Abstract
A new, simple, and rapid separation and preconcentration procedure, for determination of Pb(II), Cd(II), Zn(II), and Co(II) ions in environmental real samples, has been developed. The method is based on the combination of coprecipitation of analyte ions by the aid of the Mo(VI)–diethyldithiocarbamate–(Mo(VI)-DDTC) precipitate and flame atomic absorption spectrometric determinations. The effects of experimental conditions like pH of the aqueous solution, amounts of DDTC and Mo(VI), standing time, centrifugation rate and time, sample volume, etc. and also the influences of some foreign ions were investigated in detail on the quantitative recoveries of the analyte ions. The preconcentration factors were found to be 150 for Pb(II), Zn(II) and Co(II), and 200 for Cd(II) ions. The detection limits were in the range of 0.1–2.2 μg L−1 while the relative standard deviations were found to be lower than 5 % for the studied analyte ions. The accuracy of the method was checked by spiked/recovery tests and the analysis of certified reference material (CRM TMDW-500 Drinking Water). The procedure was successfully applied to seawater and stream water as liquid samples and baby food and dried eggplant as solid samples in order to determine the levels of Pb(II), Cd(II), Zn(II), and Co(II) ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The controlling of heavy metal levels in environmental samples is an important application in analytical chemistry since most of the heavy metals have negative or positive effects on living organisms especially on human bodies depending on their concentration levels. Although some of the metals such as iron, copper, zinc, and cobalt have a variety of biochemical functions in living organisms, they can be toxic when taken in excess. On the other hand, some metals like lead and cadmium are non-essential and their presence even in trace levels results in damaged or reduced mental and central nervous function, lower energy levels, damage the blood composition, lung, kidneys, liver, and other vital organs (Afridi et al. 2006; Cockerham and Shane 1993). Hence, the accurate and precise determination of heavy metals in environmental samples is important in order to estimate the risk level of them. For that purpose, due to its low cost, easy instrument usage, high speed, and good selectivity, flame atomic absorption spectrometry (FAAS) is a widely preferred method for determination of heavy metals at trace levels, besides different instrumental techniques such as spectrophotometry, electroanalytical techniques, and inductively coupled plasma atomic emission spectrometry (Srogi 2008). However, the lower analyte levels than the detection limits of FAAS and positive or negative effects of matrix ions on the analyte signal, limit the direct usage of FAAS in determination of metal ions. Therefore, separation/preconcentration techniques such as solid phase extraction (Elci et al. 2007), ion exchange (Murgia et al. 2011), cloud point extraction (Favre-Reguillon et al. 2011), membrane filtration (Soylak et al. 2007), liquid–liquid microextraction (Salahinejad and Aflaki 2011), and coprecipitation (Divrikli et al. 2008) are generally required prior to FAAS determination.
Coprecipitation, which occurs with retention of metal ions at trace levels onto precipitate surface via various mechanisms including surface adsorption, ion exchange, surface precipitation, and occlusion (Pereira and Arruda 2003), is a commonly utilized technique for separation and preconcentration of trace metal ions because of some advantages including simplicity, rapidity, ability to attain a high preconcentration factor, and low consumption of organic solvents. Besides, both separation and preconcentration steps can be applied in the same step and several analyte ions can be preconcentrated and separated from the matrix simultaneously by using various organic (Bulut et al. 2008, 2009; Duran et al. 2009a, b, 2011) or inorganic coprecipitants (Citak et al. 2009; Doner and Ege 2005; Peker et al. 2007) as efficient collectors of trace elements. In coprecipitation procedure, a precipitate is occurred by the combination of a carrier element such as Ni, Cu, Al, Mo, Mg, and Er, etc. with a suitable organic ligand like dithiocarbamates (Chen et al. 1997), chitosan (Minamisawa et al. 1999), violuric acid (Saracoglu et al. 2006), rubeanic acid (Soylak and Erdogan 2006), and 8-hydroxyquinoline (Bulut et al. 2010) or inorganic ligand like hydroxides (Minami et al. 2005). According to our literature survey, no coprecipitation study has been performed by using the combination of Mo(VI) and diethyldithiocarbamate (DDTC).
