is located in the National Pine Forest of Urso (MU), a 9,000-ha forested area sited less than 1 km to the south of a paper and pulp mill industrial complex (40°02′ N, 8°52′ W). The mill produces bleached kraft pulp using an elemental chlorine-free method, and the officially reported air emissions include carbon monoxide, nitrous oxide, nitrogen oxides and PM10 particulates (EPER 2009). Although no metals were presented in the mill report, previous studies comparing the same study areas detected higher mercury concentrations in feathers of both nestling (Costa et al. 2011b) and adult Great Tits (Norte et al. 2010) at the industrial area. Three homogeneous even aged plots (trees aged 70–80 years) were selected in each study area. Plots were within 2 km of each other. Nest boxes were placed at an average density of 9/ha, at equal distances from each other, resulting in 20 to 50 nest boxes per plot.
Feather and excrement sampling
In the summer of 2009, we collected fresh faeces from defecating nestlings at the age of 15 (±1) days. Nestlings were induced to defecate upon handling, and excrements were immediately collected in metal-free plastic containers. With respect to excrements, a total of 48 samples (MQ 27, MU 21) were produced by different nestlings from 18 nests (MQ 10, MU 8). With respect to feathers, a total of 70 samples (MQ 33, MU 37) from different nestlings from 12 nests (MQ 6, MU 6) and 13 samples (MQ 7, MU 6) from different adult females from 13 nests (MQ 7, MU 6) were collected. Only the two outermost tail feathers were collected from adult females and from nestlings at the age of 15 (±1) days. Nestling tail feathers measure 25–28 mm (Orell 1983), and adult female tail feathers measure ca. 58 mm (Cramp and Perrins 1993). Feathers were stored in sterile, metal-free plastic Eppendorf tubes and maintained at −20 °C until analysis. All samples were analysed separately, but nest was considered as a random factor in the statistical analysis.
Metal analyses
Prior to analysis, feathers were washed vigorously in deionized water alternated with 1 mol/l acetone to remove any external contamination and dried at room temperature for 48 h. Feather samples were weighed (range 5–80 mg), and excrement samples were dried in an oven at 50 °C for 72 h and weighed. All samples were then digested in Teflon bombs by adding 2 ml of supra-pure HNO3 and 0.5 ml of H2O2 and placed in a microwave system (Anton Paar, Multiwave 3000, Graz, Austria). The samples were then diluted to 50 ml with deionized water (Elgastat Maxima). Eight elements (As, Cd, Cu, Hg, Ni, Pb, Se and Zn) were determined with ICP-MS (Elan 6100 DRC, PerkinElmer-Sciex, Boston, USA). The detection limits for most of the metals were around 1 ng/l and below. The calibration of the instrument was done with a certified solution (Ultra Scientific, multi-element solution IMS-102, N. Kingstown, RI, USA) from LGC Promochem. Certified reference materials (mussel tissue ERM-CE278; skim milk powder BCR-063R; Ni not included) were used for method validation. The mean recoveries (±SE) in reference samples were as follows: As 90 ± 0.69 %, Cd 89 ± 1.93 %, Cu 103 ± 1.88 %, Hg 94 ± 5.21 %, Pb 96 ± 1.00 %, Se 100 ± 4.24 % and Zn 81 ± 1.04 %.
Statistics
Statistical analyses were performed with SAS statistical software 9.2 (SAS Institute 2003). Means and standard deviations are given in the tables. Differences in metal concentration in feathers and excrements of nestling Great Tits between areas (industrial vs. rural) were assessed with generalised linear models (GLIMMIX procedure in SAS) where nest was included as a random factor to account for the non-independence of the measurements in nestlings from the same brood. Pearson correlations were applied to examine relationships between metal concentrations in feathers and excrements from the same nestling, and therefore, only 30 animals were included in this analysis. Metal concentrations in feathers of adult females were compared between areas (industrial vs. rural) using a t test for unequal variances. Comparison between feathers of nestlings and females was made using generalised linear models (GLIMMIX procedure in SAS) where nest was included as a random factor. For all analysis, after checking for normality of distributions, some variables were log10 transformed to normalise distributions for the analyses and back transformed for presentation in the tables. The significance level was set at p < 0.05.
Results
Metal concentrations in nestling feathers and excrements
Mean metal concentrations in Great Tit nestlings’ excrements presented significantly higher levels of mercury in the industrial area (MU) and a marginally significantly higher level of arsenic in the rural area (MQ) (Table 1). There were no significant differences concerning the remaining analysed metals in both areas (Table 1).
