Abstract
Botrytis cinerea is a pathogen of grapes and other fruit crops which has a complex disease cycle that allows it to survive and infect host tissues through multiple pathways. This study in three vineyards and across three growing seasons investigated the different types of necrotic trash within grape bunches and their potential to act as inoculum sources to cause bunch rot. The amounts and types of bunch trash differed across a growing season, with calyptrae and stamens reducing after pre-bunch closure, while aborted and damaged berries increased towards véraison and harvest. Overall, damaged berries, leaf fragments and tendrils presented the greatest potential as inoculum sources because they had greatest levels of natural infection after véraison and the greatest sporulation potential. The numbers of conidia produced per mm2 of tissue were 2,996, 986 and 2,011 for damaged berries, leaf fragments, and tendrils. However, only aborted berries were consistently associated with development of eventual bunch rot in the three vineyards. The sources of inoculum identified in this study could initiate secondary cycles of infection and sporulation, with consequential bunch rot under moist conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botrytis cinerea has been recognised as a pathogen of grapes for centuries and is still one of the main causes of yield loss in many fruit crops. The disease cycle of this fungus is complex; it can infect a range of hosts, host tissues and through multiple pathways. It can also remain viable during the year on many alternative host plants, as a pathogen, a saprophyte colonising necrotic tissue or as dormant structures such as sclerotia. During the growing season, B. cinerea can infect and cause blights and rots or it can remain quiescent in or on host tissues until conditions favour its development. However, sporulation occurs exclusively on necrotic tissues, so they are considered to be an important inoculum source of Botrytis spp. especially if present within a grape crop (Elmer and Michailides 2004).).
Colonised overwintering crop residues, especially rachides, were found to be significant, potential sources of primary inoculum for grape flower infection (Jaspers et al. 2013). In the temperate climate of New Zealand, conditions often favour sporulation and infection during spring and early summer, when infection of inflorescences occurs commonly in host crops such as grapevines. All parts of grape inflorescences are highly susceptible to infection and this may lead directly to destruction of flowers or can lead to latent infection which becomes evident as berry rot at harvest. The significance of flower infection was confirmed in studies which showed that the amount of flower infection was strongly correlated with the proportion of bunch rot at harvest (Nair et al. 1995; Viret et al. 2001). However, other authors have reported finding no relationship between flower infection and final bunch rot (Barbetti 1980; de Kock and Holz 1991; Dubos and Roudet 2000). Despite these conflicting results, it is generally accepted that presence of B. cinerea inoculum at flowering can have a significant effect on grape yield, especially if rainy weather occurs during flowering or near to harvest.
In many regions, bunch rot is not seen between fruit set and véraison, leading to the widely held belief that developing berries are resistant to infection by B. cinerea. For example, Nair and Hill (1992) stated that the only susceptible grapevine tissues pre-véraison were leaves and floral parts. However, immature bunch tissues from flowering to pre-bunch closure may become infected and harbour the pathogen, albeit as latent infection. Holz et al. (1997; 2003) found that in South Africa during early stages of berry development, high proportions of berries, pedicels, laterals and rachides could become infected or superficially harbour the pathogen, also contributing to bunch rot at harvest. They found that incidence of latent B. cinerea was higher in bunches during the early part of the season than later in the season, which suggested that more inoculum was dispersed into bunches between bloom and bunch closure than between bunch closure and harvest. Keller et al. (2003) reported that inoculation at full bloom led to stable latent infection from pre-bunch closure to véraison (incidence ~20 %) which had increased significantly by harvest (incidence ~100 %) and so was probably due to secondary infection from other inoculum sources. It is therefore clear that de novo infections after véraison can be initiated from inoculum outside the berry. It is possible that presence of inoculum at flowering allows for later cycles of saprophytic development and sporulation on debris as the season progresses, producing conidia that infect the ripening berries.
