Abstract
Measures of oxidative stress in animals may be useful biomarkers of environmental stressors, such as anthropogenic pollution. In birds, studies of oxidative stress have focused on dietary antioxidants, primarily carotenoids, which are interesting due to their multiple physiological and pigmentary functions but therefore also unspecifically related to oxidative stress. A useful complementary biomarker may be the glutathione system, commonly used in human medicine, but rarely applied to wild, terrestrial vertebrates. In this study of urban versus rural adult and nestling great tits Parus major, we investigated both the carotenoid-based yellow plumage (by reflectance spectrometry) and the plasma levels of glutathione, the latter measured as total glutathione (tGSH) and as the ratio between oxidized and reduced glutathione (GSSG:GSH), respectively. We found that urban adults had higher current oxidative stress (GSSG:GSH) and paler yellow plumage compared to rural adults, suggesting elevated stress in the urban environment. Total glutathione levels (tGSH), however, which may indicate long-term up-regulation of the GSH reservoir, did not differ between the environments. Nestlings did not show any consistent pattern between environments in either tGSH or GSSG:GSH and, among individuals, glutathione levels were uncorrelated with carotenoid coloration. The results thus suggest some population-level correspondence between the two stress biomarkers in adult birds, but more work is obviously needed to understand how the two antioxidant systems interact in different individuals and in response to different environmental disturbances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
To identify and prevent environmental threats to humans and other animals, there is an increasing need for reliable, nonlethal stress biomarkers that detect animal health effects at an early stage. In terrestrial ecosystems, a number of recent studies show that hole-nesting passerines, notably the common and ecologically well-known tits (Parus spp.), may serve as valuable monitors of environmental stressors, such as organochlorines (Dauwe et al., 2003), heavy metals (Llacuna et al., 1995; Eens et al., 1999; Dauwe et al., 2000), and urban air pollution (Eeva and Lehikoinen, 1995; Eeva et al., 2000). Common to these and other anthropogenic pollutants is that the initial physiological effect is some form of oxidative stress (see below) associated with a specific defense mechanism. Estimates of elevated oxidative stress, either by measuring oxidative damage to cellular macromolecules or via relevant antioxidant systems, may therefore constitute promising biomarkers of environmental stress in wild animals.
Like all aerobic organisms, birds are subjected to basic oxidative processes from everyday metabolism, immune defense, and growth, which are balanced by a variety of antioxidant systems. Shifts in this balance are collectively termed oxidative stress, caused by, for example, environmental stressors such as ingested or inhaled pollutants. Several studies have shown that oxidative stress caused by urban and primarily traffic-generated air pollution (mostly NOx, ozone, soot particles) can lead to damages on proteins, carbohydrates, lipids, and DNA (Sies, 1986), also in birds (Schilderman et al., 1997). This results in a variety of lung disorders, such as damages to the tracheal epithelium (Llacuna et al., 1993), airway hypersensitivity (Sandström et al., 2000), and other responses (Klika et al., 1996; Lorz and Lopez, 1997; Saldiva and Bohm, 1998). In passerine birds, ecophysiological stress from urban air pollution is virtually unstudied, but exposure to heavy metal pollution from a copper smelter resulted in both reduced breeding success and increased fluctuating asymmetry (Eeva et al., 2000).
To counter the detrimental effects of oxidative stress, animals mount a number of antioxidant defense systems based on carotenoids, vitamin E, and other dietary antioxidants, as well as the endogenous glutathione system, catalase, and superoxide dismutase (see Noguchi and Niki, 1998). Among these defenses, carotenoids are particularly interesting due to their conflicting use as external pigments, potentially mediating color as a stress biomarker. Deposition of carotenoids in the feathers is prominent in many bird species (Brush, 1978; Goodwin, 1984; Brush, 1990), producing concentration-dependent and measurable yellow and red colors (Partali et al., 1987; Stradi et al., 1998). The intensity of external carotenoid pigmentation may thus reveal the internal allocation to antioxidant defenses, whereby pale yellow (less “chromatic”) plumage coloration may be a useful biomarker of environmental stress, as indicated by recent studies (Eeva et al., 1998; Hõrak et al., 2000, 2001).
As promising as this may seem, many questions remain to be answered before any direct links can be established between carotenoid coloration and oxidative stress. For example, it is debated whether the high circulating levels of carotenoids in many birds, in particular those with carotenoid-pigmented plumage, really can be a limiting factor for the relatively modest requirement by immunological and antioxidative functions (Hill, 1999; Navara and Hill, 2003). Furthermore, their dietary origin may suggest a correlation between plumage coloration and habitat destruction and nutrition rather than a direct impact of pollution-generated oxidative stress.
