Abstract
Pratylenchus brachyurus is one of the main limiting factors of soybean yield in Brazil, particularly because of inefficiency of the control methods when used individually. The present study aimed to assess the effect of associated methods, using seed treatment with nematicides (ST) and resistance inducers (RI), on nematode control in soybean plant in both field and greenhouse conditions. A field assay was conducted in an infested field and nematode population was assessed at sowing, 45, 75, and 100 days after sowing and the yield measured at end of crop cycle. The experiment was repeated in greenhouse. In another experiment, that was conducted in two different periods in a greenhouse, seed treatments and resistance inducers, alone or combined, were assessed under two initial populations of P. brachyurus (low IP = 500 specimens and high IP = 2000 specimens). The treatments did not reduce the number of nematodes g−1 of root in field assay, but all seed treatments effectively controlled nematode population in the greenhouse assay. Most treatments reduced the number of nematodes g−1 of root when nematode initial population was low (IP = 500) but when initial nematode population was high (IP = 2000) combinations of treatments which includes abamectin inhibited P. brachyurus reproduction. Chemical products did not affect yield but acibenzolar-S-methyl, alone or associated with other products, generally inhibit plant growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematode species of the genus Pratylenchus are widely disseminated in agricultural regions and can infect many crops. In Brazil, P. brachyurus (Godfrey) Filipjev and Sch. Stekhoven has great economic impact on soybean crops (Dias et al. 2010; Inomoto et al. 2010). This nematode is very common in tropical regions, and it is characterized as a migrating endoparasite that feeds on cortical cells of the roots, consequently darkening the parasitized tissue.
Infestations of this nematode in soybean crops have been growing over the years, particularly in the Central-Western region of Brazil (Goulart and Ferraz 2003; Dias et al. 2010). It is believed that the use of non-till systems explains most of these infestations, since greater deposition of mulching retains soil moisture, increasing the population of polyphagous nematodes (Dias et al. 2010).
Nematode control methods in large-scale crops should be well-planned, integrated, and low-cost (Dias et al. 2010). Because these parasites infect plant soon after germination, seed treatment is a low-cost option of control to promote initial root protection (Santos 2011). Furthermore, concentrated rates of the product in seed treatments reduces environmental contamination, and its use among annual crops effectively controlled various plant parasite nematodes (Monfort et al. 2006; Bessi et al. 2010; Higaki and Araujo 2012; Ribeiro et al. 2014), particularly with use of abamectin and thiodicarb (Kubo et al. 2012; Bortolini et al. 2013; Ribeiro et al. 2014; Vitti et al. 2014).
Another strategy that has attracted the attention of researchers is the use of resistance inducers in the control of nematodes. Several biotic or abiotic agents may induce resistance in plants to pathogens. Acibenzolar-S-methyl (ASM) is one of the most commonly studied chemical agents, characterized by directly activate plant defense mechanisms (Chinnasri et al. 2006). ASM reduced populations of Meloidogyne spp. in tomato, vine, and soybean (Owen et al. 2002; Silva et al. 2004; Molinari and Baser 2010; Puerari et al. 2013a) and P. brachyurus in maize crops (Puerari et al. 2015).
Seed treatments protect plant in the early stages of development, but after losing residual effect, the plant remains unprotected (Gonçalves Junior et al. 2013). However, because resistance inducers are applied in aerial part of plants, 15 to 20 days after germination, plants are not protected against early infections (Puerari et al. 2013a). It was then suggested that both methods could be used in integrated management. Thus, the present study aimed to assess the effect of seed treatment and resistance inducers, individually or combined, on the control of P. brachyurus in soybean.
Materials and methods
Four different assays were conducted in two experiments. Experiment 1 included one field assay (Exp. 1: FE assay), which was repeated in the greenhouse (Exp. 1: GH assay). The second experiment was composed of two assays (Exp. 2: Assay 1; Exp. 2: Assay 2), both performed in the greenhouse.
