Abstract
This study had two objectives: 1-evaluate the efficacy of Pasteuria thornei as seed treatment for Pratylenchus zeae control in maize; 2-measure the effects of P. thornei on preventing the yield losses caused by P. zeae. Four P. thornei doses (5 × 106, 1 × 107, 1.5 × 107, and 2 × 107 endospores/seed) were tested in two efficacy experiments, along the traditional treatment with abamectin (0.5 mg/seed) and the combination of P. thornei and abamectin (1 × 107 endospores + 0.5 mg). The treatments in two subsequent yield experiments were: one P. thornei dose (2 × 107 endospores/seed), abamectin, the combination of P. thornei and abamectin, and P. zeae -free control. Experimental plots consisted of 9L pots filled with soil infested with 9,000–11,000 specimens of P. zeae. Agronomic variables, including grain weight, were measured, as well as nematode population density. The highest concentration of P. thornei reduced nematode populations by 47–54%. Synergistic effects between P. thornei and abacmetin in nematode control was not observed. Results of the yield experiments showed that P. thornei control was similar to what was observed in the efficacy study. Abamectin reduced the yield losses caused by P. zeae, the same was true for treatments containing P.thornei in a single yield experiment. Therefore, P. thornei shows potential not only for reducing P. zeae populations densities but can also mitigate yield losses caused by this phytonematode. This work is the first to demonstrate the effectiveness of P. thornei as a biological control agent for P.zeae management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Second only to wheat and rice regarding worldwide consumption, maize (Zea mays) is a highly versatile staple food serving as a rich source of carbohydrates and fibers in both human and livestock diets (Nuss & Tanumihardjo, 2010; Ranum et al., 2014). The bulk of the world’s production of this cereal comes from three countries, USA, China and Brazil, with a production of 37 × 107 t, 26 × 107 t and 9.7 × 107 t respectively (FAOSTAT, 2019). Brazil’s maize harvest has grown, reaching 99 × 106 t in 2016, being cultivated in a total area of 17.3 × 106 ha (CONAB, 2016).
Among other factors, plant diseases contribute to a fair share of losses in maize yields, these include leaf rusts, stalk roots and phytonematodes (De Waele & Jordaan, 1988; Groth et al., 1983; Sutton, 1982). In Brazil, taking into account pathogenicity and distribution, the most important nematode species for maize are Pratylenchus zeae, P. brachyurus, Helicotylenchus dihystera, Mesocriconema spp., Meloidogyne incognita and Xiphinema spp. (Casela et al., 2006). Among the root-lesion nematodes (RLN), P. zeae causes more damage to maize roots than P. brachyurus, and when both nematodes are found in a mixed infestation, P. zeae is likely to gradually replace P. brachyurus populations (Olowe & Corbett, 1976). Although ubiquitous in Brazil’s maize plantations, there is a scarcity of information regarding yield losses in maize in areas infested by P. zeae, perhaps because above ground symptoms of RLN are often neglected or mistaken for low soil fertility (Duncan & Jones, 2006; Fosu-Nyarko & Jones, 2016). Yield losses of 50% were reported in 1960s in the municipality of Piracicaba, in maize plantations where P. zeae and P. brachyurus were present (Monteiro, 1963). Symptoms such as foliage yellowing and stunted growth in patches were able to be observed in maize growing fields with P. zeae populations of 24.2 specimens/g of soil in India (Patel et al., 2002). Such strong symptoms were not frequently reported, perhaps due to the low survival capacity of RLN. However, losses caused by RLN are becoming a growing concern thanks to the popularization of no-till agriculture, which protects soil moisture by conserving the topsoil, offering a better environment for RLN survival (Jones et al., 2016).
In Brazil, two chemical formulations are currently available for P. zeae control in maize, both for seed treatment: abamectin and a mixture of thiophanate-methyl and fluazinam (AGROFIT, 2019). Experimental formulations of the bacteria Pasteuria thornei have been tested for P. brachyurus control as soybean seed treatment in Brazil, with some degree of success (Confort & Inomoto, 2018).