The aim of this study was therefore to investigate, alongside carotenoid coloration, the glutathione system as a measure of oxidative stress in response to the urban environment and pollution. Glutathione level is commonly used as an indicator of oxidative stress in humans and laboratory animals, but rarely considered in wild birds. In blood plasma and other tissues (Kosower and Kosower, 1978; van der Vliet and Cross, 2000), glutathione plays a key role by deactivating reactive oxygen species (Meister and Anderson, 1983) and by regenerating other antioxidants (vitamin E and possibly also carotenoids) (Marcus et al., 1993). It is secreted into the blood from the liver (Wang et al., 1998) where, apart from antioxidant defense, it also serves as a cysteine reservoir for glutathione synthesis in other organs, mainly kidney, lung, and intestine (Halliwell and Gutteridge, 1999). In birds, gluthathione also appear to serve as a cysteine reservoir for muscle and feather growth (Murphy and King, 1990).
Glutathione exists in two forms; the reduced (reactive) form, conventionally termed GSH, and the oxidized form, glutathione disulfide (GSSG). We measured the sum of these two, total glutathione (tGSH) and the oxidized form (GSSG), and calculated the ratio between the oxidized and reduced form (GSSG:GSH), which is a useful indicator of current oxidative stress (Kidd, 1997; van der van der Oost et al., 2003). Among the few studies of glutathione in wild animals can be found elevated liver GSSG:GSH levels in aquatic birds living in selenium-polluted agricultural drainwater (Hoffman, 2002), and increased glutathione-dependent enzyme activities in fish from polluted harbors (Stephensen et al., 2000). Glutathione levels in response to air pollution and other sources of terrestrial ecosystem stress have, to our knowledge, not been explored in birds or any other wild terrestrial animal. In the present study, we investigated the variation in plasma glutathione levels and carotenoid coloration in a free-ranging passerine bird, the great tit Parus major, in relation to the combined direct and indirect (e.g., via nutrition) effects of the urban environment, to evaluate its potential use as a nonlethal biomarker of oxidative stress in natural populations. We predicted that we would find higher current oxidative stress (GSSG:GSH), higher tGSH and paler birds in the urban compared to the rural habitats.
METHODS
The great tit is a small (ca. 16 g) and largely resident passerine bird common in woodlands, parks, and gardens throughout Europe and Asia. During winter, they typically forage in mixed flocks over an area of approximately 4–7 ha. Movements during breeding season are within territory, which is variable in size (0.2–1.7 ha) depending on habitat (Cramp and Perrins, 1993).
The present study was carried out during the breeding season April to June 2002 in a number of urban, suburban, and rural nestbox plots in southwestern Sweden. The urban populations are situated within Göteborg city limits (Slottsskogen and Änggården), the suburban population 15 km southeast of Göteborg (Gunnebo), and the rural populations 40–50 km south of Göteborg (Råön, Hamra, and Gräppås). All nestbox plots are located in similar deciduous forest habitats dominated by oak and birch. Compared to the countryside (“rural”) areas ca. 40 km south of the city, air pollution is markedly higher in Göteborg, including, e.g., levels of NO2 (23 μg/m3 vs. 6.6 μg/m3), SO2 (1.8 μg/m3 vs. 1.1 μg/m3), and soot (8.5 μg/m3 to 2.9 μg/m3) (Kindbom et al., 2001; Persson 2002). All of these city levels have documented health impacts on humans (Medina et al., 2002; Forsberg et al., 2003).
Nestboxes were monitored every other day from the start of nestbuilding in late April, in order to record starting dates of egglaying and hatching. Measures and samples of nestlings and adults (captured with spring traps in the nestbox) were obtained when nestlings were 13–15 days old. Adult birds were sexed and aged as 1 year old (1y, subadults) or at least 2 years old (2y+) according to plumage characteristics (Svensson, 1992). Morphometrics (tarsus length to the nearest 0.1 mm, wing chord to the nearest 0.5 mm, and body mass to the nearest 0.1 g) were measured with digital calipers, ruler, and a Pesola spring balance, respectively. Approximately 200 μl blood was drawn from the neck vein with a heparinized syringe, diluted with 10 μl EDTA and kept on ice until further analyses. A few drops of blood were stored in tubes with 99% ethanol for molecular sex determination (see below).