Experiment 1: FE and GH assays
The field assay (FE) was conducted in the Midwestern region of Paraná (Araruna city), Brazil, with the following geographic coordinates: 23°55′54″ South Latitude and 52°29′47″ West Longitude, and an average altitude of 610 m above sea level. Soil was classified as sandy (6% clay, 5% silt and 89% sand). Experiment was implemented during the 2014–2015 crop season, and experimental area was 194.4 m2 composed of six 2-m-long planting rows. A randomized block design with six treatments and six repetitions was adopted. The area naturally infested by P. brachyurus, showed stunted and underdeveloped plants and low yield, in the 2013/2014 crop season.
Sowing of soybean cv. BMX Tornado was performed in October 2014, with 0.45 m spacing between rows and a density of 10 plants m−1. All agricultural treatments were performed according to the recommendations for soybean crop. Before sowing, samples of soil and forage sorghum were collected (off-season remaining plants) to estimate initial population (IP) of nematodes. Treatments used in the experiment were: control (seed treatment using fungicide Maxim Advanced®, Syngenta: fludioxonil + metalaxyl-M + thiabendazole, 25 + 20 + 150 g a.i. L−1); abamectin (Avicta Completo®, Syngenta: abamectin + thiamethoxam + fludioxonil + metalaxyl-M + thiabendazole, 500 + 350 + 25 + 20 + 15, g a.i. L−1); thiodicarb (Cropstar® + Derosal Plus®, Bayer: thiodicarb + imidacloprid + carbendazim + thiram, 450 + 150 + 150 + 350 g a.i. L−1); fipronil (Standak Top®, Basf: fipronil + pyraclostrobin + methyl tiophanate, 250 + 15 + 225 g a.i. L−1); acibenzolar-S-methyl, ASM (Bion®, Syngenta, 500 g a.i. kg−1) and abamectin + ASM. Seed treatment: control, abamectin, thiodicarb and fipronil, were applied by industrial seed treatment at the doses recommended by the manufacturers, respectively: 1.0, 1.0, 2.0 and 5.0 ml of commercial product per kg of seeds; while ASM was sprayed on the aerial part at 30 and 40 days after sowing using a knapsack sprayer with four fan nozzles (8002), at a dose of 25 g ha−1 with 200 L ha−1 flow rate.
Soil and root samples were collected 45, 75 and 100 days after sowing. One sample composed of four subsamples was collected per plot and they were homogenized and placed in plastic bags. In the laboratory, nematode extractions from 10 g of root and 100 cm3 of soil were performed according to the methods proposed by Coolen and D’Herde (1972) and Jenkins (1964), respectively. Nematode counts were performed in Peters’ chamber, under optical microscope. At the end of the crop cycle, the grains were harvested in two rows of each plot, 2 m per row, and weighed to estimate soybean yield.
The greenhouse assay was conducted at Faculdade Integrado de Campo Mourão, Paraná, Brazil, from July to November 2015. The substrate used was a mixture of soil (clayey oxisol) and sand (1:1), sterilized by solarization for 15 days (Ghini 1997) and was later distributed in 3-l pots and fertilized with NPK 8–20-20 at the rate of 0.5 g pot−1. The study was conducted in entirely randomized design with six repetitions.
Initially, the nematode was reproduced in maize due to the soybean-free period in Brazil. Thus, three seeds of maize cv. AG1051 were sown in each pot and seven days after emergence, seedlings were thinned to one plant per pot. Plants were inoculated with 1000 specimens of P. brachyurus [inoculum obtained from pure nematode population reproduced in maize, in the greenhouse, and extracted according to the method proposed by Coolen and D’Herde (1972)]. Inoculum suspension was placed in four holes at 2-cm depth and 2 cm from the shoot. Sixty days after inoculation, aerial part of the maize was removed and soybean cv. BMX Tornado was sown with the same treatments used in the field. However, ASM was applied 15 and 25 days after germination.