Mean metal concentrations in Great Tit nestlings’ feathers presented significantly higher levels of mercury and selenium in the industrial area (MU) and significantly higher levels of arsenic in the rural area (MQ) (Table 1). There were no significant differences concerning the remaining analysed metals in both areas (Table 1). The comparison of metal levels between excreta and feathers revealed that arsenic and selenium levels were significantly positively correlated (Table 1).
Metal concentrations in feathers of adult females
Mean metal concentrations in adult female feathers presented significantly higher levels of nickel in the industrial area (MU) (Table 2). There were no significant differences between the concentrations of the remaining metals in feathers from both areas (Table 2).
Comparison of metal concentrations between nestling and adult feathers
All metals, with the exception of mercury, nickel and selenium, presented significant differences between the concentration levels found in feathers of nestlings and those of adult female Great Tits (Table 3). Copper and zinc levels were higher in nestlings, while arsenic, lead and cadmium levels were higher in adult females (Table 3).
Discussion
In agreement with our previous study (Costa et al. 2011b), we found a significantly higher concentration of mercury in the industrial area MU and a significantly higher concentration of arsenic in the rural area MQ in both excrement and feathers of Great Tit nestlings. Although metal concentrations differ between sample types (excrement vs. feather), they all showed an equal tendency in both study areas, i.e. an increase in a metal concentration in nestling excrements is accompanied by an increase of the same metal concentration in nestling feathers in each study area.
Nestling feathers also presented significantly higher levels of selenium in the industrial area (MU), which is possibly related with the above-mentioned higher amount of mercury detected in this study area. In fact, the formation of Se–Hg complexes as a detoxification process (e.g. Thompson 1996) may be the source for the higher levels of selenium detected in feathers collected from the industrial area.
The use of feathers and excrements has been a common tool to observe environmental metal pollution, especially because they are non-injurious and non-invasive to birds (Burger 1993; Eens et al. 1999; Dauwe et al. 2000; Janssens et al. 2002; Bianchi et al. 2008). Feathers reflect the amount of metals present in the blood at the time of feather growth, either from current dietary sources or from mobilisation of metals from internal organs (Burger 1993), while excrements represent the unabsorbed remnants of multiple food items, often at higher concentrations than the diet items, and so they can provide a non-destructive and quantifiable means of monitoring the food chain contamination by trace metals (Spahn and Sherry 1999; Morrissey et al. 2005).
In several studies (Morrissey et al. 2005; Berglund et al. 2011), the correlation among metal levels between feathers or internal tissues and excrements was low, indicating no clear pattern with respect to metal excretion. This is in agreement with our study, where only arsenic and selenium showed a positive correlation. However, feathers and excrements partly indicate a temporally different exposure, which likely weakens the correlations. Also, the small difference in values of some metal concentrations could reduce the probability of a significant correlation.
Feather metal levels in adult female Great Tits showed some different results compared to nestlings. However, the only significantly higher levels were detected for nickel in MU, perhaps due to the low number of adult samples (MQ, n = 7; MU, n = 6), which weakens the power for statistical comparisons. However, the patterns of higher arsenic in MQ and higher levels of mercury in the industrial area (MU) were the same as those found in nestlings, although differences were not significant. One cannot rule out external contamination and differences in metabolism, since some elements like nickel can be regulated by homeostatic control in nestlings (Nyholm 1995; Dauwe et al. 2004; Berglund et al. 2011).
When we compared contaminant levels of adult and nestling feathers, we expected levels of metals to be higher in adults than in nestlings, especially because adults have had longer time to acquire and bioaccumulate contaminants (Burger et al. 2009). However, we found significantly higher levels of zinc and copper in nestlings, and only arsenic, lead and cadmium were significantly higher in adults. Interestingly, the metals are here divided by their redox activity (Koivula et al. 2011). Copper is a redox active metal, while arsenic, lead and cadmium are redox inactive. Adult and nestling feathers present a slightly different composition because, at the time of sample collection, adult feathers are completely formed, while in 15-day-old nestlings, feathers are still growing and have an active blood circulation supporting their growth (Burger and Gochfeld 1992). Once the feather has reached its full size, the blood supply is no longer needed, and the vessels shrivel up. The redox inactive metals have strong affinity to the sulfhydryl groups of keratin (a key protein in feather), and this is probably why we found higher levels of these metals in adult feathers. Nestling feathers, on the other hand, include blood vessels and blood that might contain more copper and zinc than pure keratin.