Reflectance Spectrometry and ObjectiveColorimetry
Spectral reflectance of the yellow (Partali et al., 1987) ventral plumage was measured with a S2000 spectrometer system (Ocean Optics Inc., Dunedin, FL), controlled by the software C-spec (Ancal Inc., Las Vegas, NV) and using a DH 2000 UV/VIS light source together with a 7 × 400 μm bundled, fiber optic reflectance probe, fitted with a cylindrical plastic sheath to block out external light and provide a standardized scanning distance and a 4-mm-wide measuring spot. A reference scan from a WS-2 white standard (>98% reflectance within wavelengths 300–800 nm) (Avantes, Eerbek, Netherlands) was obtained before each individual bird was measured. The fiber optic probe was held at a 90° angle against the plumage. Five scans (removing the probe between each) were taken from the yellow flank plumage (just below the white bar on the folded right wing), chosen for being the best developing and most easily measured ventral plumage tract on great tit nestlings. From the raw spectral reflectance data, we computed, and averaged for each individual, several objective colorimetrics (Andersson and Prager, 2005). The measure relevant to this study is “carotenoid chroma” (R700–R450)/R700. This is the relative difference in reflectance between the wavelengths of minimum (700 nm) and maximum (450 nm) absorptance of the two main carotenoids in great tit plumage (lutein and zeaxanthin). This measure has the benefit of both being a strong correlate (r > 0.8) of perceived chroma (e.g., in the human CIELab color space, and in segment- or PCA-based methods) and also being the best spectrometric estimate of actual carotenoid concentration [S. Andersson, unpublished results].
Sex Determination of Nestlings
DNA was extracted from the blood samples using the Sigma Gene EluteTM Mammalian Genomic DNA Kit (Sigma- Aldrich, Stockholm, Sweden). The PCR-protocol developed by Griffiths et al. (1998) was used with minor modifications. The primer pair P2 and P8 amplifies homologous sections of the avian genes CHD-Z (present in both sexes) and CHD-W (only present in females), which include introns of different lengths. The amplified products were then separated by electrophoresis in a 2% agarose gel and visualized with ethidium bromide staining under UV light. A single CHD-Z band was visible in all samples but only females also showed a second, distinctive CHD-W band.
Measuring Total and Oxidized Glutathione in Plasma
Blood samples were centrifuged (1800 rpm/10 minutes), 50 μl plasma transferred to new tubes in which proteins were precipitated by adding 25 μl 1.2% 5-sulfosalicylic acid (SSA). After 15 minutes on ice, tubes were centrifuged for 5 minutes at 1800 rpm. Liquid were then stored at −80°C until assay.
Total and oxidized glutathione was measured as in Baker et al. (1990), adapted to a microplate reader by Vandeputte et al. (1994; see also Stephensen et al., 2002). The samples assigned for GSSG analysis were treated with 5 μl of 2-vinylpyridine (2-VP) to derivatise GSH in order to prevent it from interfering with the GSSG measurement. GSH standards were prepared daily, a 10-mM GSH stock solution was diluted with 1.2% SSA to the concentrations 0.5, 1, 5, and 10 μM. Reaction mixtures containing, respectively, 0.34 mM NADPH dissolved in a stock buffer (143 mM NaH2PO4 and 6.3 mM EDTA, pH 7.4) and 10 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) for tGSH measurements or 1 mM DTNB for GSSG measurements, were added to wells containing samples and the reactions were started by adding 20 μl of 17 U/ml glutathione reductase (GR). Standards, samples, and daily reagent stocks were kept on ice until transfer to a 96-well microtiter plate. The plate was immediately placed in a “SpectraMax” 190 plate reader from Molecular Devices (Palo Alto, CA). Absorptance change was monitored in room temperature at 415 nm during 7 minutes and compared to a simultaneously obtained standard curve from GSH with known concentration. Glutathione (GSH), glutathione reductase (GR), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), NADPH, 2-vinylpyridine (VP), 5-sulfosalicylic acid (SSA), EDTA, and NaH2PO4 were purchased from Sigma. Ninety-six-well round-bottom microtiter plates were obtained from VWR International AB (Stockholm, Sweden).
Data Handling and Statistical Analyses
Plasma samples for glutathione analyses were initially obtained from 60 adult and 135 nestling great tits from 36 different nestboxes (i.e., “families”), 12 in each of the three habitat types (urban, suburban, and rural). Forty-seven of the nestling samples were excluded, however, due to failed glutathione assays, such as both replicates belonging to microplate rows in which all wells (treated and measured together) for unknown reasons showed negative concentrations. For a further 22 nestling and 6 adult samples, only the tGSH measure could be used while the assay of oxidized glutathione (GSSG) failed. Except for obviously failed assays, all positive values (even when close to zero) were used, taking the average of the two replicates, or using one of the replicates if the other had failed or was negative. The final sample sizes, means, and standard errors for the three variables (tGSH, GSSG:GSH, and “carotenoid chroma”), are shown in Figure 1. Because adults and nestlings differ with respect to the time-period of exposure to pollutants and differ in many biological aspects (such as physiology and behavior), we did separate analyses for nestling and adult birds.
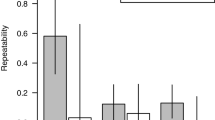
Average levels in great tits of (a) glutathione (tGSH, nmol/ml), (b) oxidative stress (GSSG:GSH), and (c) carotenoid coloration (“carotenoid chroma” R700–R450)/Raverage). Each chart shows actual (univariate) means ± standard error and sample sizes for urban, suburban, and rural environments, split on nestling (left panels) and adults (right panels). Significant pairwise post hoc comparisons (see Table 1 for model effects) derive from Student’s t-tests and are indicated with *P < 0.01.