Sixty days after sowing, plants were collected, and aerial part was separated from root. Plant height and fresh and dry weight of the aerial part were determined. Dry weight was obtained after drying in an oven with air circulation at 65 °C, for three days. Roots were washed and weighed, and root fresh weight was obtained. Then, roots were subjected to nematode extraction according to the previously mentioned methodology. Soil samples (100 cm3) were also collected from each pot, and nematodes were extracted according to the methodology proposed by Jenkins (1964). Nematode counts were performed as previously cited.
Experiment 2 – Assay 1 and assay 2
Experiment was conducted in greenhouse, under the same conditions described in the previous experiment (Exp. 1: GH assay), although it was conducted in a differente period, that is, from November 2014 to March 2015 (Exp. 2: Assay 1) and from July to November 2015 (Exp. 2: Assay 2). Both assays were arranged in an entirely randomized design with nine treatments and five repetitions, with two levels of initial population (IP): 500 and 2000 nematodes plant−1, called low and high IP, respectively. Treatments were abamectin, thiodicarb, ASM, citric biomass (Ecolife®, Quinabra: 1.5 ml of commercial product per litre, with 200 L ha−1 flow rate), abamectin + ASM, abamectin + citric biomass, thiodicarb + ASM, and thiodicarb + citric biomass, using untreated plants as controls. Another aspect that differed from Exp. 1: GH assay was the fact that inducers were applied only once, 15 days after germination, and that total population obtained by the sum of nematodes in root + soil was considered in the assessment.
Data obtained in the experiments were subjected to analysis of variance using SISVAR software (Ferreira 2008), and means were compared by Scott–Knott test at 5% probability. For analysis, nematological data were transformed by √(x + 1).
Results
Experiment 1: FE and GH assays
Treatments did not reduce number of P. brachyurus on the soybean roots in the FE assay, regardless of the period of assessment (Table 1). However, in the GH assay, except for ASM treatment applied alone, all treatments reduced nematode reproduction compared to control (Table 1). In the FE assay, soil nematode population was reduced 45 days after sowing, when seeds were treated chemically.
Treatments did not affect soybean yield in the FE assay (Table 2). Regarding vegetative growth parameters assessed in greenhouse, lower height was observed for treatments with thiodicarb and fipronil, and fresh and dry weight of aerial part of the plant were reduced by abamectin, when applied alone (Table 2).
Experiment 2: Assay 1 and assay 2
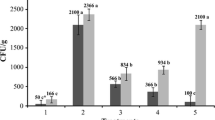
In general, treatments were effective in reducing number of nematodes g−1 of root when applied in low initial population (IP = 500) of nematodes, except for the thiodicarb and citric biomass in Assay 1 and thiodicarb + ASM in Assay 2 (Table 3). However, to high population of nematodes (IP = 2000), only the treatment with abamectin + citric biomass effectively reduced number of nematodes g−1 of root in Assay 1. In Assay 2, nematode control was obtained using of abamectin, citric biomass, abamectin + citric biomass and abamectin + ASM (Table 3).
Concerning total nematode population, under IP = 500, treatments with ASM, thiodicarb + citric biomass, and abamectin + ASM reduced nematode population in Assay 1. In Assay 2, in addition to the referred treatments, thiodicarb + citric biomass controlled the P. brachyurus population. With IP = 2000 in Assay 1, treatments effectively reduced total nematode population, except for thiodicarb used alone. The best results were obtained to application of abamectin and abamectin + citric biomass. However, in Assay 2, abamectin + citric biomass and abamectin + ASM reduced total nematode population (Table 3).
Regarding vegetative growth parameters, in general, treatments using ASM, alone or combined with other products, resulted in lower plant growth, considering height, fresh and dry weight of aerial part, as well as fresh root weight (Table 4). This tendency was observed regardless of the initial nematode population.
Discussion
In the field assay, treatments did not reduce the nematodes number per gram of root. Two factors may have contributed to these results. Firstly, there was a low level of rainfall in the days after planting; it did not rain during the first 10 days, and total rainfall was 45 mm in the first 25 days after planting. In addition, initial population was low when the experiment was implemented (Table 1). However, data obtained in the greenhouse showed the potential of the seed treatments, since all chemical treatments reduced P. brachyurus reproduction in soybean if compared to the control treatment.
