Abstract
Botryosphaeria stem blight is an economically important disease of blueberry worldwide. In this study, factors affecting inoculum production, infection and disease progression of Neofusicoccum spp. in blueberries were investigated. Under laboratory conditions conidia of the main three Neofusicoccum species (N. australe, N. parvum and N. ribis) were released from pycnidia at 15–30 °C and under relative humidities (RHs) of 80–100%, with greatest numbers released by N. parvum. The greatest numbers of oozing pycnidia and conidial release occurred at higher temperatures (25–30 °C) and RHs (92–100%). Inoculation of green shoots with different N. parvum and N. ribis conidial concentrations (50 μL of 5 × 104−5 × 106 conidia/ mL) caused 100% incidence but lesion lengths increased with increasing concentrations. Wound age affected N. ribis lesion development, with lesions only observed for 0–7-day-old wounds in soft green shoots and 0–4-day-old wounds for both hard green shoots and trunks. Colonisation length decreased with increasing wound age. Lesions developed on wounded shoots when plants were exposed to 20 or 25 °C and 90 or 100% RH during the early infection processes; and in non-wounded shoots spot-like lesions were observed although N. ribis colonised the stem tissue. Seasons (summer, autumn and winter) had no effect on susceptibility of wounded plants to N. ribis. External lesions only developed in summer-inoculated plants and colonisation length was lower in winter-inoculated plants. Information on host and environmental factors that affect disease development determined by the study will be used to inform the development of control strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The blueberry industry has been established for over two decades in New Zealand after the introduction of both Vaccinium corymbosum (highbush) and V. ashei (rabbiteye) cultivars from North America (Poll and Wood 1985). Since then the growth of the industry has been rapid and currently there are over 60 growers and 400 ha planted in New Zealand. Traditionally most of the plantings have occurred in the upper North Island, however new areas are being planted in the South Island (www.blueberriesnz.co.nz). Blueberry stem blight and twig dieback caused by Botryosphaeriaceae species are increasingly being recognised as major issues for blueberry production worldwide (Xu et al. 2015). In New Zealand this disease is an emerging problem; dieback and crown rot were estimated to affect about 18% of blueberry plants in the main production areas, costing about $500,000 annually due to yield losses and replanting costs (Sammonds et al. 2009). The Botryosphaeriaceae species associated with stem blight of blueberry differ between countries and are probably related to the differential effects of environmental conditions on inoculum production and infection by the different pathogen species, as well as the influence of management practices and prevalence of alternative hosts. In south-eastern USA B. corticis and B. dothidea were reported to be the cause of blueberry stem blight (Creswell and Milholland 1988; Milholland 1972a; Smith 2009) and in Florida L. theobromae and N. ribis were the prevalent species (Wright and Harmon 2010). Neofusicoccum arbuti, N. australe and N. parvum were reported to be associated with blueberry stem dieback in Chile (Espinoza et al. 2009), whilst in south west China B. dothidea, N. parvum and Lasiodiplodia theobromae were reported as the cause of dieback and stem blight (Xu et al. 2015). Sampling of necrotic blueberry stems from New Zealand farms resulted in the isolation of N. australe, N. luteum, N. parvum and N. ribis, with N. australe being the most common species recovered (Tennakoon et al. 2017a). All the recovered species were pathogenic on blueberry shoots, with N. ribis and N. parvum being the most pathogenic. Some Botryosphaeriaceae spp. can also act as endophytes, becoming saprotrophic or pathogenic when conditions are favourable (Smith et al. 1996), indicating the potential for infected plants to act as new inoculum sources when used to set up blueberry farms. In nurseries, Botryosphaeriaceae infections of propagating material may take place in the field and during propagation, with Botryosphaeriaceae spp. isolated from asymptomatic nursery plants and propagation material (Tennakoon et al. 2017a). In grapevines, van Niekerk et al. (2006) also stated that early infections, which take place during the propagation of planting material, may stay latent until the plants undergo abiotic or biotic stress. This may also be the case with blueberry.
For infection of mature bushes, the sources of primary inoculum are likely to be the pycnidia which form on current-season’s infected rachides, fruit, blighted shoots, petioles and leaf lesions, as reported for pistachio by Michailides (1991). In pistachio, these constitute the main sources of primary inoculum and the same pycnidia can provide viable conidia for summer and autumn infections for up to 6 years (Michailides and Morgan 2004). Under humid conditions, pycnidia of Botryosphaeriaceae species produce conidia that are exuded in gelatinous matrices forming cirrhi, which are ribbon-like masses of spores (Phillips 2002). However, the effect of temperature and relative humidity on the release of conidia from pycnidia on Neofusicoccum spp. infected blueberry tissue has not been determined. The spores in cirrhi are then released by water splash, which is provided by rain or irrigation. Dispersal by splash is normally effective for only a few meters, although the dispersing droplets may be further dispersed by wind. In the USA, Cline (2013) reported that B. dothidea overwintered as fruiting bodies in dead and infected blueberry stems, with spores being carried by wind and rain from infected stems to wounds on healthy plants. Infection is generally believed to be through wounds, which could be caused by pruning and trimming, mechanical injury (such as caused by harvesters or wind-blown grit), freezing injury and herbicide injury. Recently, Tennakoon et al. (2015) demonstrated herbicide injury to provide suitable sites for infection of blueberry stems by N. ribis. However, the factors that increase the infection and development of Botryosphaeriaceae spp. in blueberries have not been well studied.