To investigate the relationship between glutathione and carotenoid coloration as stress biomarkers, we first included “carotenoid chroma” as a covariate in both the nestling and adult models. However, it did not reach statistical significance and was therefore removed from these models and analyzed separately as a dependent variable (see below).
Independent effects on tGSH and GSSG:GSH, and “carotenoid chroma” variation were identified by entering the discrete variables of environment (urban, suburban, rural), family (nestbox ID), and sex (m, f) in JMP 5.1 (SAS Institute Inc., Cary, NC; 2003), log-transformed to approximately normal distributions. To control for family (nestbox) effects, nestbox ID was nested within habitat type and treated as a random variable (i.e., used as F-ratio denominator in the test of habitat effects). Additional variables (breeding data, tarsus, body condition [ln mass/3 × ln tarsus], adult age) and interactions were then entered one at a time and retained only if they contributed significantly to the model. Least square estimation was used throughout, to allow testing of the nestbox effect, but all other effects were confirmed by repeating the models with REML (restricted maximum likelihood) error estimation. This slightly increased some P-values, but did not change any significance levels or conclusions.
RESULTS
Variation in Total Glutathione Levels (tGSH)
Absolute mean values for nestlings and adults in the three areas, of the two main response variables as well as the measure “carotenoid chroma,” are shown in Figure 1.
The nestbox (“family”) effect on tGSH was highly significant in nestlings but not in adults (see Table 1). Likewise, the habitat effect was only found among nestlings (Table 1), entirely due to the greatly elevated tGSH levels in suburban nestlings, which was almost as high as in adults (Fig. 1a). Although not visualized in Figure 1, there was also a significant sexual difference in adult great tits, with higher tGSH in females (F1,21 = 7.85, n = 0, P = 0.011) but there was no sexual difference among nestlings (F1,69 = 0.00, n = 87, P = 0.98). Finally, adults had significantly higher levels of tGSH than nestlings (ANOVA; Age: F1,142 = 26.76, P < 0.0001; Age × Environment: F2,142 = 3.80, P = 0.025; Environment: F2,142 = 5.69, P = 0.0042; see Fig. 1a).
Variation in the Ratio of Oxidized to Reduced Glutathione (GSSG:GSH)
The GSSG:GSH ratio, the most widely used estimate of current oxidative stress, was negatively related to tGSH among adults but there was no such relationship among nestlings (Linear regressions of log[GSSG:GSH] vs. log[tGSH]; adults, F1,52 = 11.94, n = 54 , P = 0.001; nestlings, F1,69 = 0.18, n = 71, P = 0.67). The nestbox (“family”) effect was highly significant on GSSG:GSH ratio in nestlings but not in adults (Table 1). There was a significant habitat effect in adults, with urban birds showing higher oxidative stress, but no significant effect in nestlings (Fig. 1b and Table 1). A significant sex effect in adults (Table 1) was due to higher ratios in males than in females (univariate mean GSSG:GSH ratio; 1.08 in males vs. 0.56 in females), whereas there was an opposite but not significant pattern in nestlings (0.84 in males vs. 0.96 in females).
The body condition (ln mass/3 × ln tars) followed the pollution gradient for both adults (F2,59 = 3.045, P = 0.055) and nestlings (F2,132 = 18.49, P < 0.0001), with urban birds in poorer condition than suburban and rural birds. However, there was no bivariate correlation between condition and oxidative stress ratio (see Methods).
Glutathione and Carotenoid Coloration
To demonstrate the urban-rural color variation in the present sample, we ran the same models as above with “carotenoid chroma” as dependent variable. Similar to the glutathione levels, carotenoid chroma showed a significant nestbox (“family”) effect in nestlings (F17,108 = 3.63, n = 131, P < 0.0001) but not in adults (F29,16 = 1.55, n = 51, P = 0.18). As shown in Figure 1c, by the univariate means and model post hoc comparisons, habitat had a significant and consistent effect in both nestlings (F2,17 = 6.73, n = 131, P = 0.007) and adults (F2,36 = 4.46, n = 51, P = 0.019), with urban birds being “palest” (least pigmented), suburban birds intermediate, and rural birds most chromatic yellow. There was no trend of a bivariate relationship between tGSH , current oxidative stress ratio, and carotenoid content in feathers in separate analyses of adult or nestling great tits (see Methods).