Bacteria of the gram-positive genus Pasteuria are obligate parasites of nematodes and crustaceans (Dickson et al., 2009). Each particular species of Pasteuria is attuned to a specific nematode genus (Chen & Dickson, 1998). This bacterium is known to parasitize all the most notorious nematode pests in agriculture, attaching itself to the nematode’s cuticle, penetrating its body and then colonizing the entirety of the host’s pseudocoelom, ultimately leading to a loss of reproductive capabilities of the host (Stirling, 2014). As a soilborne microorganism, Pasteuria spp. occurs naturally in nematode infested soil worldwide (Gonzaga & Santos, 2008; Stirling et al., 2017), circumventing some of the dangers in introducing exotic species for biological control purposes (Van Lenteren et al., 2006). Currently in the USA the seed treatment Clariva™, containing Pasteuria nishizawae isolate PN1 is commercially available for the control of Heterodera glycines, the soybean cyst nematode (Lund et al., 2018). The same isolate had been shown to control H. schachtii in sugar beet (Eberlein et al., 2020), and it is also registered in the EU under Commission Implementing Regulation (EU) 2018/1278.
Given the importance of root lesion nematodes as maize pathogens, and the scarcity of tools for its control, the goal of this study was to evaluate the efficacy of P. thornei as a potential biological control agent for P. zeae control.
Material and methods
Four greenhouse experiments were conducted during the years of 2015 and 2016 in the municipality of Piracicaba, Brazil in order to evaluate the effect of Pasteuria thornei as a maize seed treatment for Pratylenchus zeae control. Efficacy experiments were carried out in a greenhouse to determine the effect of P. thornei on P. zeae density. Yield experiments were designed to evaluate the effect of P. thornei on agronomic characteristics and yield. The currently available seed treatment with abamectin was included in the experiments and each experiment was repeated once. Soil temperatures for the duration of the experiments were obtained from an electronic thermometer and maximum and minimum temperature ranges were recorded daily (Table 1).
Seeds
Seven seed treatments were included using maize seeds of the hybrid Celeron TL: i) P. thornei 5 × 106 endospores per seed, ii) P. thornei 1 × 107 endospores, iii) P. thornei 1.5 × 107 endospores, iv) 2 × 107 endospores, v) abamectin 0.5 mg per seed (Avicta 500 FS™ 1.0 mg per seed) (Ab),, vi) P. thornei + abamectin (1 × 107 endospores + 0.5 mg per seed), vii) nontreated seeds (Ctrl + Pz). Additionally, the seeds received a commercial fungicide and insecticide seed treatment with 0.5 µL of Maxim XL™ (mixture of metalaxyl-M at 10 g/L and fludioxonil at 20 g/L) and 2.0 µL of Cruiser 350 FS™ (thiametoxam at 350 g/L) per seed.
The P. thornei isolate was obtained from an unspecified site in the USA. Mass multiplication of the P. thornei isolate used in this work is achieved through an in vitro fermentation technique, in which the natural conditions for its reproduction inside the nematode are emulated. As of the moment of this publication the details of this in vitro procedure for industrial scale are still undisclosed (Daniela Ribeiro, pers. com., 10 November 2017).
The seeds were supplied by Syngenta Proteção de Cultivos Ltda, and the seed coating procedures were performed individually for each treatment in November of 2014. Seeds of hybrid Celeron TL were treated in batches of 1 kg, following the order: 1) Maxim XL ™, 2) Cruiser 350 FS™, 3) Avicta 500 FS™ and 4) P. thornei. Double layered plastic bags were used to apply the slurries to the seeds, which were manually shaken for 2 min, ensuring the best homogeneity possible. For proper drying of the treatments, a 1-h interval ensued after each coating. The seeds were stored in a refrigerator at a temperature of 16 ºC upon receive.
Nematode collection
The P. zeae isolate was collected from sugarcane roots in a field in the municipality of Jaú (São Paulo state, Brazil) by Roberto Kubo (Scientific Researcher of Instituto Biológico de São Paulo) and maintained in pearl millet (Pennisetum glaucum), sorghum (Sorghum bicolor) and sugarcane roots (Saccharum sp.) in a greenhouse environment. The alternation of hosts is an attempt to maintain the nematode with a broad infection spectrum.