In the present study, a simple and rapid coprecipitation procedure is proposed for the separation and preconcentration of Pb(II), Cd(II), Zn(II), and Co(II) ions by using DDTC, as an organic coprecipitant, and Mo(VI), as carrier element, prior to their FAAS determinations. The influences of the various analytical parameters such as the effects of pH, quantity of DDTC and Mo(VI), standing time, centrifugation rate and time, and sample volume, etc. were investigated on the recoveries of the examined metal ions. After validation of the method by certified reference materials and spike tests, it was applied to determine the analyte ions in several solid and liquid environmental samples.
Experimental
Apparatus
The analyte measurements were performed by using a Unicam model AA-929 flame atomic absorption spectrometer with an air/acetylene flame. The instrumental parameters were those recommended by the manufacturer. The pH measurements were taken using Hanna pH-211 (HANNA instruments, Romania) digital pH meter with glass electrode. Sigma 3-16P (Sigma Laborzentrifugen GmbH, Germany) model centrifuge was used to centrifuge solutions. Milestone Ethos D (Milestone Inc., Italy) closed vessel microwave system (maximum pressure, 1,450 psi; maximum temperature, 300 °C) was operated for digestion of the solid samples.
Reagents and solutions
All of the chemicals used in this work were of analytical grade from Merck (Darmstadt, Germany) or Fluka (Buchs, Switzerland). Distilled/deionized water was obtained from Sartorius Milli-Q system (arium® 611UV) and used for all the experiments. Dilute HNO3 and NaOH solutions were used for pH adjustments. A stock solution of Mo(VI) (1.0 g L−1) was prepared daily by dissolving appropriate amounts of (NH4)6Mo7O24.4H2O in small amounts of 0.5 mol L−1 HNO3 and diluting to 50 mL with distilled/deionized water. Stock standard solutions of the analytes (1.0 g L−1) were prepared by dissolving appropriate amounts of nitrate salts of analytes in 1 % nitric acid. The stock metal ions solution was diluted daily for obtaining reference and working solutions. A 0.5 % (w/v) solution of DDTC, as coprecipitating agent, was prepared daily in ethanol. The drinking water standard (CRM-TMDW-500), used as a certified reference material in the experimental studies, was obtained from High-Purity Standard Inc.
Working model
Prior to preconcentration of analyte ions from real samples, Mo(VI)-DDTC coprecipitation method was tested with model solutions. For that purpose, approximately 50 mL of an aqueous solution containing 50 μg of Pb(II), 10 μg of Zn(II) and Cd(II), and 25 μg of Co(II) ions was placed in a centrifuge tube and 0.75 mL of 1.0 g L−1 Mo(VI) solution as a carrier element was added to this solution. After adjustment of the pH to 4.5, 0.6 mL of DDTC solution (0.5 % (w/v)) was added to the tube. After standing for 10 min, the solution was centrifuged at 2,750 rpm for 15 min. The supernatant was removed and the precipitate remained adhering to the tube was dissolved with 1.0 mL of conc. HNO3. The final volume was completed to 5.0 mL with distilled/deionized water, and then the content was analyzed by FAAS for determination of analyte ions.
Analysis of real samples
The present procedure was applied to various solid and liquid samples; baby food and dried eggplant as solid samples, stream water (Çaykara–Trabzon/Turkey) and seawater (Black Sea–Trabzon/Turkey) as liquid samples. The method was also applied to CRM-TMDW-500 Drinking Water as certified reference material. Various amounts of analyte ions were spiked to solid and liquid real samples.
The stream and seawater samples were collected in prewashed polyethylene bottles. After filtration through a Millipore cellulose nitrate membrane of pore size 0.45 μm, the samples were acidified with 1 mL of 1 % nitric acid, and stored at 4 °C in a refrigerator. Before the analysis, the pH of the liquid samples was adjusted to 4.5. Then, appropriate amounts of DDTC were added and the procedure given above was applied. The final volume of the solutions was diluted to 5 mL with distilled/deionized water. The levels of the analyte ions in the samples were determined by FAAS.
Twenty five milliliters of CRM-TMDW-500 Drinking Water were taken and the preconcentration procedure given in Working model section was applied to these samples. The solid samples were digested with a closed microwave digestion system. For this, 0.50 g of baby food and dried eggplant samples were weighed with sensitivity of 0.1 mg into teflon vessels separately. Added into the vessels were 4.5 mL of HCl, 1.5 mL of HNO3, 2 mL H2O2, and 1 mL HF. Digestion conditions for the microwave system for the samples were applied as (45 bar) 6 min for 250 W, 6 min for 400 W, 6 min for 650 W, 6 min for 250 W; vent, 3 min. After microwave digestion, the volume of the samples was made up to 100 mL with distilled/deionized water and the presented method was applied. The final volume was 5 mL.