To-date, research on bunch trash has focussed on preventing infection of berries by B. cinerea present in necrotic tissues such as aborted flowers, flower parts or aborted fruit trapped within bunches, or in already infected berries (Northover 1987; Wolf et al. 1997). The senescing and necrotic flower parts are suitable substrates for B. cinerea colonisation and inoculum production and may also become attached to, or rest adjacent to, the developing berries, with infection occurring by mycelial spread. The necrotic matter found within bunches at harvest also contain tissues which originate from outside the bunch, namely tendrils and leaf fragments. In New Zealand, canopies are routinely trimmed in mid- and late-summer and this generates leaf fragments which can fall onto and into bunches during the season. These necrotic tissues can then be colonised by B. cinerea and so may provide secondary inoculum if weather conditions are suitable for colonisation and sporulation (Northover 1987; Wolf et al. 1997). All of these trash types have been reported as components of bunch trash, however, the relationship between the presence of bunch trash and berry infection has not always been consistent, either spatially or temporally (Wolf et al. 1997). This study therefore investigated the potential of the different types of bunch and leaf trash to become infected and to act as inoculum sources, as well as the relationship between the types of bunch trash and the incidence of B. cinerea infection in the bunch.
Methods
Characteristic of vineyards
Bunch trash surveys were carried out in three growing seasons in three commercial Sauvignon blanc vineyards where no specific botrytocides were applied during the experiments. The vineyards, called Vineyards A, B and C, respectively, were in the northern, mid and southern parts of the Wairau Plains, which are in Marlborough, New Zealand. This region has a temperate climate; during the growing season (October 2013 to April 2014) mean minimum and maximum daily temperatures were 22.6 and 9.5 °C, respectively. Rae (1987) described relatively even rainfall throughout the year with different annual amounts across the Wairau Plains. He indicated that annual rainfall for Vineyards A, B and C is usually approximately 1000, 1000 and 700 mm, respectively. The management practices of these vineyards differed somewhat due the preferences of the different growers. However, the pruning, shoot thinning and leaf removal practices were similar. Canopy density measurements made in each vineyard before harvest in Season 2, using the point quadrat system described by Smart and Robinson (1991), reflected the canopy management practices and the vigour of the sites. The respective numbers of leaf layers and percent gaps between leaves were 2.4 and 0 % for Vineyard A, 1.9 and 5 % for Vineyard B, and 1.2 and 7 % for Vineyard C, which indicated that canopies of Vineyard A were most dense and of Vineyard C the least dense. Fertilisation and irrigation were applied as needed, according to the rainfall and soil types, which were a deep silt loam for Vineyard A, a silt loam for Vineyard B and a relatively stony loam for Vineyard C. The experimental vines were trained with vertical shoot positioning in all vineyards.
Sampling of bunch trash
In Season 1, 100 bunches were sampled post-véraison (7th February) from the Vineyard A and again at harvest (10th April). Five rows and five bays (a bay containing five vines) from within each row were selected at random, and on each occasion two bunches were removed from each side of the vines in each bay. In Season 2, bunches were sampled at post-véraison from Vineyards A, B and C (23rd, 21st and 20th February, respectively) and at harvest (14th, 15th and 11th April, respectively). The sampling design was the same as for the previous season, except that one bunch was taken from each side of the canopy (not two as in the previous season) due to low crop yields; therefore 50 bunches were taken each time in each vineyard. In Season 3, the same vineyard blocks were used with the same sampling design as in the Season 2, except that samples were also taken at pre-bunch closure (PBC) (13th, 12th and 15th January), as well as post-véraison (23rd, 21st and 26th February) and harvest (17th, 16th and 21st April), from Vineyards A, B and C, respectively. Bunches were processed in the same way in all seasons. Individual vines were treated as the replicate units for statistical analysis.