Abamectin controlled P. brachyurus in Exp. 1: GH assay and in both assays of the Experiment 2, since number of nematodes g−1 of root decreased when two levels of inoculum were applied. Effectiveness of the treatment abamectin in reducing P. brachyurus has been shown by other authors (Ribeiro et al. 2014; Bortolini et al. 2013). This molecule was also efficient in the control of other nematodes, such as Rotylenchulus reniformis (Linford and Oliveira) and Meloidogyne incognita (Kofoid and White) Chitwood (Monfort et al. 2006; Bessi et al. 2010). There are also reports of 80% reduction in infestation by Pratylenchus zeae Graham in maize, 90% of M. incognita in cotton and 50% of Heterodera schachtii Schmidt in sugar beet (Cabrera et al. 2009). Abamectin does not act systematically on the plant, so beneficial effects observed came from the action of the product on soil nematodes, which decreases over time (Bessi et al. 2010). Additionally, abamectin may inhibit hatching of nematodes, paralyze juveniles (Cayrol et al. 1993; Faske and Starr 2006) and cause damage to sensory organs, making impossible for parasite to recognize the site of penetration (Gourd et al. 1993; Silva et al. 2004).
Thiodicarb showed different results and effectively controlled P. brachyurus only in the Exp. 1: GH assay and Exp. 2: Assay 2, under low initial nematode population. However, several studies have demonstrated the potential of the active ingredient to control Meloidogyne spp., P. brachyurus and Heterodera glycines Ichinohe in soybean (Higaki and Araujo 2012; Bortolini et al. 2013; Corte et al. 2014), R. reniformis and Meloidogyne spp. in cotton (Kubo et al. 2012), and M. incognita and M. javanica in bean plants (Gonçalves Junior et al. 2013).
Fipronil-based product, used in both assays of Experiment 1, is not registered as nematicide, but was effective in controlling P. brachyurus in greenhouse, not differing from the other seed treatments and corroborating previous studies that demonstrated its efficiency in reducing populations of P. brachyurus in soybean and cotton (Ribeiro et al. 2014; Bortolini et al. 2013). Further studies should be performed to understand the mode of action of this product on parasites and on host.
Abamectin also effectively controlled nematodes when associated with ASM in Exp. 1: GH assay and in both assays of the Experiment 2, corroborating the results of preliminary assessments reported by Lopes et al. (2015). Under high populations, association of both inducers with abamectin promoted greater reduction of nematode per gram of root. Other product associations also controlled nematode when compared to the control, but did not differ from the products used alone.
Application of ASM alone did not control nematodes in both assays of the Experiment 1, but it controlled P. brachyurus in greenhouse, under low initial population. Studies indicate that the product is effective in the control of nematodes in different plant pathosystems (Silva et al. 2004; Molinari and Baser 2010; Puerari et al. 2013a; Puerari et al. 2015), and promoted reductions of 38.3 to 86.5% in the P. brachyurus reproduction in maize (Puerari et al. 2015). It is already known that ASM does not have nematicidal action, but it activates the mechanisms related to systemic acquired resistance (SAR), associated with accumulation of salicylic acid and expression of various pathogenesis-related protein genes (Sticher et al. 1997).
However, to ensure the effect of ASM on nematode-plant interaction, application must be performed during the initial stage of vegetative growth, because this is a period in which the plant undergoes extensive damage by soil pathogens (Silva et al. 2004). Consequently, timing of application could be one hypothesis that explains ineffectiveness of the product in field assay. ASM treatment in this study was applied late, unlike other trials in which treatments were applied before or few days after inoculation (Puerari et al. 2013a; Puerari et al. 2015). Another aspect that deserves consideration is low effectiveness of the product when initial nematode populations are higher, as observed in Exp. 1: GH assay, whose IP was 1000 specimens, and in one of the assays of Experiment 2, using IP = 2000 specimens. Moreover, it should be emphasized that the mode of action of this product to control root lesion nematodes has not been elucidated yet.