Results and discussion
Effect of pH on the coprecipitation efficiency of the analyte ions
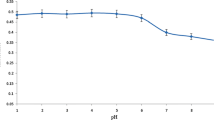
The pH of the aqueous solution is an important factor to obtain the quantitative coprecipitation efficiency for heavy metal ions. Hence, the effects of the pH on the Mo(VI)-DDTC coprecipitation system were investigated in the pH range of 1.0–10.0 by using 0.75 mg (0.75 mL of 1.0 g L−1) of Mo(VI) and 3.0 mg (0.6 mL of 0.5 % (w/v)) of DDTC. The pH of the model solutions were adjusted by the addition of dilute HNO3 and NaOH solutions. The quantitative recovery values (>95 %) were obtained in the pH range of 4.0–10.0 for all investigated metal ions hence all subsequent works were performed at pH 4.5 (Fig. 1).
Effect of DDTC amount
The effects of DDTC amount on the coprecipitation efficiency of analyte ions were examined in the DDTC amount range of 0–10.0 mg. The recovery of Cd(II), Co(II), and Zn(II) ions increased with increasing the amounts of DDTC from 0 to 10.0 mg and the quantitative recovery values were observed in the DDTC amount range of 2.0–10.0 mg while the recovery of Pb(II) ions increased with increasing the DDTC amount from 0 to 5.0 mg, then it suddenly decreased after the DDTC amount is >5.0 mg (Fig. 2). These results indicated that for quantitative and simultaneous recovery of Cd(II), Co(II), Zn(II), and Pb(II) ions, DDTC is necessary. All subsequent experiments were performed by using 3.0 mg (0.6 mL of 0.5 %, w/v) of DDTC solution.
Effect of amounts of Mo(VI) as carrier element
The influences of the amount of Mo(VI) as carrier element on the quantitative recoveries of analyte ions were also investigated in the Mo(VI) amount range of 0–2.0 mg. The recovery values for all the studied analyte ions increased with increasing the amount of Mo(VI). Without using Mo(VI), the recovery values for Cd(II), Zn(II), and Pb(II) ions were below 60 % and for Co(II) below 75 % (Fig. 3). These results showed that the addition of Mo(VI) is necessary for the quantitative recovery of the analyte ions by the presented coprecipitation method. Quantitative recovery values were obtained for the analyte ions in the Mo(VI) amount range of 0.6–2.0 mg. In light of these results, all further works were performed with 0.75 mg (0.75 mL of 1 g L−1) of Mo(VI) ions as carrier element.
Effects of standing time, centrifuge rate, and time
Because of the importance of standing time, centrifugation time, and rate on the formation of the precipitate, these parameters also should be optimized. For that purpose, 0.75 mg of Mo(VI) (0.75 mL of 1.0 g L−1) and 3.0 mg (0.6 mL of 0.5 %, w/v) of DDTC were added into a series of aqueous solution (50 mL) containing analyte ions. After the pH of the solutions was adjusted to 4.5, they were kept standing from 0 to 20 min. After 10 min of standing time, the quantitative recoveries for all analyte ions were obtained. This period of standing time was enough for the formation of precipitate and adsorption of trace metal ions on the precipitate.
The effects of the centrifugation rate on the recoveries of analyte ions were evaluated in the range of 1,500–3,500 rpm under optimal conditions. The quantitative recoveries were obtained for all the studied analyte ions at 2,750 rpm. And the effects of centrifugation time were tested in the range of 5–25 min at 2,750 rpm. After 15 min, quantitative recoveries were obtained, so all the other works were performed at 2,750 rpm for 15 min.
Effects of sample volume
The sample volume is an important parameter to obtain a high preconcentration factor in the analysis of real samples. Therefore, the sample volume on the recoveries of the studied analyte ions were investigated in the sample volume range of 50–1,500 mL containing 50 μg of Pb(II), 10 μg of Zn(II) and Cd(II), and 25 μg of Co(II) ions by using model solutions. As can be seen from Fig. 4, the analyte ion recoveries were quantitative up to 750 mL for Zn(II), Pb(II), and Co(II) ions while 1,000 mL for Cd(II) ions. The preconcentration factor is calculated by the ratio of the highest sample volume and the lowest final volume, and it was found as 150 for Zn(II), Pb(II) and Co(II) ions and 200 for Cd(II) ions when the final volume was 5.0 mL.