DISCUSSION
To our knowledge, this is the first study to describe plasma glutathione levels in a wild, terrestrial bird population, and in relation to another promising, noninvasive biomarker of environmental stress, carotenoid pigmentation. As predicted, current oxidative stress, indicated by the ratio of oxidized to reduced glutathione (GSSG:GSH) was elevated in urban compared to rural adult birds. Among nestlings, however, levels were lower and there was no corresponding variation, but instead a significantly higher level in the suburban habitat. As regards carotenoid coloration, our spectrometric analyses revealed paler yellow plumage in the urban and more polluted habitats, confirming a previous, similar relation between pollution and subjectively assessed great tit coloration (Eeva et. al., 1998). Thus, both GSSG:GSH and carotenoid coloration deserve further exploration as biomarkers of environmental stress in bird populations.
Our results also indicate that both the total concentration of gluthathione (tGSH) and the measure of current oxidative stress (GSSG:GSH) vary considerably between individuals. The average glutathione levels (Fig. 1) for nestlings (150–450 nmol/ml) are in line with studies on young domesticated birds (Enkvetchakul et al., 1995). As far as we know, there are no similar references on adult levels, which in this study were somewhat higher (590–650 nmol/ml) than in nestlings. Although the individual variation was large, adults in the urban areas (the two Göteborg city parks, Slottsskogen and Änggården) exhibited significantly higher oxidative stress level (GSSG:GSH) than suburban and rural populations. While GSSG:GSH thus might serve as a useful biomarker of either direct oxidative stress or indirect effects of the urban environment, total glutathione (tGSH) did not show a similar trend. To the extent that tGSH can be seen as a measure of upregulation of the glutathione system, it seems that such long-term responses have a more complex and individually variable relationship to environmental stress.
Breeding females generally had higher tGSH levels than males, primarily in suburban and urban areas (Table 1). This may indicate a temporarily elevated stress associated with egg production which is known to be costly (e.g., Visser and Lessells, 2001). This requires further studies, but some support for this interpretation comes from earlier studies where fish are able to increase their tGSH level in 5 days (Stephensen et al., 2002), and where humans elevate their glutathione levels in response to oxidative stress (Dickinson et al., 2004). Supposedly, female great tits are more sensitive to additional costs in terms of oxidative stress during and just after the costly egg production, and may therefore respond stronger to the additional stress from air pollution. Although both tGSH and GSSG:GSH seem to indicate stress, there are a number of unsolved problems. When the tGSH levels differ between, for example, populations or sexes, interpretation of the GSSG:GSH ratio can be incorrect. Thus, the higher oxidative stress ratio in males compared to females may relate to the lower male tGSH levels rather than differences in stress.
Adults had generally higher tGSH levels than nestlings (Fig. 1a), which could be an adaptive response to the physical strain and other stressors (e.g., predators, parasites, adverse climate, or poor nutrition during preceding winter) experienced by breeding adults, as compared to 13-day-old nestlings sitting mostly immobile, warm, and safe in the nestbox along with a not fully developed defense system. It might, however, also derive from a difference in protein limitation (particularly cystein, as discussed below in relation to environmental effects) between adults and chicks, probably not in ingested amounts (which, if anything, should be higher in chicks) but rather from biased allocation to growth in nestlings.
Among nestlings, there were significant family effects on glutathione levels, as expected from sharing genes, parental effects, and environment, but no consistent difference in relation to degree of air pollution (Fig. 1). The suburban nestlings had the highest GSSG:GSH ratio, along with also having the highest tGSH concentration (Fig. 1). Some additional effect, besides the simple rural-urban environmental gradient, seems to have affected the suburban nestling tGSH levels. One possibility is variation between habitats in dietary access to cystein, which is the limiting amino acid for glutathione synthesis (see below). However, we know of no study showing habitat variation in cysteine availability.
The obvious alternative explanation to the elevated glutathione measures in suburban nestlings is that they, in this area (Gunnebo, approximately 5 km SE of the city center), indeed are exposed to more or qualitatively different anthropogenic stress compared to both urban and rural nestlings. One possibility might be pollution (SOx, NOx) from a nearby paper mill, Klippan Bruk AB, 3–4 km west (i.e., usually upwind) of the nestbox area. This may add to, and also photochemically interact with, traffic-generated pollution (Kley et al., 1999). Why this would not equally affect the adult birds, we do not know, but possibly their longer history of pollution exposure (during the preceding winter) or other stressors, adds up to less than for the urban birds.
As regards the relationship with carotenoid pigmentation, we detected no significant correlation between individual measures of plumage color and either of the glutathione measurements. However, on a population-level, we found that urban adult great tits, which had the highest oxidative stress level (GSSG:GSH) (Fig. 1b) also had (on average) the palest yellow flank plumage (Fig. 1c). Among nestlings, however, urban birds were again the least pigmented, but the highest average stress level was found in the suburban habitat. Thus, while carotenoid coloration may act as an indicator of pollution exposure, it is uncertain whether this is mediated by oxidative stress. Instead, carotenoid coloration may be primarily (or entirely) dependent on differences in carotenoid nutrition between habitats (see, e.g., Partali et al., 1987). Air pollution is known to decrease the abundance of different insects (Eeva et al., 1998), and thus the caterpillars that are the major source of carotenoids (lutein and zeaxanthin) for great tits (Partali et al., 1987). However, whether it is through direct oxidative stress or through poorer diet, carotenoid coloration may still be a useful indicator of environmental disturbance.