Latent infection of berries
Bunches were dissected in the laboratory by clipping the berries below their pedicels, leaving the bunch skeletons. To determine the incidence of B. cinerea latent infection of the berries at post-véraison, they were surface sterilised in a 0.05 % sodium hypochlorite solution containing two drops per L of Tween 20 (polyoxyethylenesorbitan monolaurate BDH) for 5 min, and then rinsed twice in sterile water for 2 min. All of the berries from every bunch were incubated in a saturated atmosphere by being placed into one plastic tray (190 × 230 mm) lined with moist paper towels. The trays were enclosed in new plastic bags and randomly allocated to positions on a laboratory bench under natural light and ambient temperature (19–24 °C). After 5 days incubation, the berries were individually observed with a hand lens for presence of characteristic B. cinerea sporulation.
Bunch trash infestation
All trash from the bunch skeletons was carefully removed and separated into seven categories: aborted flowers (<2 mm diameter), aborted berries (2–8 mm diameter), damaged berries (>8 mm diameter), leaf fragments, tendrils, stamens and calyptrae. All trash components were fully necrotic except damaged berries which were detached from the bunch. These often had small necrotic edges around the points of damage but no obvious signs of B. cinerea. Immediately after being extracted, the specific trash types from any bunch were separately incubated on plastic trays lined with moist paper towels and enclosed in new plastic bags at room temperature under natural light. After 5 days, the trash was assessed with a hand lens for the presence of B. cinerea sporulation, and the infested proportion calculated for each type of trash.
Sporulation potential of bunch trash
A randomly selected subsample of 10 pieces of sporulating tissue of each trash type from each vineyard was used to assess the sporulation potential of each trash type, and so its capacity to initiate secondary infection cycles. The 10 pieces of each type were placed together into a Universal bottle containing 5 ml of sterile distilled water with Tween 20 (2 drops per l). The bottles were agitated for 2 min by hand, and the spore concentration of the suspension determined using a haemocytometer. For each trash type, the sporulation potential was calculated as the infested proportion per bunch multiplied by the number of trash pieces per bunch and by and the mean sporulation potential per mm2 of tissue (numbers of spores counted in the wash water divided by the calculated area of trash pieces washed). This was done for each trash type and for each sampling time.
To assess the surface area of each trash type, a sample of 20–30 pieces, depending on availability, was taken at random from 30 bunches from each of the three vineyards in Season 3. The calyptrae and leaf fragments were glued flat onto filter paper, and then scanned and the mean surface areas calculated with image analysis using a Magiscan (1989 model, Joyce-loebel Ltd. Nell, England) and Genius software (1993; (EMBL, Heidelberg). The remaining trash types which were considered to be made up of columns (e.g., tendrils) or spheres (e.g., berries). They were measured with a calliper and the appropriate geometric equations used to determine approximate surface areas of cylinders (2 πr2+ 2 Πr) and spheres (4 πr2). The mean surface areas of the pieces of trash types were determined because it allowed for comparison of their overall sporulation potentials by surface areas, which differed greatly between trash types.
To investigate the reason for lack of sporulation originating from stamens, a subsample of 30 stamens was selected at random at each sampling time, with no more than two stamens from each bunch. The stamens were placed in mesh bags and surface sterilised as for the berries, except that 0.035 % sodium hypochlorite was used. The sterilised stamens were placed onto plates of malt extract agar (MEA; Difco) amended with 0.25 g/l chloramphenicol (Sigma Chemical Co.) and incubated at 20 °C with diurnal 12/12 h light. The stamens were assessed for presence of typical B. cinerea sporulation after 6 days.