Since mercury and arsenic levels showed a consistent pattern in feathers and excrements of nestling Great Tits, we may well say that these substrates present a good method for the evaluation of these elements in the study areas. Arsenic is a toxic non-essential element that readily bioaccumulates. Several reported values for arsenic in the feathers and excrements of Great Tit are higher than the levels found in the present study (Janssens et al. 2003; Dauwe et al. 2004; Eeva et al. 2006, 2009), and no toxic effect was found to be directly related to arsenic values. Mercury is considered to be very toxic for wild animals, and at higher levels of contamination, it can adversely affect birds by reducing their fecundity, growth and body length (Eisler 1987). Although the values found in the present study were within the ranges previously reported for mercury levels in feathers and excrements of Great Tit inhabiting metal-contaminated sites (Janssens et al. 2002, 2003), we found no direct adverse effects on the breeding biology of the Great Tit (Costa et al. 2011a). Nevertheless, considering the well-known hazardous effect of these elements on the environment, the regular monitoring of the study areas is essential.
In conclusion, nestlings seem a more optimal choice for the evaluation of local pollution, since we can sample data from a defined area and time period. Furthermore, because the nestlings stay within the nest boxes, there is a very limited possibility of external airborne deposition from industrial sources, which can happen to adults. In addition, we found that nestlings’ feathers show a different metal profile, which is partly due to the fact that their feathers are still growing and show a different tissue composition in comparison to fully grown feathers. Therefore, the developmental stage of feathers is important to consider when such results are interpreted.
References
Berglund, Å. M. M., Koivula, M. J., & Eeva, T. (2011). Species- and age-related variation in metal exposure and accumulation of two passerine bird species. Environmental Pollution, 159(10), 2368–2374.
Bianchi, N., Ancora, S., Fazio, N., & Leonzio, C. (2008). Cadmium, lead, and mercury levels in feathers of small passerine birds: noninvasive sampling strategy. Environmental Toxicology and Chemistry, 27(10), 2064–2070.
Burger, J. (1993). Metals in avian feathers: bioindicators of environmental pollution. Review of Environmental Toxicology, 5, 203–311.
Burger, J. (1994). Heavy metals in avian eggshells: another excretion method. Journal of Toxicology and Environmental Health, 41, 207–220.
Burger, J., & Gochfeld, M. (1985). Comparison of nine heavy metals in salt gland and liver of greater scaup (Aythya marila), black duck (Anas rubripes) and mallard (Anas platyrhynchos). Comparative Biochemistry and Physiology, 81, 287–292.
Burger, J., & Gochfeld, M. (1992). Trace element distribution in growing feathers: additional excretion in feathers sheaths. Archives of Environmental Contamination and Toxicology, 23, 105–108.
Burger, J., Gochfeld, M., Jeitner, C., Burke, S., Volz, C., Snigaroff, R., Snigaroff, D., Shukla, T., & Shukla, S. (2009). Mercury and other metals in eggs and feathers of glaucous-winged gulls (Larus glaucescens) in the Aleutians. Environmental Monitoring and Assessment, 152, 179–194.
Costa, R. A., Eeva, T., Eira, C., Vaqueiro, J., & Vingada, J. V. (2011). Effects of air pollution from paper and pulp industry on breeding success of Great tit in maritime pine forests. Ecoscience, 18(2), 115–123.
Costa, R. A., Petronilho, J. M. S., Soares, A. M. V. M., & Vingada, J. V. (2011). The use of passerine feathers to evaluate heavy metal pollution in Central Portugal. Bulletin of Environmental Contamination and Toxicology, 86, 352–356.
Cramp, S., & Perrins, C. M. (1993). The birds of the Western Palearctic VII. London, UK: Oxford University Press.
Dauwe, T., Bervoets, L., Blust, R., Pinxten, R., & Eens, M. (2000). Can excrement and feathers of nestling song birds be used as biomonitors for heavy metal pollution? Archives of Environmental Contamination and Toxicology, 39, 227–234.
Dauwe, T., Jassens, E., Bervoets, L., Blust, R., & Eens, M. (2004). Heavy-metal concentrations in female laying Great Tits (Parus major) and their clutches. Archives of Environmental Contamination and Toxicology, 49, 249–256.
Denneman, W. D., & Douben, P. E. T. (1993). Trace metals in primary feathers of the barn owl (Tyto alba guttattus) in the Netherlands. Environmental Pollution, 82, 301–310.
Eens, M., Pinxten, R., Verheyen, R. F., Blust, R., & Bervoets, L. (1999). Great and blue tits as indicators of heavy metal contamination in terrestrial ecosystems. Ecotoxicology and Environmental Safety, 44, 81–85.
Eeva, T., Belskii, E., & Kuranov, B. (2006). Environmental pollution affects genetic diversity in wild bird population. Mutation Research, 608, 8–15.
Eeva, T., Ahola, M., & Lehikoinen, E. (2009). Breeding performance of blue tits (Cyanistes caeruleus) and great tits (Parus major) in a heavy metal polluted area. Environmental Pollution, 157, 3126–3131.