Efficacy experiments (EE)
Infected roots of millet and sorghum were processed in a blender for 60 s, and the resulting aqueous suspension was sifted through 60 and 500 mesh sieves (0.250- and 0.025-mm aperture) in order to separate the females, juveniles and eggs from the larger pieces of root tissue. The purified aqueous solution underwent a centrifugation process in a centrifuge containing 120 cm3 tubes (10 cm high × 3.9 cm diam.) at 550 g (Coolen & D’Herde, 1972). Populational density quantification in the inoculum was performed by counting the number of nematodes with the aid of a Peter’s slide under a light microscope (Olympus CH2) 100 × magnification.
Eighty-four plastic cups (twelve cups per treatment) with a volume of 500 cm3 (13.5 cm deep × 9 cm diam.) were filled with steam-treated sandy-loam soil (121 °C for 2 h), with six 2-cm-deep holes being made in the soil in each cup. In each hole a maize seed was sown. For experiment 1 (EE1), an initial population (Pi) of 4,000 nematodes consisting of mixed life stages of P. zeae was inoculated into two oblique holes of 2 and 4 cm respectively, in order to evenly maximize root exposure. Inoculation took place five days after germination. The aqueous suspension containing the infective individuals for each plot was calibrated to a maximum of 2 mL. Nematode densities were determined 60 and 90 days after the inoculation (DAI) by extracting nematodes from roots of six pots per treatment using the method described above for the inoculum. Concerning soil nematodes, as a rule, the number of Pratylenchus obtained from the soil represent a very small fraction of the total nematode population, based in the experience of the authors. Thus, the effort to recover Pratylenchus specimens from soil seems to be not worthwhile. The nematodes were counted using a light microscope at 100 × magnification with the aid of a Peters slide in order to estimate the P. zeae population 60 and 90 DAI (P60 and P90). This experiment was replicated once (EE2) with a lower Pi (1,000) due to lower inoculum availability at that time.
Yield experiments (YE)
Based on the results from the efficacy experiments five treatments were established for the YE: iv) P. thornei 2 × 107 endospores per seed; v) abamectin 0.5 mg per seed; vi) abamectin + P. thornei (0.5 mg + 1 × 107 endospores per seed); vii) nontreated seeds (Ctrl + Pz), vii) control without nematode (Ctrl – Pz). The seeds used here were from the same batches as those used in the efficiency experiments for each respective treatment.
The P. zeae isolate for these experiments derived from the same population used in the previous EE and were maintained in roots of sugarcane, which were the host available at the time of the yield experiments. Ten grams of root were separated from the soil, disintegrated for 1 min at low speed by a kitchen blender and incorporated in 8L of a steam-treated sandy-loam soil (121 °C for 2 h), in a 9-L-ceramic pot (R = 13 / r = 9 / h = 24). These minimally processed roots were used as P. zeae inoculum attempting to emulate field condition. In addition, based in the personal experience of the authors, usually Pratylenchus spp. produces more pronounced symptoms on hosts when incorporated in soil this way. The initial population (Pi) of P. zeae was estimated based on four samples of 10 g of the sugarcane roots, processed by Coolen and D’Herde (1972). Therefore, in the YE1, the estimated Pi for each pot was 9,000 specimens of P. zeae, including females, juveniles and eggs. Twenty-four ceramic pots containing steam-treated soil plus sugarcane roots were used in all treatments with P. zeae (corresponding to six pot-replications per treatment with nematode). Six pot-treatments with steam-treated soil were used as control without nematode. Three seeds were sown in each pot; however, they were thinned to only two plants 14 days after sowing (DAS). Taking into account the average stand of 60,000 plants per ha in commercial maize fields in Brazil, and two plants per experiment, a pre-sowing fertilization of 10 g of a NPK fertilizer 4–14-8 (Adubos Vera Cruz, Ibaté, São Paulo state) per pot, corresponding to 0.4 g N, 1.4 g P2O5, and 0.8 g K2O per pot.