Citric biomass was more efficient at controlling P. brachyurus when associated with seed treatments, even under high initial nematode population, at least in one of the assays. The potential of this product to resistance induction was mentioned in studies on the control of M. javanica in susceptible soybean cultivars. When applied seven days and one day before inoculation (Puerari et al. 2013b). Resistance induction of this product was also observed in P. brachyurus in maize applied seven days and one day before and seven days after inoculation (Puerari et al. 2015). It is believed that the effect of Ecolife® as resistance inducer is explained by the fact that citric extracts promote the production of phytoalexin glyceolin (Motoyama et al. 2003), which may induce resistance to root-knot nematodes (Kaplan et al. 1980).
Both Bion® (ASM) and Ecolife® (citric biomass) are recommended for applications in aerial part, although some authors consider that this is a limiting factor, since nematodes infections occur at an early stage (Puerari et al. 2013a). However, in the present study, soil was previously infested by nematodes and both products effectively controlled the population of P. brachyurus under low population. However, ASM and Bion did not promote reduction of the nematode when applied alone under high population. Despite the need for further studies, it was possible to observe that integrated management is important to control high nematode populations. Other practices should be used to reduce initial populations, e.g., growing non-host or antagonistic plants. Therefore, seed treatment and application of resistance inducers may contribute to the appropriate management of P. brachyurus populations in soybean, when initial population is low.
Treatments did not present positive impact on vegetative growth parameters in Experiment 1. One hypothesis to explain these results is that seed treatment fifteen days before sowing might have caused some plant phytotoxicity. Despite the lack of studies with the products used in this study, some authors showed that insecticides, such as carbofuran, thiamethoxam and acephate, used in seed treatment reduced the development of soybean seedlings due to phytotoxic effect on seeds stored for 45 days (Dan et al. 2010). The insecticide thiamethoxam presented also adverse effect on the height of bean seedlings when the seeds were treated 10 and 30 days before sowing (Guimarães et al. 2005).
Concerning Experiment 2, effect of the treatments on vegetative growth parameters in soybean did not show in a pattern, except for ASM treatments, which usually reduced plant growth. Similar results were obtained in studies with soybean cultivars inoculated with M. javanica (Puerari et al. 2013a). This reduction can be explained by the fact that the plants consumed energy to activate resistance mechanisms (Dietrich et al. 2005). In addition, phytotoxic effect of ASM on vegetables was suggested by Cole (1999).
Conclusion
Our study indicates that both seed treatment and resistance inducers, applied singly or combined, controlled P. brachyurus in soybean. Under high initial population, the products association reduced the number of nematode g−1 of root. In general, treatment with acibenzolar-S-methyl presented adverse effect on the vegetative growth of soybean.
References
Bessi, R., Sujimoto, F. R., & Inomoto, M. M. (2010). Seed treatment affects Meloidogyne incognita penetration, colonization and reproduction on cotton. Ciência Rural. doi:10.1590/S0103-84782010000600030.
Bortolini, G. L., Araujo, D. V., Zavislak, F. D., Junior, J. R., & Krause, W. (2013). Controle de Pratylenchus brachyurus via tratamento de semente de soja. Enciclopédia Biosfera, Centro Científico Conhecer, 9, 818–830.
Cabrera, J. A., Kiewnick, S., Grimm, C., Dababat, A. A., & Sikora, R. A. (2009). Efficacy of abamectin seed treatment on Pratylenchus zeae, Meloidogyne incognita and Heterodera schachtii. Journal of Plant Diseases and Protection, 116, 124–128.
Cayrol, J. C., Djian, C., & Frankowski, J. P. (1993). Efficacy of abamectin B1 for the control of Meloidogyne arenaria. Fundamental Applied Nematology, 16, 239–246.
Chinnasri, B., Sipes, B. S., & Schimitt, D. P. (2006). Effects of inducers of systemic acquired resistance on reproduction of Meloidogyne javanica and Rotylenchulus reniformis in pineapple. Journal of Nematology, 38, 319–325.