Data analysis
The raw data of each bunch trash type which were found to fit a normal distribution were analysed by ANOVA to determine effects of sampling time and vineyard. The data included surface area standardised data [estimated total surface area (mm2) per bunch], the numbers and proportions infested per bunch and estimated total sporulation potential per bunch. Numbers of different trash types were not analysed because of the differences in sizes of most trash types. Rather, mean surface area and sporulation potential of each trash type was considered of greater importance in determining the risk of infection and so was used in the analysis. These data were analysed to assess the effect of sampling time and vineyard on inoculum potential of the trash pieces. Data of damaged berries were included in the analysis even though Northover (1987) and Padgett and Morrison (1990) had considered them to be diseased berries. However, they acknowledged that these berries could become detached and remain within a bunch, thereby having the same potential function as bunch trash. Significant factors (P ≤ 0.05) indicated by ANOVA were further examined for difference between means of the treatments using Fisher’s LSD test. Correlation analysis using data from all vineyards was used to determine the significance of the relationship between the mean trash types within each bunch (total as well as infested) and the numbers of infected berries per bunch at the post-véraison sampling.
Sporulation potential of leaf trash from the canopy
During Season 3, the sporulation potential of leaf trash within the canopy was determined in eight commercial Sauvignon blanc vineyards which were scattered across the Marlborough region. Samples were collected post-véraison (18th February) and at harvest (17th April), when leaf trash was likely to be present due to the trimming and leaf plucking operations which are typically carried out during late summer and early autumn. Within each vineyard, five bays within each of four rows were selected at random. Within each bay, 10 sampling canopy positions were selected at random, and at each position the closest, necrotic whole leaf was removed (200 per time and per vineyard). Leaf samples were pooled for each bay and were incubated in a saturated atmosphere as before. After 5 days, the leaves from each bay were washed together in a plastic jar containing 40 ml of sterile water and Tween 20 (2 drops per l), by agitating for 3 min on a rotary shaker (Chiltern Scientific, Wanganui, New Zealand) at 170 rpm. The wash suspensions were decanted into 50 mL polypropylene centrifuge tubes (Nalge Nunc International, Naperville, IL, USA) and centrifuged at 805 × g for 5 min. The supernatants were then decanted off and the spore pellets resuspended in 10 ml of sterile water and Tween 20 (2 drops per l). From each conidial suspension, three separate samples were removed and mounted on two sections of a haemocytometer for counting. The mean number of conidia per leaf was calculated and data analysed using ANOVA to determine the effects of vineyard and sampling time.
Results
Bunch trash
The numbers of the different trash types were relatively similar across the three seasons and three vineyards (Table 1). The mean number of pieces of each trash type per bunch also remained relatively similar between véraison and harvest in all seasons (Table 1).
The mean surface areas per piece of the trash types did not differ between vineyards, sampling times or seasons. However, the total surface areas per bunch of the different trash types were significantly affected by sampling times in both Seasons 2 and 3 (P < 0.05; Table 2). In both seasons, there was a trend for increasing surface areas of aborted berries, aborted flowers and tendrils towards harvest, but decreasing surface areas of damaged berries and leaf fragments over that time although the individual effects were not significant.
Bunch trash infestation
Proportions of trash types infested with B. cinerea were not affected by season, but were significantly affected by vineyard source in Season 2 for aborted berries, damaged berries and calyptrae and in Season 3 for aborted flowers (Table 3). No stamens were found to be colonised. The proportions of infested tissue pieces did not differ between Seasons 2 and 3, at either post-véraison or harvest, for any of the trash types (Table 4). However, infestation of calyptrae was significantly higher in Season 1, when only Vineyard A was sampled, than in Seasons 2 and 3 (P < 0.05). Sampling times affected the mean proportions of infested trash types, but there were no clear trends for all seasons. In Season 2, the proportions of infested aborted flowers decreased significantly between post-véraison and harvest (P < 0.01), whereas the proportion of infested calyptrae and damaged berries increased between these times (P < 0.01). In Season 3, the proportion of aborted berries that were infested decreased from pre-bunch closure to post- véraison (P < 0.05). The proportion of infested aborted flowers and calyptrae also differed between sampling times, with post-véraison values being lower than at pre-bunch closure and harvest (P < 0.01; Table 4).