Eisler, R. (1987). Mercury hazards to fish, wildlife, and invertebrates: a synoptic review. Contaminant Hazard Reviews. Biological Report no 85 (1.10). U.S. Fish and Wildlife Service, Patuxent Wildlife Research Center Laurel:1–63.
EPER (2009). European pollutant emission register. http://www.eper.cec.eu.int (cited 10/November/2009).
Hahn, E., Hahn, K., & Stoepler, M. (1993). Bird feathers as bioindicators in areas of the German Environmental Specimen Bank—bioaccumulation of mercury in food chains and exogenous deposition of atmospheric pollution with lead and cadmium. Science of the Total Environment, 139(140), 259–270.
Haahtela, T., Marttila, O., Vikka, V., Jäppinen, P., & Jaakkola, J. J. K. (1992). The South Karelia air pollution study: acute health effects of malodorous sulfur air pollutants released by a pulp mill. American Journal of Public Health, 82, 603–605.
Harris, M. L., & Elliott, J. E. (2000). Reproductive success and chlorinated hydrocarbon contamination in tree swallows (Tachycineta bicolor) nesting along rivers receiving pulp and paper mill effluent discharges. Environmental Pollution, 110, 307–320.
Isaksson, C., Örnborg, J., Stephensen, E., & Andersson, S. (2005). Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. Ecohealth, 2, 138–146.
Janssens, E., Dauwe, T., Bervoets, L., & Eens, M. (2002). Inter and intraclutch variability in heavy metals in feathers of Great tit nestlings (Parus major) along a pollution gradient. Archives of Environmental Contamination and Toxicology, 43, 323–329.
Janssens, E., Dauwe, T., Pinxten, R., Bervoets, L., Blust, R., & Eens, M. (2003). Effects of heavy metal exposure on the condition and health of nestlings of the great tit (Parus major), a small songbird species. Environmental Pollution, 126, 267–274.
Koivula, M. J., Kanerva, M., Salminen, J., Nikinmaa, M., & Eeva, T. (2011). Metal pollution indirectly increases oxidative stress in great tit (Parus major) nestlings. Environmental Research, 111, 362–370.
Morrissey, C. A., Bendell-Young, L. I., & Elliott, J. E. (2005). Assessing trace-metal exposure to American dippers in mountain streams of southwestern British Columbia, Canada. Environmental Toxicology and Chemistry, 24(4), 836–845.
Norte, A. C., Sheldon, B. C., Sousa, J. P., Tavares, P. C., Pereira, M. E., Duarte, A. C., & Ramos, J. A. (2010). Are Great Tits (Parus major) inhabiting the vicinity of a pulp mill healthy? Impacts on physiology and breeding performance. Archives of Environmental Contamination and Toxicology, 59, 502–512.
Nyholm, N. E. I. (1995). Monitoring of terrestrial environmental metal pollution by means of free-living insectivorous birds. Annali di Chimica, 85, 343–351.
Orell, M. (1983). Nestling growth in the Great Tit, Parus major, and the Willow Tit, Parus montanus. Ornis Fennica, 60, 65–82.
SAS Institute (2003). The SAS System for Windows. Release 9.2. SAS Inst., Cary, NC.
Soimasuoa, R., Jokinen, I., Kukkonenb, J., Petänend, T., Ristola, T., & Oikarid, A. (1995). Biomarker responses along a pollution gradient: effects of pulp and paper mill effluents on caged whitefish. Aquatic Toxicology, 31, 329–345.
Spahn, S. A., & Sherry, T. W. (1999). Cadmium and lead exposure associated with reduced growth rates, poorer fledging success of Little Blue heron chicks (Egretta caerulea) in South Louisiana wetlands. Archives of Environmental Contamination and Toxicology, 37, 377–384.
Thompson, D. R. (1996). Mercury in birds and terrestrial mammals. In W. N. Beyer, G. H. Heinz, & A. W. Redmon-Norwood (Eds.), Environmental contaminants in wildlife. Boca Raton, FL: Interpreting tissue concentrations. CRC Press.
Acknowledgments
This study was supported by the Portuguese Foundation for Science and Technology (FCT) with a grant SFRH/BD/42532/2007 to R. A. Costa. Also, C.E. and J.V. were respectively supported by grants SFRH/BPD/27014/2006 and SFRH/BD/31425/2006 from FCT. T.E. was supported by a grant from the Academy of Finland (project 8119367). The authors wish to thank all people that helped during this long-term fieldwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, R.A., Eeva, T., Eira, C. et al. Assessing heavy metal pollution using Great Tits (Parus major): feathers and excrements from nestlings and adults. Environ Monit Assess 185, 5339–5344 (2013). https://doi.org/10.1007/s10661-012-2949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2949-6