Cole, D. L. (1999). The efficacy of acibenzolar-S-methyl, an inducer of systemic acquired resistance, against bacterial and fungal disease of tobacco. Crop Protection, 18, 267–273.
Coolen, W. A., & D’Herde, C. J. (1972). A method for the quantitative extraction of nematodes from plant tissue (p. 77). State of Nematology and Entomology Research Station: Ghent.
Corte, G. D., Pinto, F. F., Stefanello, M. T., Gulart, C., Ramos, J. P., & Balardin, R. S. (2014). Tecnologia de aplicação de agrotóxicos no controle de fitonematoides em soja. Ciência Rural. doi:10.1590/0103-8478cr20130738.
Dan, L. G. M., Dan, H. A., Barroso, A. L. L. B., & Braccini, A. L. (2010). Qualidade fisiológica de sementes de soja tratadas com inseticidas sob efeito do armazenamento. Revista Brasileira de Sementes, 32, 131–139.
Dias, W. P., Garcia, A., Silva, J. F. V., & Carneiro, G. E. S. (2010). Nematoides em Soja: Identificação e Controle (p. 8). Londrina: Embrapa Soja. (Circular Técnica 76).
Dietrich, R., Ploss, K., & Heil, M. (2005). Growth responses and fitness cost after induction of pathogen resistance depend on environmental condition. Plant, Cell and Environment. doi:10.1111/j.1365-3040.2004.01265.x.
Faske, T. R., & Starr, J. L. (2006). Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to abamectin. Journal of Nematology, 38, 240–244.
Ferreira, D. F. (2008). Sisvar: Um programa para análises e ensino de estatística. Revista Symposium, 6, 36–41.
Ghini, R. (1997). Desinfestação do solo com o uso de energia solar: solarização e coletor solar (p. 29). Jaguariúna: Embrapa CNPDA. Circular Técnica 1.
Gonçalves Junior, D. B., Roldi, M., Namur, F. M., & Machado, A. C. Z. (2013). Tratamento de sementes de feijoeiro no controle de Pratylenchus brachyurus, Meloidogyne incognita e M. javanica. Nematologia Brasileira, 37, 53–56.
Goulart, A. M. C., & Ferraz, L. C. C. B. (2003). Comunidades de nematoides em Cerrado com vegetação original preservada ou substituída por culturas. 1 Diversidade Trófica. Nematologia Brasileira, 27, 123–128.
Gourd, T. R., Schmitt, D. P., & Barker, K. R. (1993). Use of nematodes as biomonitors of nonfumigant nematicide movement thrugh field soil. Journal of Nematology, 25, 63–70.
Guimarães, R. N., Porto, T. B., Pereira, J. M., Barbosa, L. A., Fernandes, P. M., Costa, R. B., & Barros, R. G. (2005). Efeito do Tratamento de Sementes com Inseticidas na Emergência e Altura de Plântulas de Feijão. Congresso Nacional de Pesquisa de Feijão (pp. 94–99). Goiânia: Embrapa Arroz e Feijão.
Higaki, A. W., & Araujo, F. F. (2012). Bacillus subtilis e abamectina no controle de nematoides e alterações fisiológicas em algodoeiro cultivado em solos naturalmente infestados. Nematropica, 42, 295–303.
Inomoto, M. M., Asmus, G. L., & Silva, R. A. (2010). Importância e manejo dos nematoides da soja. Rondonópolis: Fundação MT.
Jenkins, W. R. (1964). A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter, 48, 692.
Kaplan, D. T., Keen, N. T., & Thomason, I. J. (1980). Studies on the mode of action of glyceollin in soybean incompatibility to the root-knot nematode. Meloidogyne incognita. Physiology Plant Pathology. doi:10.1016/S0048-4059(80)80003-8.
Kubo, R. K., Machado, A. C. Z., & Oliveira, C. M. G. (2012). Efeito do tratamento de sementes no controle de Rotylenchulus reniformis em dois cultivares de algodão. Arquivos do Instituto Biológico. doi:10.1590/S1808-16572012000200012.