Effects of matrix ions
Most of the heavy metal ions may present together with the interfering matrix ions in the environmental real samples. Hence, the effects of different foreign ions on the present separation and preconcentration procedure were evaluated under the optimum experimental conditions. For these studies, different amounts of each foreign ions were added to the model solutions containing 50 μg of Pb(II), 10 μg of Zn(II) and Cd(II), and 25 μg of Co(II) ions and the presented procedure was applied. The presence of interfering anions and cations and also transition metal ions at milligram per liter levels has no significant interference effects on the coprecipitation of the studied analyte ions (Table 1). In the light of these results, it can be concluded that the proposed separation and preconcentration method, based on the coprecipitation by Mo(VI)-DDTC precipitate, can be applied to the samples containing high amount of salts and some transition metal ions at concentration levels given in Table 1.
Analytical performance of the method
The precision of the method was evaluated as the relative standard deviation (RSD). In order to evaluate the precision of the determination of the analyte ions (50 μg of Pb(II), 10 μg of Zn(II) and Cd(II), and 25 μg of Co(II) ions in 50 mL aqueous solution) the procedure was repeated 10 times under optimum conditions mentioned above. From the results, the RSD values (percentage) were found to be 3.6, 3.1, 4.1, and 2.5 for Zn(II), Cd(II), Co(II), and Pb(II) ions, respectively. The limit of detections (LOD), defined as the concentration that gives a signal equivalent to three times the standard deviation of 10 replicate measurements of the blank samples, were calculated by dividing the instrumental detection limit by the preconcentration factors (150 for Zn(II), Co(II) and Pb(II), 200 for Cd(II)) and found to be 0.2, 0.1, 0.6, and 2.2 μg L−1 for Zn(II), Cd(II), Co(II), and Pb(II) ions, respectively.
Method validation and applications to real samples
In order to evaluate the accuracy of the method, different amounts of the analyte ions were spiked in 50 mL of sea water, stream water, 0.50 of baby food and dried eggplant samples. The coprecipitation procedure given above was applied to the samples. Final volume of the solutions was 5.0 mL. The results were given in Tables 2 and 3. In most cases, a good agreement was obtained between the added and measured analyte amounts. The results showed that the presented coprecipitation method can be applied for the separation and preconcentration of the analyte ions in environmental liquid and solid samples.
The certified reference material (CRM-TMDW-500 Drinking Water) was also used for the method validation. Good agreement was obtained between analytical and certified values (Table 4).
After verification of the accuracy of the present method, the coprecipitation procedure was applied to baby food and dried eggplant as solid samples and stream and seawater as liquid samples. After applying the present separation and preconcentration procedure, based on the coprecipitation by Mo(VI)-DDTC precipitate, to the environmental real samples, the obtained results were tabulated in Table 5.
Conclusions
The developed method, based on the coprecipitation of Zn(II), Co(II), Pb(II), and Cd(II) ions by the aid of Mo(VI)-DDTC precipitate, offers a simple, rapid, and low-cost separation and preconcentration technique for accurate and precise determination of analyte ions in environmental solid and liquid samples. The coprecipitated analyte ions can be sensitively determined by atomic absorption spectrometry without any influence of Mo(VI) and DDTC. A comparison of the proposed method with other coprecipitation methods (Duran et al. 2009a; Saracoglu and Soylak 2010; Elci et al. 1997; Citak et al. 2009; Soylak and Balgunes 2008; Cui et al. 2007; Narin et al. 2007; Ghaedi et al. 2008; Divrikli et al. 2007; Soylak et al. 2010 ) is summarized in Table 6 in terms of some analytical parameters including preconcentration factor, limit of detection, and relative standard deviation. In most cases, the proposed coprecipitation method has high preconcentration factor, low RSD, and relatively low LOD values when compared the other methods reported in Table 6. The presented method was successfully employed for determination of Zn(II), Co(II), Pb(II), and Cd(II) ion levels in seawater, stream water, baby food, and dried eggplant samples.