Sporulation potential of bunch trash
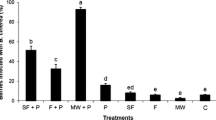
Sporulation levels differed between infested trash types. Over all three seasons, mean numbers of conidia per mm2 were 198 ± 36 for aborted berries, 152 ± 29 for aborted flowers, 2996 ± 469 for damaged berries, 986 ± 166 for leaf fragments, 2011 ± 164 for tendrils and 249 ± 81 for calyptrae. The sporulation potential per bunch also differed between trash types at each sampling time in Seasons 2 and 3 (Table 5) with greatest sporulation being from damaged berries at all sampling times except pre-bunch closure when they were absent.
The correlation analysis using data from all vineyards found that the number of infected berries per bunch at the post-véraison sampling reflected both the total number of aborted and mean number of aborted infested berries per bunch (Table 6). There was no relationship between the other trash types and number of infected berries per bunch. The number of berries infected per bunch at the post-véraison sampling was not significantly related to the total surface area of all trash per bunch (P = 0.354), nor the total sporulation potential of trash per bunch (P = 0.532).
Sporulation Potential of leaf trash from the canopy
The mean sporulation potential of necrotic leaves in the canopy did not differ between vineyards (P = 0.076), or sampling times (P = 0.421). The mean numbers of conidia produced per leaf were 11.7 ± 2.3 × 106 at post-véraison, and 9.3 ± 1.7 × 106 at harvest.
Discussion
Quantities of the different types of bunch trash were greater at pre-bunch closure and had decreased by post- véraison and harvest when they were similar. This was mainly attributed to the loss of stamens and calyptrae which had probably fallen out of bunches, as was postulated by Nair et al. (1988) who completed a similar study but collected the trash by shaking bunches, not dissecting them. The surface areas of the different trash types were also different depending on sampling times and season, with greater total areas per bunch at post-véraison than at harvest and smallest total areas at pre-bunch closure. These differences were due to the berries growing larger during the season and also increasing numbers of damaged berries as the tighter bunches with berry-to-berry pressure can increase splitting damage (Considine and Kriedemann 1972). The greater surface areas of damaged berries shown in Season 2 than Season 3, at post-véraison and harvest, were probably due to the warmer temperatures which occurred at flowering in Season 2 than in Season 3 (means of 16.9 and 14.8 °C, as determined by Bacchus programme; R. Agnew pers. comm. 2014). As a result of the temperatures, there were increased numbers of berries initiated in each bunch in Season 2. The greater total surface areas of all trash in Season 2 than Season 3 were also probably due to trash retention in tight bunches.
Incidence of B. cinerea also varied between types of bunch trash, with greatest overall, infestation incidences being in damaged berries and leaf fragments (44 and 45 %, respectively). Since damaged berries had the greatest sporulation potential per mm2 of trash tissue in bunches at post-véraison and harvest (means of 3.4 and 4.6 per mm2, respectively), they appeared likely to create a greater risk for fruit infection. However, the correlation analysis showed that damaged berries were not positively related to the numbers of infected berries per bunch at the post-véraison sampling. This apparent anomaly may have been caused by a number of factors. By the time bunches were most susceptible at full ripeness, the sporulation potential of damaged berries may have been depleted by previous periods of sporulation, such that conidial production was low. Sosa-Alvarez et al. (1995) reported that B. cinerea sporulated more frequently with rising temperatures in spring and Rotem et al. (1978) suggested that cooler temperatures preserve sporulation potential of some saprophytic plant pathogens in the longer term, because the nutrients within the mycelium were not depleted by the sporulation events. However, necrotic leaves collected from the canopies, rather than within the bunches, had greater sporulation capacity than any of the trash types from within bunches, and are produced later in the season so may provide tissues suitable for colonisation and sporulation, thereby acting as sources of secondary inoculum for infecting mature berries. In New Zealand, grapevine canopies are usually trimmed 2–3 times between pre-bunch closure and harvest to increase air-flow and sun exposure and so leaf fragments are abundant. Provision of suitable moisture and temperature are also needed for sporulation by B. cinerea on necrotic tissues, which are 15–25 °C with 95 % relative humidity for 12–24 h, and reflect the temperature ranges and moisture levels known to occur during bunch rot epidemics (Nair et al. 1988). Under New Zealand conditions, inoculum levels are likely to be high by the time of harvest (autumn) when the berries are particularly susceptible to infection. In a Swiss vineyard inoculated at flowering, Keller et al. (2003) found greater incidence of berry infection at harvest (100 %) than at pea-size and véraison (~20 %), which led them to conclude that secondary infections had occurred during the season, creating abundant inoculum for penetration of berries near to harvest. However in the drier South African and Mediterranean climates (Coertze et al. 2001 and Calvo‐Garrido et al. 2013, respectively), provision of viable surface inoculum on intact berries at véraison had little effect on the development of bunch rot, probably because wounds and free moisture were necessary for infection by air-borne conidia (Coertze and Holz 2002). Nair et al. (1988) reported that optimal rates of berry infection by B. cinerea occurred at 15–25 °C with 95 % relative humidity for 12–24 h, which reflect the temperature ranges and moisture levels known to occur during bunch rot epidemics. In cool climates such as in New Zealand, late season rainfall which occurs within a few weeks of harvest can cause sudden and excessive uptake of water by the grapevines, resulting in increased internal turgor of the berries, which split or crack (Considine and Kriedemann 1972). The presence of free moisture and wounds in mature berries inevitably results in bunch rot caused by B. cinerea (Jarvis 1980).
Floral debris has been regarded as a potential inoculum source of B. cinerea to the developing berries (Nair et al. 1988), however the relationship between infected trash trapped within a bunch and berry infection at harvest was not proven in this study. In contrast, Wolf et al. (1997) demonstrated that removal of floral debris from within the grape bunch at about fruit-set, using a back-pack leaf blower, reduced incidence and severity of berry infection at harvest, but only in some vineyards and some sampling seasons. Further, they could also have underestimated pathogen incidence since they assessed incidence of B. cinerea and severity of rot by symptoms visible on berries directly after removal from the field, without moist incubation as was used in this study. In another experiment, Calvo‐Garrido et al. (2013) demonstrated that when iprodione was applied at flowering, the necrotic bunch tissues assessed (aborted flowers, aborted fruit and calyptrae) had 0 % B. cinerea incidence overall compared to the unsprayed control (8.3 %). The regression model (R2 = 0.95) they developed indicated the significance of variables accounting for bunch rot at harvest, which were predominantly the proportion of fruit with latent infection (49 %), followed by infested calyptrae (25 %) and infested aborted fruits (20 %). Recently Tardaguila et al. (2008) investigated mechanical thinning with a machine harvester on which the bow rods were adjusted to strike the vine trunks. The vibration caused some of the berries to be detached, thereby creating more open bunches. They showed that the treatment significantly reduced berry number per bunch and bunch compactness, with reduction of Botrytis bunch rot incidence in a Grenache block in one season. Similar experiments have recently been carried out in New Zealand, with resulting reductions in bunch rot and in amounts of bunch debris (Dion Mundy pers. comm, 2014). Gibberellic acid applications have also been shown to consistently lengthen bunch stems, opening up bunches and reducing cluster compactness, with a significant reduction in the incidence of bunch rot for both Chardonnay and Vignoles (Hed et al. 2009).
The presence or absence of suitable weather conditions is a likely reason for the differences reported between the results of this study and those of Wolf et al. (1997) and Calvo‐Garrido et al. (2013), since epidemics of Botrytis bunch rot are known to be associated with presence of rainy weather after véraison (Coertze and Holz 2002). It is also possible that berry infection and the retention of some bunch trash types are both influenced by the same external factor. Damp or overcast weather has been reported to be conducive for trash retention, particularly stamens and calyptrae (Elmer and Michailides 2004). Alternatively, the bunch architecture may influence both; for example, cultivars with more compact bunches have been shown to be more commonly infected by B. cinerea (Marois et al. 1986; Vail and Marois 1991), possibly because trash is less likely to fall from a more compact bunch than an open bunch (Nair and Hill 1992).