Lopes, A. P. M., Dias-Arieira, C. R., Mattei, D., Puerari, H. H., Cardoso, M. R., & Rodrigues, D. B. (2015). Associação de tecnologias para o manejo de Pratylenchus brachyurus em soja. Congresso Brasileiro de Nematologia. Londrina: Sociedade Brasileira de Nematologia.
Molinari, S., & Baser, N. (2010). Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Protection. doi:10.1016/j.cropro.2010.07.012.
Monfort, W. S., Kirkpatrick, T. L., Long, D. L., & Rideout, S. (2006). Efficacy of a novel nematicidal seed treatment against Meloidogyne incognita on cotton. Journal of Nematology, 38, 245–249.
Motoyama, M. M., Schwan-Estrada, K. F., Stangarlin, J. R., Fiori-Tutida, A. C. G., & Scapim, C. A. (2003). Indução de fitoalexinas em soja e em sorgo e efeito fungitóxico de extratos cítricos sobre Colletotrichum lagenarium e Fusarium semitectum. Acta Scientiarum Agronomy. doi:10.4025/actasciagron.v25i2.2062.
Owen, K. J., Green, C. D., & Deverall, B. J. (2002). A benzothiazole applied to foliage reduces development and egg deposition by Meloidogyne spp. in glasshouse-grown grapevine roots. Australian Plant Pathology. doi:10.1071/AP01068.
Puerari, H. H., Dias-Arieira, C. R., Cardoso, M. R., Hernandes, I., & Brito, O. D. C. (2015). Resistance inducers in the control of root lesion nematodes in resistant and susceptible cultivars of maize. Phytoparasitica. doi:10.1007/s12600-014-0447-9.
Puerari, H. H., Dias-Arieira, C. R., Dadazio, T. S., Mattei, D., Silva, T. R. B., & Ribeiro, R. C. F. (2013a). Evaluation of acibenzolar-S-methyl for the control of Meloidogyne javanica and effects on the development of susceptible and resistant soybean. Tropical Plant Pathology. doi:10.1590/S1982-56762013000100006.
Puerari, H. H., Dias-Arieira, C. R., Tavares-Silva, C. A., Arieira, J. O., Biela, F., & Poletine, J. P. (2013b). Ecolife® and manganese phosphate in the control of Meloidogyne javanica and in the development of soybean cultivars susceptible and resistant to the nematode. Nematropica, 43, 105–112.
Ribeiro, L. M., Campos, H. D., Dias-Arieira, C. R., Neves, D. L., & Ribeiro, G. C. (2014). Effect of soybean seed treatment on the population dynamics of Pratylenchus brachyurus under water stress conditions. Bioscience Journal, 30, 616–622.
Santos, J. M. (2011). O manejo de nematoides em soja na agricultura sustentável. Boletim: Passarela da Soja, 3, 8.
Silva, L. H. C. P., Campos, J. R., Dutra, M. R., & Campos, V. P. (2004). Aumento da resistência de cultivares de tomate e Meloidogyne incognita com aplicações do acibenzolar-S-metil. Nematologia Brasileira, 28, 199–206.
Sticher, L., Mauch-Mani, B., & Metraux, J. P. (1997). Systemic acquired resistance. Annual Review of Phytopathology. doi:10.1146/annurev.phyto.35.1.235.
Vitti, A. J., Resende Neto, U. R., Araújo, F. G., Santos, L. C., Barbosa, K. A. G., & Rocha, M. R. (2014). Effect of soybean seed treatment with abamectin and thiabendazole on Heterodera glycines. Nematropica, 44, 74–80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Homiak, J.A., Dias-Arieira, C.R., Couto, E.A.A. et al. Seed treatments associated with resistance inducers for management of Pratylenchus brachyurus in soybean. Phytoparasitica 45, 243–250 (2017). https://doi.org/10.1007/s12600-017-0575-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-017-0575-0