Three root nematode evaluations were carried out along the yield experiments, in order to measure the evolution of P. zeae population densities over the course of the maize cycle: two non-destructive evaluations at 45 / 90 DAS and a final destructive evaluation at the end of the experiment, 110 DAS. At 45 and 90 DAS, two root samples were collected from each pot with the aid of a plastic cylinder (4.8 cm diam.) at a soil depth of 15 cm and 6 cm apart from the plants, at two opposite points. The roots were separated from the soil, weighted, and processed by Coolen and D’Herde (1972) method. The two holes made in the sampling were filled with steam-treated sandy-loam soil (271 cm3 per hole), enabling the root system to regrow for subsequent evaluations. A final destructive evaluation was carried out at the time of maize harvest, 110 DAS. Ten grams of roots were taken from the bulk of the radicular system of each pot and processed for nematode recovery. The reproductive variable used for comparing the treatments in YE1 was the number of P. zeae (females, juveniles, and eggs) per gram of roots 45, 90 DAS and at harvest (Ng45, Ng90 and NgHv). Productivity was measured through the weighting of the total grains of two threshed corncobs (TGW) in each pot, harvested at physiological maturity (R6), ie., when kernels have attained maximum dry weight at 30 to 35% moisture (O’Keeffe, 2009). Additionally, measurements were taken for fresh root weight (FRW), as well as the leaves + stalk weight, after drying for 72 h at 60 ºC (LSDW) in a dry oven to reach a constant mass.
The YE was replicated once (YE2) with a higher Pi (11,000 specimens of P. zeae per pot), and two additional nitrogen topdressing, at V3-4 (plants with 3–4 leaves) and V8-9 (plants with 8–9 leaves), using 5.2 g of urea (Adubos Vera Cruz, Ibaté, São Paulo state) per pot, corresponding to 2.4 g N. Crop management was enhanced in YE2, due to the very small cobs produced in the previous installment. In YE2, the R6 stage was reached earlier than in YE1, thus the final evaluation was carried out 101 DAS.
Statistical analysis
All experiments were set in a completely randomized design, with seven treatments and six replications (EE) and five treatments and six replications (YE). The values of P60 and P90 (EE1 and EE2), Ng45, Ng90, NgHv, TGW, FRW and LSDW (YE1 and YE2) were subjected to analysis of variance (ANOVA), and the means were compared using the Tukey Honestly Significant Difference (HSD), performed on the R software package (R Core Team, 2017). All HSD analysis were tested for homogeneity of variance through a Levene test. Raw data was used for agricultural parameters, while a Box-Cox transformation was necessary to perform the analysis on nematological data. Linear regression between NgHv and TGW was obtained, in order to evaluate the potential effect of P. zeae on the maize yield.
Results
Efficacy experiments (EE)
The nematode populational growth of both EE1 and EE2 (Table 2) were similar when comparing the 90 DAI evaluations, despite the difference in the initial population (Pi). The nontreated seeds control (Ctrl + Pz) showed a final population 25–26 times larger than the initial one for EE1 and EE2, this similarity despite the discrepancy of 3,000 individuals in the original inoculum is a possible indication that an upper limit for populational growth was not reached, as quantity and quality of food was available in this host under the given experimental conditions (Seinhorst, 1970). This similarity is an important guideline for an accurate comparison across all treatments in both installments of the experiments.
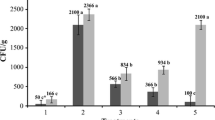
As expected, the control showed the highest P. zeae population densities over EE1 and EE2, in both evaluation dates (P60 and P90). The treatment with the lowest P. thornei concentration (5 × 106 endospores per seed) did not suppress the P. zeae populations in 3 out of 4 evaluations (Table 2), the exception being the P90 evaluation of the experiment 1, in which this treatment (5 × 106 endospores per seed) showed a suppression potential of nearly 30% in comparison with Ctrl + Pz. This performance was not observed consistently over other evaluations. Intermediate P. thornei doses of 1 × 107 and 1.5 × 107 behaved similarly to each other. The concentration of 1 × 107 performed a slightly higher control than the lowest concentration, deviating from the control in P60 and P90 of EE1, and P60 of EE2, respectively. The endospore concentration of 1.5 × 107 reduced P. zeae population densities in all evaluations except in P60 of EE2, in which doses ranging from 5 × 106 to 1.5 × 107 showed means very close to control. The highest concentration (Pt2 × 107) was the only P. thornei exclusive treatment to demonstrate a positive effect on the reduction of P. zeae populations on all evaluations, with reduction rates ranging 40–55% when compared to Ctrl + Pz.
The two treatments containing the abamectin commercial formulation (only abamectin and abamectin + P. thornei 107 endospores per seed) showed a superior control potential over all treatments containing only P. thornei, with reduction rates of P. zeae population surpassing 90%. Of those two, the treatment Ab + Pt107 did not differ statistically from the Ab. However, Ab + Pt107 showed a much higher efficacy in reducing P. zeae populations than its abamectin free counterpart, Pt107 (Table 2).