References
Afridi, H. I., Kazi, T. G., Jamali, M. K., Kazi, G. H., Arain, M. B., Jalbani, N., et al. (2006). Evaluation of toxic metals in biological samples (scalp hair, blood and urine) of steel mill workers by electrothermal atomic absorption spectrometry. Toxicology and Industrial Health, 22, 381–393.
Bulut, V. N., Duran, C., Gundogdu, A., Soylak, M., Yildirim, N., & Elci, L. (2008). A new approach to separation and pre-concentration of some trace metals with co-precipitation method using a triazole. Talanta, 76, 469–474.
Bulut, V. N., Ozdes, D., Bekircan, O., Gundogdu, A., Duran, C., & Soylak, M. (2009). Carrier element-free coprecipitation (CEFC) method for the separation, preconcentration and speciation of chromium using an isatin derivative. Analytica Chimica Acta, 632, 35–41.
Bulut, V. N., Arslan, D., Ozdes, D., Soylak, M., & Tufekci, M. (2010). Preconcentration, separation and spectrophotometric determination of aluminium(III) in water samples and dialysis concentrates at trace levels with 8-hydroxyquinoline–cobalt(II) coprecipitation system. Journal of Hazardous Materials, 182, 331–336.
Chen, H., Jin, J., & Wang, Y. (1997). Flow injection on-line coprecipitation preconcentration system using copper(II) diethyldithiocarbamate as carrier for flame atomic absorption spectrometric determination of cadmium, lead and nickel in environmental samples. Analytica Chimica Acta, 353, 181–188.
Citak, D., Tuzen, M., & Soylak, M. (2009). Simultaneous coprecipitation of lead, cobalt, copper, cadmium, ironand nickel in food samples with zirconium(IV) hydroxide prior to their flame atomic absorption spectrometric determination. Food and Chemical Toxicology, 47, 2302–2307.
Cockerham, L. G., & Shane, B. S. (1993). Basic environmental toxicology (1st ed.). Boca Raton: CRC Press.
Cui, Y., Chang, X., Zhu, X., Luo, H., Hu, Z., Zou, X., et al. (2007). Chemically modified silica gel with p-dimethylaminobenzaldehyde for selective solid-phase extraction and preconcentration of Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by ICP-OES. Microchemical Journal, 87, 20–26.
Divrikli, U., Kartal, A. A., Soylak, M., & Elci, L. (2007). Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations. Journal of Hazardous Materials, 145, 459–464.
Divrikli, U., Soylak, M., & Elci, L. (2008). Determination of total chromium by flame atomic absorption spectrometry after coprecipitation by cerium (IV) hydroxide. Environmental Monitoring and Assessment, 138, 167–172.
Doner, G., & Ege, A. (2005). Determination of copper, cadmium and lead in seawater and mineral water by flame atomic absorption spectrometry after coprecipitation with aluminum hydroxide. Analytica Chimica Acta, 547, 14–17.
Duran, C., Bulut, V. N., Gundogdu, A., Ozdes, D., Yildirim, N., Soylak, M., et al. (2009a). Carrier element-free coprecipitation with 3-phenly-4-o-hydroxybenzylidenamino-4,5-dihydro-1,2,4-triazole-5-one for separation/preconcentration of Cr(III), Fe(III), Pb(II) and Zn(II) from aqueous solutions. Journal of Hazardous Materials, 167, 294–299.
Duran, C., Bulut, V. N., Ozdes, D., Gundogdu, A., & Soylak, M. (2009b). A novel method for speciation of chromium: coprecipitation without carrier element by using a triazole derivative. Journal of AOAC International, 92, 257–262.
Duran, C., Ozdes, D., Sahin, D., Bulut, V. N., Gundogdu, A., & Soylak, M. (2011). Preconcentration of Cd(II) and Cu(II) ions by coprecipitation without any carrier element in some food and water samples. Microchemical Journal, 98, 317–322.
Elci, L., Şahin, U., & Öztaş, S. (1997). Determination of trace amounts of some metals in samples with high salt content by atomic absorption spectrometry after cobalt-diethyldithiocarbamate coprecipitation. Talanta, 44, 1017–1023.
Elci, L., Sahan, D., Basaran, A., & Soylak, M. (2007). Solid phase extraction of gold(III) on Amberlite XAD-2000 prior to its flame atomic absorption spectrometric determination. Environmental Monitoring and Assessment, 132, 331–338.