Finally, if the measure tGSH indeed reflects a general mobilization of the glutathione system in response to oxidative stress, how can we explain why urban birds do not have higher levels of tGSH than rural birds? One possible physiological answer is that base levels of GSH in adult birds are already high enough to accommodate the increased antioxidant defense, i.e., without increasing the supply of GSH in the plasma. Another possibility is that there is some environmental or physiological limitation on tGSH levels that is reached by adults in all three habitats. One responsible mechanism could be cysteine availability which, as mentioned, is the rate-limiting amino acid of GSH synthesis (Meister and Anderson, 1983). The significant effects of sex and family suggest that genetic and small-scale ecological factors may be important for plasma glutathione levels. More detailed studies of individual regulation and constraints of GSH levels in wild animals are clearly needed.
CONCLUSIONS
As judged from the glutathione antioxidant activity in blood plasma, adult great tits breeding in urban habitats were, on average, exposed to higher oxidative stress than rural birds, and also had significantly paler yellow plumage. Among nestlings, plumage was also palest in the urban environment, but the strongest oxidative stress (although generally lower than in adults) was found in a suburban habitat. While much of the substantial individual variation thus remains unexplained, the glutathione system and carotenoid pigmentation deserve further attention as promising biomarkers of environmental stress in wild bird populations.
References
Andersson S, Prager M (2005) Quantification of avian coloration, Part 1. In: Mechanisms and Measurements, Hill GE, McGraw KJ (editors), Cambridge, MA: Harvard University Press, in press
MA Baker GJ Cerniglia A Zaman (1990) ArticleTitleMicrotiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples Analytical Biochemistry 190 360–365 Occurrence Handle10.1016/0003-2697(90)90208-Q Occurrence Handle2291479
WG Bottje S Wang KW Beers D Cawthon (1998) ArticleTitleLung lining fluid antioxidants in male broilers: age-related changes under thermoneutral and cold temperature conditions Poultry Science 77 1905–1912 Occurrence Handle9872595
A Brush (1978) Avian pigmentation M Florkin TS Bradley A Brush (Eds) Chemical Zoology, Vol 10 Academic Press New York 141–164
AH Brush (1990) ArticleTitleMetabolism of carotenoid pigments in birds FASEB Journal 4 2969–2977 Occurrence Handle2394316
S Cramp CM Perrins (1993) The Birds of the Western Palearctic, Vol VIII Oxford University Press Oxford
T Dauwe L Bervoets R Blust R Pinxten M Eens (2000) ArticleTitleCan excrement and feathers of nestling songbirds be used as biomonitors for heavy metal pollution? Archives of Environmental Contamination and Toxicology 39 541–546 Occurrence Handle10.1007/s002440010138 Occurrence Handle11031316
T Dauwe SG Chu A Covaci P Schepens M Eens (2003) ArticleTitleGreat tit (Parus major) nestlings as biomonitors of organochlorine pollution Archives of Environmental Contamination and Toxicology 44 89–96 Occurrence Handle10.1007/s00244-002-1243-y Occurrence Handle12434223
DA Dickinson AL Levonen DR Moellering EK Arnold HQ Zhang VM Darley-Usmar et al. (2004) ArticleTitleHuman glutamate cysteine ligase gene regulation through the electrophile response element Free Radical Biology and Medicine 37 1152–1159 Occurrence Handle10.1016/j.freeradbiomed.2004.06.011 Occurrence Handle15451055
M Eens R Pinxten RF Verheyen R Blust L Bervoets (1999) ArticleTitleGreat and blue tits as indicators of heavy metal contamination in terrestrial ecosystems Ecotoxicology and Environmental Safety 44 81–85 Occurrence Handle10.1006/eesa.1999.1828 Occurrence Handle10499992
T Eeva E Lehikoinen (1995) ArticleTitleEgg shell quality, clutch size and hatching success of the great tit (Parus major) and the pied flycatcher (Ficedula hypoleuca) in an air pollution gradient Oecologia 102 312–323 Occurrence Handle10.1007/BF00329798
T Eeva E Lehikoinen M Ronka (1998) ArticleTitleAir pollution fades the plumage of the great tit Functional Ecology 12 607–612 Occurrence Handle10.1046/j.1365-2435.1998.00221.x