Yield experiments (YE)
Populational growth of P. zeae followed a steeper growth curve in YE1 in comparison with its replicate. While the Ctrl + Pz multiplied 32-fold comparing P. zeae densities at 45 DAS and at harvest (NgHv/Ng45 ratio) in YE1, while in YE2 the same time interval between evaluations led only to an 1.8 times increase (Table 3). Regarding the efficacy in reducing P. zeae reproduction, all of the three seed treatments showed statistically significant results by the time of harvest and did not differ from each other. Using the Ctrl + Pzas a base of comparison, a reduction rate of 51–59% in NgHv was observed for the abamectin treatment in YE1 and YE2. The biological treatment (P. thornei 2 × 107 endospores per seed) showed reduction rates of 44–46% for YE1 and YE2. When combined, abamectin and P. thornei (0.5 mg + 107 endospores per seed) reduced P. zeae population by a factor of 45% in the YE1 and 31% for its replicate.
The Ng45 evaluations in YE1 and YE2 showed a decrease in P. zeae population for all seed treatments containing abamectin, in comparison to Ctrl + Pz. Also, the seed treatment containing solely P. thornei (2 × 107 endospores per seed) showed a substantial reduction of P. zeae in Ng45 in YE2. However, in YE1 the same treatment did not differ statistically from the Ctrl + Pz (Table 3).
Agronomic measurements as depicted in Table 4 shows that fresh root weight (FRW) by the time of harvest did not differ statistically in the first experiment, regardless of the treatment. In YE2, fresh roots of Ctrl Pz showed the highest weight, placing it apart from Ctrl + Pz. The three tested seed treatments however were placed in the same group as the Ctrl + Pz, with both treatments containing exclusively abamectin (Ab) and P. thornei (Pt2 × 107) participating in an intermediate between Ctrl + Pz and Ctrl—Pz.
In YE1, LSDW of the Ctrl – Pz was the highest, distancing itself from all other treatments, except Pt2 × 107. In YE2, LSDW of this same treatment was again the highest. The treatments Ab and Ab + Pt107 showed LSDW higher than the Ctrl + Pz, while the treatment Pt2 × 107 was placed in an intermediate group (Table 4).
Yield measurements (TGW) reflected the effects of P. zeae infestation on maize and the mitigation potential of the tested treatments. In both experiments, the Ctrl + Pz showed lower yields than the Ctrl—Pz, reducing TGW in the inoculated plots by over 58% for YE1 and 49% for YE2. In YE1 only the treatment Ab was set apart from Ctrl + Pz seeds, with an increase of 62%. However, the nematode infection in Ab treatment still caused a reduction in yield of 33% when compared to the Ctrl—Pz treatment. Whereas the other two seed treatments, consisting of abamectin + P. thornei (Ab + Pt 107) and P. thornei alone (Pt 2 × 107) did not differ statistically from the Ctrl + Pz. In YE2, however, treatments Pt 2 × 107 and Ab + Pt 107 performed well, with both placed in the same statistical group as the Ctrl—Pz treatment. The treatment Pt2 × 107 also showed positive results in YE2, with TGW lower than the Ctrl—Pz treatment (18% reduction), but higher than the Ctrl + Pz, which showed a reduction on yield of 49% as mentioned, in this installment two plots of the Ctrl—Pz treatment were lost before harvesting due stalk breakage during the grain fill stage (Fig. 1 and Table 4). It is noteworthy that P. zeae infestation had strong detrimental effects on most of the agronomic characteristics measured in this study, with exception of FRW for YE1. However, the maize roots of Ctrl + Pz exhibited extensive lesion symptoms typical of Pratylenchus infection, whereas maize roots of the Ctrl—Pz remained with a healthy aspect until harvest in both YE1 and YE2 (Fig. 2).