The overall objective of this study was to determine the factors affecting inoculum production, infection and disease development in blueberry by Neofusicoccum spp. Initially, the effect of temperature and relative humidity on sporulation of two isolates of each of the three main Neofusicoccum species infecting blueberry in New Zealand (N. australe, N. parvum and N. ribis) from lesions on blueberry stem tissue was determined. The effect of different conidial numbers on wound infection and the effect of wound age on susceptibility of different tissue types was evaluated. The interaction between wounding, temperature and relative humidity during the early infection period on N. ribis disease development was also assessed. Finally the influence of wounding at different times of the year on susceptibility to N. ribis infection and disease development was determined. This information on host and environmental factors that affect disease development will inform the development of disease management strategies including the appropriate timing of cultural practices and application of control products to provide effective control.
Materials and methods
Origin and maintenance of fungal isolates
Neofusicoccum australe isolates LUPP1321 and LUPP1364, N. ribis isolates LU1340, LUPP1348 and LUPP1365 and N. parvum isolates LUPP1249, LUPP1288 and LUPP1363 were all originally isolated from blueberry in a New Zealand nationwide survey (Tennakoon et al. 2017a) and obtained from the Lincoln University culture collection. Fungal isolates were stored in 20% glycerol at −80 °C and routinely cultured on potato dextrose agar (PDA; DIFCO™, New Jersey, USA) at 20 °C.
Effect of temperature and relative humidity on sporulation from lesions
Soft green shoots of rabbiteye (V. ashei) cultivar ‘Dolce Blue’ were detached, wounded and inoculated with mycelium plugs of two pathogenic isolates each of N. australe (isolates LUPP1321, LUPP1364), N. ribis (isolates LUPP1348, LUPP1365) and N. parvum (isolates LUPP1288, LUPP1363) and allowed to develop lesions and pycnidia as described by Tennakoon et al. (2017b). To determine the effect of different relative humidities (RH) and temperatures on numbers of oozing pycnidia and conidia, different saturated salt solutions and water were used to achieve RHs of 80–81% [(NH4)2SO4], 92–96% (KNO3) and 100% (water) (Greenspan 1977). Ten milliliters of each saturated salt solution were poured into separate 25-mL tubes and a filter paper strip was placed into the solution of each tube to act as a wick, to maintain uniform vapour inside the tube. Shoot sections (15 mm) with lesions and pycnidia cut from the center of each lesion were suspended in each tube on a string and tubes were incubated at 15 °C, 20 °C, 25 °C or 30 °C for 24 h. Shoot sections were held with tweezers to allow counting of oozing pycnidia under a stereo microscope (× 10 magnification) and each section was then placed into a 1.5 mL Eppendorf tube containing 1 mL of sterile water amended with 0.01% Tween 80 (BDH Chemicals LTD, UK). The tubes were vortexed for 30 s to disperse the conidia into the water and the concentrations of conidia in the suspensions were determined using a haemocytometer. There were four replicates for each isolate and RH treatment which were arranged in a complete randomized design (CRD) for each temperature supplied by individual incubators which had all been set to provide 12 h light and 12 h dark. Data were analyzed for each temperature as this factor could not be randomised within the layout.
Effects of different conidial numbers on wound infection
The effect of different conidial concentrations of N. parvum (isolates LUPP1249, LUPP1288, LUPP1363) and N. ribis (isolates LUPP1340, LUPP1348, LUPP1365) on wound infection was determined using two year old potted blueberry plants (cultivar ‘Dolce Blue’) in February 2014. For each species a mixed isolate conidial suspension, containing equal concentrations of each isolate, was prepared as described by Amponsah et al. (2014). Four dilutions for each species were then prepared (5 × 104, 1 × 105, 5 × 105 and 5 × 106 conidia/ mL) based on haemocytometer counts.
The soft green shoots and hard green shoots emerging from the main stem were wounded (~1–2 mm deep and 4–6 mm diameter) at 10 cm from their bases using a sterile scalpel. Wounds were immediately inoculated with 50 μL drops of one of the conidial suspensions (equivalent to approximately 2.5 × 103, 5 × 103, 2.5 × 104 and 2.5 × 105 conidia per inoculation site) and control plants were inoculated with sterile water. Each inoculation point was covered with Parafilm™ by wrapping it to form a lip which held the solution in place. Each plant was then covered with a transparent plastic bag which was sprayed inside with a fine mist of water and left for 48 h to provide humid conditions conducive to spore germination and infection. Ten replicate plants were used for each treatment and two shoots of each type were inoculated on each plant. The plants were arranged in a CRD in an open area similar to field conditions at the Lincoln University nursery and the soil watered by hand as required. Lesion lengths were measured with a digital caliper (Mitutoyo, Kanagawa, Japan), after 14 days for soft green shoots and after 30 days on the bark of the hard green shoots. For the hard green shoots, the bark was then removed and the lesions that developed in the wood were measured. Then the colonisation length (entire length from which the pathogen was recovered from) was assessed by surface sterilising the shoots by dipping in 70% ethanol for 30 s and air dried in a laminar flow cabinet for 10 min. Isolations were made onto PDA using 1 cm stem pieces cut from above and below the inoculation point. The plates were incubated in 12 h light and 12 h dark conditions at 25 °C for 3–5 days and N. ribis and N. parvum isolates growing from the stem pieces identified by colony appearance.