T Eeva S Tanhuanpaa C Rabergh S Airaksinen M Nikinmaa E Lehikoinen (2000) ArticleTitleBiomarkers and fluctuating asymmetry as indicators of pollution-induced stress in two hole-nesting passerines Functional Ecology 14 235–243 Occurrence Handle10.1046/j.1365-2435.2000.00406.x
B Enkvetchakul NB Anthony WG Bottje (1995) ArticleTitleLiver and blood glutathione in male broiler-chickens, turkeys, and quail Poultry Science 74 885–889 Occurrence Handle7603965
Forsberg B, Modig L, Svanberg P-A, Segerstedt B (2003) Hälsokonsekvenser av ozon- en kvantifiering av det marknära ozonets korttidseffekter på antalet sjukhusinläggningar och dödsfall i Sverige. Rapport på uppdrag av Statens folkhälsoinstitut
TW Goodwin (1984) The Biochemistry of Carotenoids, Vol II: Animals EditionNumber2 Chapman & Hall New York
R Griffiths MC Double K Orr RJG Dawson (1998) ArticleTitleA DNA test to sex most birds Molecular Ecology 7 1071–1075 Occurrence Handle10.1046/j.1365-294x.1998.00389.x Occurrence Handle9711866
GE Hill (1999) ArticleTitleIs there an immunological cost to carotenoid-based ornamental coloration? American Naturalist 154 589–595 Occurrence Handle10.1086/303264 Occurrence Handle10561131
DJ Hoffman (2002) ArticleTitleRole of selenium toxicity and oxidative stress in aquatic birds Aquatic Toxicology 57 11–26 Occurrence Handle10.1016/S0166-445X(01)00263-6 Occurrence Handle11879935
P Hõrak I Ots H Vellau C Spottiswoode AP Moller (2001) ArticleTitleCarotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits Oecologia 126 166–173 Occurrence Handle10.1007/s004420000513
P Hõrak H Vellau I Ots AP Moller (2000) ArticleTitleGrowth conditions affect carotenoid-based plumage coloration of great tit nestlings Naturwissenschaften 87 460–464 Occurrence Handle10.1007/s001140050759 Occurrence Handle11129946
PM Kidd (1997) ArticleTitleGlutathione: systemic protectant against oxidative and free radical damage Alternative Medicine Review 2 155–176
Kindbom K, Svensson A, Sjöberg K, Pihl Karlsson G (2001) Trends in air concentration and deposition at background monitoring sites in Sweden—major inorganic compounds, heavy metals and ozone. IVL report B1429. Available: http://hera.naturvardsverket.se/opac401/sv/opac/visa_exemplar. asp?bokid=0000038381&Host_nr=1
D Kley M Kleinmann H Sanderman S Krupa (1999) ArticleTitlePhotochemical oxidants: state of the science Environmental Pollution 100 19–42 Occurrence Handle10.1016/S0269-7491(99)00086-X Occurrence Handle15093111
E Klika DW Scheuermann MHAD Groodt-Lasseel I Bazantova A Switka (1996) ArticleTitlePulmonary macrophages in birds (barn owl, Tyto tyto alba), domestic fowl (Gallus gallus f. domestica), quail (Coturnix coturnix), and pigeons (Columbia livia) Anatomical Record 246 87–97 Occurrence Handle10.1002/(SICI)1097-0185(199609)246:1<87::AID-AR10>3.0.CO;2-0 Occurrence Handle8876827
S Llacuna A Gorriz M Durfort J Nadal (1993) ArticleTitleEffects of air-pollution on passerine birds and small mammals Archives of Environmental Contamination and Toxicology 24 59–66 Occurrence Handle10.1007/BF01061089 Occurrence Handle8466292
S Llacuna A Gorriz C Sanpera J Nadal (1995) ArticleTitleMetal accumulation in 3 species of passerine birds (Emberiza-cia, Paras-major, and Turdus-merula) subjected to air-pollution from a coal-fired power-plant Archives of Environmental Contamination and Toxicology 28 298–303 Occurrence Handle10.1007/BF00213105
C Lorz J Lopez (1997) ArticleTitleIncidence of air pollution in the pulmonary surfactant system of the pigeon (Columba livia) Anatomical Record 249 206–212 Occurrence Handle10.1002/(SICI)1097-0185(199710)249:2<206::AID-AR7>3.0.CO;2-V Occurrence Handle9335466
SR Marcus MV Chandrakala HA Nadiger (1993) ArticleTitleInteraction between vitamin-E and glutathione in rat-brain—effect of acute alcohol administration Journal of Nutritional Biochemistry 4 336–340 Occurrence Handle10.1016/0955-2863(93)90078-B
Medina S, Plasència A, Artazcoz L, Quénel P, Katsouyanni K, Mücke H-G, et al. (2002) APHEIS Health Impact Assessment of Air Pollution in 26 European Cities. APHEIS 225 p. Available: http://www.apheis.nat/pages/communications.htm
A Meister ME Anderson (1983) ArticleTitleGlutathione Annual Review of Biochemistry 52 711–760 Occurrence Handle10.1146/annurev.bi.52.070183.003431 Occurrence Handle6137189
ME Murphy JR King (1990) ArticleTitleDiurnal changes in tissue glutathione and protein pools of molting white-crowned sparrows—the influence of photoperiod and feeding schedule Physiological Zoology 63 1118–1140