Cobs from YE2: A - Ctrl - Pz – Control without Pratylenchus zeae. B - Ctrl + Pz – Nontreated control. C – Pt2 × 107 – Pasteuria thornei 1 × 107 endospores per seed. D – Pt1 × 107 + Ab – mixture of 1 × 107endospores of P. thornei and 0.5 mg of abamectin per seed). E – Ab – 0.5 mg of abamectin per seed
The correlations between P. zeae densities and TGW were highest during the NgHv evaluation in both YE1 (adjusted R-squared = 0.66) and YE2 (0.61) being highly significant according to t-test at P = 0.001 (Fig. 3). These productivity correlations could be seen as early as 45 DAS, when comparing Ng45 and TGW for YE1 (adjusted R-squared = 0.37) and YE2 (0.57). The 90 DAS analysis showed similar correlation rates when comparing Ng90 and TGW for YE1 (adjusted R-squared = 0.58) and YE2 (0.41).
Discussion
The efficacy experiments showed that lower concentrations of P. thornei, 5 × 106 and 1 × 107 endospores per seed, presented inconsistent results in reducing P. zeae population growth, often resulting in final populational means for P. zeae very close to the untreated control. Whereas the treatments containing higher concentrations of the biological control agent, especially the dose of 2 × 107 endospores/seed, showed more reliable results, reducing P. zeae populations by nearly half when compared to the untreated treatment in both experiments. The chemical treatment abamectin (0.5 mg a.i. per seed), which is currently available in Brazil, showed the best results in controlling P. zeae, with reduction rates of up to 95%, such rates are high but not too distant from similar results in other works (Bortolini et al., 2013; Cabrera et al., 2009). However, it is important to notice that, in both efficacy experiments, this effect was highly likely to be exacerbated by the number of seeds in one plot, due to an overlapping of the effect of each seed treatment.
The treatment containing P. thornei + abamectin (Ab + Pt107) showed nearly identical results to the treatment containing abamectin alone (Ab), in all of the evaluations and experiments. This points to an absence of a synergist effect between chemical and biological treatments in this case, also likely to be related to the high effectiveness of abamectin in reducing P. zeae numbers, which could either mask the effects of P. thornei or deprive this biological control agent of its obligatory host, impeding it from reaching its full control potential. It is important to notice, however, that species of the genus Pasteuria specialize in the parasitism of a single genus of phytonematode, and abamectin is a broad range nematicide (Cabrera et al., 2009). Therefore, the combination of both treatments might still prove beneficial in the occurrence of a multi-species infestation where Pratylenchus levels are high, especially if in the long run P. thornei establishes itself in the soil as its Meloidogyne parasitizing counterpart Pasteuria penetrans (Stirling, 2014).
Regarding the YE, the first noteworthy result of the populational counts for this experiment is how differently abamectin affected the densities of P. zeae in the roots. In the efficacy experiments reduction rates of up to 95% were observed in P. zeae populations, due to the treatment of the seeds with abamectin at a concentration of 0.5 mg per seed. The same did not happen with P. zeae populations of the analog treatment in YE1 and YE2, with this chemical treatment showing reduction rates ranging from 51–59%, numbers that are considerably lower when compared to the ET experiments. While the treatments containing abamectin behaved differently when compared to the efficacy experiments, Pt2 × 107 showed very similar results. In the efficacy experiments, this same treatment presented reduction rates of 41–45% when compared to the untreated control, against 31–45% reduction in the productivity experiments. This shows a consistency in P. thornei control potential as a biological agent, and sets a new perspective on how biological control for the genus Pratylenchus might be achieved through its use as a seed treatment.
Despite the differences in soil fertility management for YE1 and YE2, results showed similar trends when analyzing the effects of P.zeae infestation on the nontreated control treatments (Ctrl + Pz x Ctrl—Pz). The reduction in productivity (TGW) was around 58 and 46% when compared to the uninoculated control, for experiments 3 and 4 respectively. In general, yield losses were lower across all treatments in YE2 when compared to YE1, likely due to a better fertilizer management for this installment. This is an interesting take on the phytonematode phenomena, in which crops cultivated on soils of poor fertility are much more likely to be negatively affected by the presence of this parasite. The correlations between NgHv and TGW demonstrated that the detrimental effect of P. zeae parasitism was consistent through both experiments, and also that the P. zeae control provided by seed treatments resulted in higher yields.
Our results did not show additive or synergistic effects between abamectin and P. thornei for controlling P. zeae or mitigating the yield losses at all dosages used.
Most of the data regarding yield reduction caused by P. zeae on maize originates from observational studies or field experiments, correlating productivity with P. zeae population in naturally infested areas (Egunjobi & Bolaji, 1979; Kagoda et al., 2011; Monteiro, 1963). Probably, the current experiments are the first to report the damage of P. zeae on maize roots, and its effects on plant growth and yield, under controlled conditions.