This study has provided further evidence of the potential links in the complex disease cycle of Botrytis bunch rot in grapes. It has demonstrated that bunch trash comprises abundant flower, berry and leaf tissues, for which the amount, percent infestation and sporulation changes during the growing season. Necrotic leaf material lodged within the canopy was also shown to have high rates of infestation and sporulation which could also provide an ongoing source of inoculum for infection of mature fruit. However, there was no consistent, positive relationship between inoculum potentials of the bunch trash components and berry infection at post-véraison. This was not surprising since numerous reports have indicated that many factors affect infection, including those associated with variety, such as bunch compactness, thickness of berry skins and canopy vigour. Further, epidemic development requires favourable weather conditions and berries to be wounded, often due to splitting. When moist weather occurs near to harvest, the sources of inoculum elucidated by this study could be sufficient to initiate secondary cycles of infection and sporulation, with consequential bunch rot and yield losses.
References
Barbetti, M. J. (1980). Bunch rot of Rhine Riesling grapes in the lower south-west of Western Australia. Australian Journal of Experimental Agriculture and Animal Husbandry, 20, 247–251.
Calvo‐Garrido, C., Usall, J., Viñas, I., Elmer, P. A., Cases, E., & Teixidó, N. (2013). Potential secondary inoculum sources of Botrytis cinerea and their influence on bunch rot development in dry Mediterranean climate vineyards. Pest Management Science, 70, 922–930.
Coertze, S., & Holz, G. (2002). Epidemiology of Botrytis cinerea on grape: wound infection by dry, airborne conidia. South African Journal of Enology and Viticulture, 23, 72–77.
Coertze, S., Holz, G., & Sadie, A. (2001). Germination and establishment of infection on grape berries by single airborne conidia of Botrytis cinerea. Plant Disease, 85, 668–677.
Considine, J. A., & Kriedemann, P. E. (1972). Fruit splitting in grapes: determination of the critical turgor pressure. Crop and Pasture Science, 23, 17–23.
de Kock, P. J., & Holz, G. (1991). Colonization of table grapes by Botrytis cinerea in the Western Cape province. Phytophylactica, 23, 73–80.
Dubos, B. & Roudet, J. (2000). First results of the research network on vine grey rot epidemiology in France. Proceedings of the XIIth International Botrytis Symposium, 44
Elmer, P. A., & Michailides, T. J. (2004). Epidemiology of Botrytis cinerea in orchard and vine crops. In Y. Elad, B. Williamson, P. Tudzynski, & N. Delen (Eds.), Botrytis: Biology, pathology and control (pp. 243–272). Netherlands: Springer.
Hed, B., Ngugi, H. K., & Travis, J. W. (2009). Relationship between cluster compactness and bunch rot in Chardonnay and Vignoles grapes. Plant Disease, 93(11), 1195–1201.
Holz, G., Coertze, S., & Basson, E. J. (1997). Latent infection of Botrytis cinerea in grape pedicels leads to postharvest decay. Phytopathology, 87, S43.
Holz, G., Gütschow, M., Coertze, S., & Calitz, F. J. (2003). Occurrence of Botrytis cinerea and subsequent disease expression at different positions on leaves and bunches of grape. Plant Disease, 87, 351–358.
Jarvis, W. R. (1980). Epidemiology. In R. J. Coley-Smith, K. Verhoeff, & W. R. Jarvis (Eds.), Biology of botrytis (pp. 219–250). London: Academic Press.
Jaspers, M. V., Seyb, A. M., Trought, M. C. T., & Balasubramaniam, R. (2013). Overwintering grapevine debris as an important source of Botrytis cinerea inoculum. Plant Pathology, 62, 130–138.