KJ Navara GE Hill (2003) ArticleTitleDietary carotenoid pigments and immune function in a songbird with extensive carotenoid-based plumage coloration Behavioral Ecology 14 909–916 Occurrence Handle10.1093/beheco/arg085
N Noguchi E Niki (1998) Chemistry of active oxygen species and antioxidants AM Papas (Eds) Antioxidant Status, Diet, Nutrition, and Health CRC Press Boca Raton, FL 3–20
T Part (2001) ArticleTitleThe effects of territory quality on age-dependent reproductive performance in the northern wheatear, Oenanthe oenanthe Animal Behaviour 62 379–388 Occurrence Handle10.1006/anbe.2001.1754
V Partali S Liaaenjensen T Slagsvold JT Lifjeld (1987) ArticleTitleCarotenoids in food-chain studies. 2. The food-chain of Parus spp monitored by carotenoid analysis. Comparative Biochemistry and Physiology. B: Biochemistry and Molecular Biology 87 885–888
Persson K (2002) Luftkvaliteten i Sverige sommaren 2001 och vintern 2001/02. Resultatet från mätningar inom URBAN-projektet. IVL report B1478. Available: http://www.ivl.se/rapporter/pdf/B1478.pdf
PHN Saldiva GM Bohm (1998) ArticleTitleAnimal indicators of adverse effects associated with air pollution Ecosystem Health 4 230–235 Occurrence Handle10.1046/j.1526-0992.1998.98098.x
T Sandström A Blomberg R Helleday B Rudell (2000) ArticleTitleAllergy and automobile pollution: experiments in animals. Revue Francaise D Allergologie et D Immunologie Clinique 40 47–51
H Sies (1986) Biochemistry of Oxidative Stress, Angewandte Chemie-International Edition, Volume 25 VCH Publishers Deerfield Beach, FL 1058–1071
E Stephensen J Sturve L Forlin (2002) ArticleTitleEffects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comparative Biochemistry and Physiology C: Toxicology and Pharmacology 133 435–442
E Stephensen J Svavarsson J Sturve G Ericson M Adolfsson-Erici L Forlin (2000) ArticleTitleBiochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland Aquatic Toxicology 48 431–442 Occurrence Handle10.1016/S0166-445X(99)00062-4 Occurrence Handle10794829
R Stradi J Hudon G Celentano E Pini (1998) ArticleTitleCarotenoids in bird plumage: the complement of yellow and red pigments in true woodpeckers (Picinae). Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology 120 223–230 Occurrence Handle10.1016/S0305-0491(98)10033-0
L Svensson (1992) Identification Guide to European Passerines Märstatryck Stockholm
R Oost Particlevan der J Beyer NPE Vermeulen (2003) ArticleTitleFish bioaccumulation and biomarkers in environmental risk assessment: a review Environmental Toxicology and Pharmacology 13 57–149 Occurrence Handle10.1016/S1382-6689(02)00126-6
C Vandeputte I Guizon I Genestiedenis B Vannier G Lorenzon (1994) ArticleTitleA microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells—performance study of a new miniaturized protocol Cell Biology and Toxicology 10 415–421 Occurrence Handle10.1007/BF00755791 Occurrence Handle7697505
ME Visser CM Lessells (2001) ArticleTitleThe costs of egg production and incubation in great tits (Parus major) Proceedings of the Royal Society of London. Series B: Biological Sciences 268 1271–1277 Occurrence Handle10.1098/rspb.2001.1661
Acknowledgments
We thank Lars Förlin for valuable suggestions at the onset of the project, and for comments on the manuscript. We also thank Stefanie Bergmann, Anders Malmsten, and Uno Unger for field assistance; Anette Johansson and Mats Hulander for help in the lab; Joachim Sturve for discussions; and Tobias Uller for statistical advice and comments on the manuscript. Furthermore, we thank the anonymous reviewers for useful comments on the manuscript. Financial support was provided by the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (FORMAS) to S.A., and Helge Ax:son Johnsons stiftelse to C.I. The study was conducted in compliance with Swedish laws and regulations, including ethical permit from Centrala Försökdjursnämnden, CFN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isaksson, C., Örnborg, J., Stephensen, E. et al. Plasma Glutathione and Carotenoid Coloration as Potential Biomarkers of Environmental Stress in Great Tits. EcoHealth 2, 138–146 (2005). https://doi.org/10.1007/s10393-005-3869-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-005-3869-5