Abamectin is currently available as a seed treatment for P. zeae control in Brazil. Under controlled conditions, a mixture of thiametoxam and abamectin (0.7 mg + 0.583 mg per maize seed) was effective in controlling P. zeae (Confort & Inomoto, 2015). However, previously to the current results, the beneficial effect of abamectin on maize yield under P. zeae infestation was not yet reported.
The experiments comprised here are also the first to demonstrate the effectiveness of P. thornei as a BCA for the management of P. zeae on maize. Indeed, our results proved that P. thornei used in seed treatment show potential in preventing productivity losses in maize under P. zeae infested soils. Currently, P. thornei is not available as commercial nematicide. However, this work should give support for upcoming studies aiming to use P. thornei as a biological control agent for the control of root lesion nematodes, and also stimulate colleagues in the pursue of objective results regarding the effects of RLN on maize.
References
AGROFIT – Sistema de Agrotóxicos Fitossanitários do Ministério da Agricultura. (2019). A report on the avaible commercial products for Pratylenchus zeae control on maize, Brasília, Brasil. https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed Dec 2019.
Bortolini, G. L., Araújo, D. D., Zavislak, F. D., Romano Júnior, J., & Krause, W. (2013). Controle de Pratylenchus brachyurus via tratamento de semente de soja. Enciclopédia Biosfera, 9, 818–830.
Cabrera, J. A., Kiewnick, S., Grimm, C., Dababat, A. A., & Sikora, R. A. (2009). Efficacy of abamectin seed treatment on Pratylenchus zeae, Meloidogyne incognita and Heterodera schachtii. Journal of Plant Diseases and Protection, 116, 124–128. https://doi.org/10.1007/BF03356298
Casela, C. R., Ferreira, A. S., & Pinto, N. F. A. (2006). Doenças na Cultura do Milho. Embrapa Milho e Sorgo, Sete Lagoas MG, Circular Técnica 83:11-12.
Chen, Z. X., & Dickson, D. W. (1998). Review of Pasteuria penetrans: Biology, ecology and biological control potential. Journal of Nematology, 30, 313–340.
CONAB – Companhia Nacional de Abastecimento. (2016). Observatório Agrícola: Acompanhamento da Safra Brasileira. Monitoramento Agrícola – Safra 2018/19. 3.12:104–115.
Confort, P. M. S., & Inomoto, M. M. (2015). Evaluation of maize seed treatment for Pratylenchus zeae control. Nematology, 17, 667–670. https://doi.org/10.1163/15685411-00002898
Confort, P. M. S., & Inomoto, M. M. (2018). Pasteuria thornei, a novel biological seed treatment for Pratylenchus brachyurus control in soybean. Nematology, 20, 519–523. https://doi.org/10.1163/15685411-00003156
Coolen, W. A., and D’Herde, C. J. (1972). A method for the quantitative extraction of nematodes from plant tissue. State Nematology and Entomology Research Station.
De Waele, D., & Jordaan, E. M. (1988). Plant-parasitic nematodes on field crops in South Africa. Revue Nématol, 11, 65–74
Dickson, D. W., Preston J. F. III, Giblin-Davis, R. M., Noel G. R., Dieter-Ebert, G. R. D., & Bird G. W. (2009). Family Pasteuriaceae Laurent (1890). In: P. De Vos, G. M. Garrity, D. Jones, N. R. Krieg, W. Ludwig, F. A. Rainey, K. H. Schleifer, W. B. Whitman (Eds.), Bergey's manual of systematic bacteriology, the firmicutes (vol 3, pp 328-347). Springer.
Duncan, L. W., and Jones, M. G. K. (2006). Migratory endoparasitic nematodes. Pages 120–131 In: Plant Nematology. CABI Publishing
Eberlein, C., Heuer, H., & Westphal, A. (2020). Biological suppression of populations of Heterodera schachtii adapted to different host genotypes of sugar beet. Frontiers in Plant Science, 11, 812. https://doi.org/10.3389/fpls.2020.00812
Egunjobi, O. A., & Bolaji, E. I. (1979). Dry season survival of Pratylenchus spp. in maize fields in Western Nigeria. Nematologia Mediterranea, 7, 129–135.