Keller, M., Viret, O., & Cole, F. M. (2003). Botrytis cinerea infection in grape flowers: defence reaction, latency, and disease expression. Phytopathology, 93, 316–322.
Marois, J. J., Nelson, J. K., Morrison, J. C., Lile, L. S., & Bledsoe, A. M. (1986). The influence of berry contact within grape clusters on the development of Botrytis cinerea and epicuticular wax. American Journal of Enology and Viticulture, 37, 293–296.
Nair, N. G., & Hill, G. K. (1992). Bunch rot of grapes caused by Botrytis cinerea. In J. Kumar, H. Chaube, U. Singh, & A. Mukhopadhyay (Eds.), Plant diseases of international importance: Diseases of fruit crops (pp. 147–169). New Jersey: Prentice-Hall Inc.
Nair, N. G., Emmett, R. W., & Parker, F. E. (1988). Some factors predisposing grape berries to infection by Botrytis cinerea. New Zealand Journal of Experimental Agriculture, 16, 257–263.
Nair, N. G., Guilbaud-Oulton, S., Barchia, I., & Emmett, R. (1995). Significance of carry over inoculum, flower infection and latency on the incidence of Botrytis cinerea in berries of grapevines at harvest in New South Wales. Australian Journal of Experimental Agriculture, 35, 1177–1180.
Northover, J. (1987). Infection sites and fungicidal prevention of Botrytis cinerea bunch rot of grapes in Ontario. Canadian Journal of Plant Pathology, 9, 129–136.
Padgett, M., & Morrison, J. C. (1990). Changes in the grape berry exudates during fruit development and their effect on the mycelial growth of Botrytis cinerea. Journal for the American Society of Horticultural Science, 115, 269–273.
Rae, S.N., ed. (1987). Water and soil resources of the Wairau: Water resources. Vol. 1. Blenheim: Marlborough Catchment and Regional Water Board.
Rotem, J., Cohen, Y., & Bashi, E. (1978). Host and environmental influences on sporulation in vivo. Annual Review of Phytopathology, 16, 83–101.
Smart, R., & Robinson, M. (1991). Sunlight into wine: A handbook for winegrape canopy management (p. 88). Adelaide: Winetitles.
Sosa-Alvarez, M., Madden, L. V., & Ellis, M. A. (1995). Effects of temperature and wetness duration on sporulation of Botrytis cinerea on strawberry leaf residues. Plant Disease, 79, 609–615.
Tardaguila, J., Petrie, P. R., Poni, S., Diago, M. P., & de Toda, F. M. (2008). Effects of mechanical thinning on yield and fruit composition of Tempranillo and Grenache grapes trained to a vertical shoot-positioned canopy. American Journal of Enology and Viticulture, 59(4), 412–417.
Vail, M. E., & Marois, J. J. (1991). Grape cluster architecture and the susceptibility of berries to Botrytis cinerea. Phytopathology, 81, 188–191.
Viret, O., Pezet, R., Cole, M. & Keller, M. (2001). Latency, disease incidence and localization of Botrytis cinerea Pers. during the early stages of bloom infection of grapes. Proceedings of the 11th Congress of the Mediterranean Phytopathological Union pp 276–278.
Wolf, T. K., Baudoin, A. B. A. M., & Martinez-Ochoa, N. (1997). Effect of floral debris removal from fruit clusters on Botrytis bunch rot of Chardonnay grapes. Vitis, 36, 27–33.
Acknowledgments
We wish to thank the Foundation for Technology and Research (NZ) for funding this PhD (AMS) study and the many grape growers who allowed the experiments to be conducted within their vineyards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaspers, M.V., Seyb, A.M., Trought, M.C.T. et al. Necrotic grapevine material from the current season is a source of Botrytis cinerea inoculum. Eur J Plant Pathol 144, 811–820 (2016). https://doi.org/10.1007/s10658-015-0726-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0726-4