Favre-Reguillon, A., Murat, D., & Draye, M. (2011). Study on the cloud point extraction of Gd(III) with 8-hydroxyquinoline. Separation Science and Technology, 46, 611–615.
Ghaedi, M., Shokrollahi, A., Ahmadi, F., Rajabi, H. R., & Soylak, M. (2008). Cloud point extraction for the determination of copper, nickel and cobalt ions in environmental samples by flame atomic absorption spectrometry. Journal of Hazardous Materials, 150, 533–540.
Minami, T., Sohrin, Y., & Ueda, J. (2005). Determination of chromium, copper and lead in river water by graphite-furnace atomic absorption spectrometry after coprecipitation with terbium hydroxide. Analytical Sciences, 21, 1519–1521.
Minamisawa, H., Kuroki, H., Arai, N., & Okutani, T. (1999). Coprecipitation of ruthenium with chitosan and its determination by graphite furnace atomic absorption spectrometry. Analytica Chimica Acta, 398, 289–296.
Murgia, S. M., Selvaggi, R., & Poletti, A. (2011). Determination of trace transition metals in environmental matrices by chelation ion chromatography. Environmental Monitoring and Assessment, 174, 313–326.
Narin, I., Surme, Y., Bercin, E., & Soylak, M. (2007). SP70-α-benzoin oxime chelating resin for preconcentration-separation of Pb(II), Cd(II), Co(II) and Cr(III) in environmental samples. Journal of Hazardous Materials, 145, 113–119.
Peker, D. S. K., Turkoglu, O., & Soylak, M. (2007). Dysprosium(III) hydroxide coprecipitation system for the separation and preconcentration of heavy metal contents of table salts and natural waters. Journal of Hazardous Materials, 143, 555–560.
Pereira, M. G., & Arruda, M. A. Z. (2003). Trends in preconcentration procedures for metal determination using atomic spectrometry techniques. Microchimica Acta, 141, 115–131.
Salahinejad, M., & Aflaki, F. (2011). Optimization and determination of Cd (II) in different environmental water samples with dispersive liquid–liquid microextraction preconcentration combined with inductively coupled plasma optical emission spectrometry. Environmental Monitoring and Assessment, 177, 115–125.
Saracoglu, S., & Soylak, M. (2010). Carrier element-free coprecipitation (CEFC) method for separation and pre-concentration of some metal ions in natural water and soil samples. Food and Chemical Toxicology, 48, 1328–1333.
Saracoglu, S., Soylak, M., Peker, D. S. K., Elci, L., Santos, W. N. L., Lemos, V. A., et al. (2006). A pre-concentration procedure using coprecipitation for determination of lead and iron in several samples using flame atomic absorption spectrometry. Analytica Chimica Acta, 575, 133–137.
Soylak, M., & Balgunes, H. (2008). Gadolinium hydroxide coprecipitation system for the separation–preconcentration of some heavy metals. Journal of Hazardous Materials, 155, 595–600.
Soylak, M., & Erdogan, N. D. (2006). Copper(II)–rubeanic acid coprecipitation system for separation–preconcentration of trace metal ions in environmental samples for their flame atomic absorption spectrometric determinations. Journal of Hazardous Materials, 137, 1035–1041.
Soylak, M., Divrikli, U., Saracoglu, S., & Elci, L. (2007). Membrane filtration—atomic absorption spectrometry combination for copper, cobalt, cadmium, lead and chromium in environmental samples. Environmental Monitoring and Assessment, 127, 169–176.
Soylak, M., Unsal, Y. E., Kizil, N., & Aydin, A. (2010). Utilization of membrane filtration for preconcentration and determination of Cu(II) and Pb(II) in food, water and geological samples by atomic absorption spectrometry. Food and Chemical Toxicology, 48, 517–571.
Srogi, K. (2008). Developments in the determination of trace elements by atomic spectroscopic techniques. Analytical Letters, 41, 677–724.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tufekci, M., Bulut, V.N., Elvan, H. et al. Determination of Pb(II), Zn(II), Cd(II), and Co(II) ions by flame atomic absorption spectrometry in food and water samples after preconcentration by coprecipitation with Mo(VI)-diethyldithiocarbamate. Environ Monit Assess 185, 1107–1115 (2013). https://doi.org/10.1007/s10661-012-2618-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2618-9