FAOSTAT. (2019). FAOSTAT database. FAO. http://www.fao.org/faostat/en/#data/QC. Accessed Dec 2019.
Fosu-Nyarko, J., & Jones, M. G. K. (2016). Advances in understanding the molecular mechanisms of root lesion nematodes host interactions. Annual Review of Phytopathology, 54, 253–278. https://doi.org/10.1146/annurev-phyto-080615-100257
Gonzaga, V., & Santos, J. M. (2008). Detecção de Pasteuria thornei em Pratylenchus brachyurus e P. zeae. Nematologia Brasileira, 33, 103–105.
Groth, J. V., Zeyen, R. J., Davis, D. W., & Christ, B. J. (1983). Yield and quality losses caused by common rust (Puccinia sorghi Schw.) in sweet corn (Zea mays) hybrids. Crop Protection, 2, 105–111. https://doi.org/10.1016/0261-2194(83)90030-3
Jones, M. G. K., Sadia, I., & Fosu-Nyarko, J. (2016). Belowground defense strategies against migratory nematodes. In: C. M. Vos, & K. Kazan (eds.), Signaling and communications in plants: Belowground Defense strategies in plants. Springer. https://doi.org/10.1007/978-3-319-42319-7_11
Kagoda, F., Derera, J., Tongoona, P., Coyne, D. L., & Talwana, H. L. (2011). Grain yield and heterosis of maize hybrids under nematodes infested and nematicide treated conditions. Journal of Nematology, 43, 209–219.
Lund, M. E., Mourtzinis, S., Conley, S. P., & Ané, J. M. (2018). Soybean cyst nematode control with Pasteuria nishizawae under different management practices. Agronomy Journal, 110, 2534–2540.
Monteiro, A. R. (1963). Pratilencose do milho. Revista de Agricultura, 38, 177–187. https://doi.org/10.2134/agronj2018.05.0314
Nuss, E. T., & Tanumihardjo, S. A. (2010). Maize: A paramount staple crop in the context of global nutrition. Comprehensive Reviews in Food Science and Safety, 9, 417–436. https://doi.org/10.1111/j.1541-4337.2010.00117.x
O’Keeffe, K. (2009). Kernel development. In: Edwards, J. (ed) Maize, growth and development (pp 41–46). NSW Department of Primary Industries.
Olowe, T., & Corbett, D. C. M. (1976). Aspects of the biology of Pratylenchus brachyurus and P. zeae. Nematologica, 22, 202–211.
Patel, N. B., Patel, D. J., & Patel, A. D. (2002). Effect of Pratylenchus zeae on maize. Indian Phytopathology., 55, 333–334.
Ranum, P., Peña-Rosa, J. P., & Garcia-Casal, M. N. (2014). Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences, 1312, 105–112. https://doi.org/10.1111/nyas.12396
R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. http://www.R-project.org/
Seinhorst, J. W. (1970). Dynamics of population of plant parasitic nematodes. Annual Review of Phytopathology, 8, 131–156.
Stirling, G. R., Wong, E., & Bhuiyan, S. (2017). Pasteuria, a bacterial parasite of plant-parasitic nematodes: Its occurrence in Australian sugarcane soils and its role as a biological control agent in naturally-infested soil. Australasian Plant Pathology, 46, 563–569. https://doi.org/10.1007/s13313-017-0522-z
Stirling, G. R. (2014). Biological control of plant parasitic nematodes. In: Soil ecosystem management in sustainable agriculture, 2nd ed. CAB International. https://doi.org/10.1079/9781780644158.0000
Sutton, J. C. (1982). Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Canadian Journal of Plant Pathology, 4, 195–209. https://doi.org/10.1080/07060668209501326
Van Lenteren, J. C., Bale, J., Bigler, F., Hokkanen, H. M. T., & Loomans, A. J. M. (2006). Assessing risks of releasing exotic biological control agents of arthropod pests. Annual Review of Entomology, 51, 609–634. https://doi.org/10.1146/annurev.ento.51.110104.151129
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interest to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Confort, P.M.S., Inomoto, M.M. Effect of seed treatment with the biological agent Pasteuria thornei on the control of Pratylenchus zeae in Maize. Eur J Plant Pathol 166, 275–284 (2023). https://doi.org/10.1007/s10658-023-02659-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02659-2