Effect of wound age on susceptibility of different tissue types
This experiment was conducted during July 2014 using 2-year-old blueberry plants (cultivar ‘Dolce Blue’). The soft green shoots, hard green shoots and the woody trunk (main stem) were wounded and inoculated after 0, 1, 4, 7, 10, 14 and 28 days. The wounds were inoculated with 50 μL drops of a mixed isolate conidial suspension (106/mL) of N. ribis (isolates LUPP1340, LUPP1348, LUPP1365). Six plants were used for each treatment and tissue type and two shoots were inoculated on each plant. The controls were inoculated 1 and 28 days after wounding with sterile water. Plants were immediately covered with separate new plastic bags as before and plants were arranged in a CRD in an open area at the Lincoln University nursery and maintained as described previously. Lesion lengths were measured using a digital caliper after 14 days on soft green shoots, and after 30 days on hard green shoots and woody trunks. The 11-cm shoot sections, cut from 5 cm above to 5 cm below the inoculation point, were then surface sterilised as described previously, and isolation onto PDA was conducted using tissue pieces cut at 1 cm intervals, but excluding 1 cm around the inoculation point. The plates were incubated as described previously and N. ribis isolates identified by colony appearance.
Effect of wounding and environmental factors on infection
The two growth chambers (Conviron PGV36; Controlled Environments Limited), used for this experiment were maintained at 20 °C and 25 °C. Each chamber was used to provide two relative humidities in turn, 90% and 99–100%. To ensure a relative humidity of 99–100% a misting unit was installed which sprayed the chamber walls with fine water droplets for 30 s every hour. The RH and temperature were measured using Tinytag® relative humidity and temperature data loggers (Gemini Data Loggers, UK). Two-year-old potted blueberry plants cultivar ‘Dolce Blue’ were used for this experiment which was conducted in March 2014. Hard green shoots were wounded using a sterile scalpel as described previously or non-wounded, the inoculation site being marked with a permanent marker pen and wrapped with Parafilm™ to form a lip as described previously. The sites were immediately inoculated with 50 μL drops of a mixed isolate conidial suspension (106/mL) using same three isolates of N. ribis as previously outlined. Control plants were inoculated with sterile water. Plants were arranged in a CRD in each growth chamber, for each temperature and RH treatment, and incubated for 48 h. Plants were then transferred into a shade house where they were exposed to natural conditions and the soil watered by hand as required. Six plants were used for each treatment and two shoots were inoculated on each plant. Non-wounded shoots and wounded shoots were observed weekly for disease progression. At 30 days after inoculation, the lesion lengths were measured with a digital caliper and isolations were carried out as described previously.
Effect of wounding at different times of the year on susceptibility
Potted 2-year-old blueberry plants (cultivar ‘Dolce Blue’) were used for this experiment which was conducted from August 2014 to March 2015. Hard green shoots and trunks of the plants were wounded using a sterile scalpel and wrapped with Parafilm™ to form a lip, then immediately inoculated as described previously with 50 μL drops of a mixed isolate conidial suspension (106/mL) of N. ribis (isolates LUPP1348 and LUPP1365). Control plants were inoculated with water. The inoculated plants were covered with separate new polythene bags misted inside with water and left in place for 48 h. Six replicate plants were used for each treatment and two shoots were inoculated on each plant. Treatments were set up at different times of the year, being winter (August), summer (December) and autumn (March). Plants for each season were placed separately in a CRD in a shade house and examined for disease development weekly until 30 days. The external lesion lengths were then measured with a digital caliper and isolations were carried out from the bark and wood separately, as described in previously.
Statistical analysis
As the data were shown to be normally distributed raw data were analysed. Data of lesion lengths and pathogen isolation distances, pycnidial and conidial numbers, were analysed by general analysis of variance (ANOVA) using Genstat 16 to determine treatment effects. In experiments where two stems were inoculated on the same plant, these were used to calculate a mean value for that replicate and this used in the analysis. Comparisons between means of individual treatments used Fisher’s protected LSDs at P ≤ 0.05. Binomial data for presence and absence were analysed by generalized linear mixed model (GLM) using Genstat 16 and SEDs used to determine significance of differences between means. The correlation between the numbers of oozing pycnidia and conidia were analysed by linear regression using Genstat 16.
Results
Effect of temperature and relative humidity on sporulation from lesions
In general, two N. parvum isolates oozed the highest number of pycnidia and conidia across all the relative humidities and temperatures compared to N. australe and N. parvum isolates (data not shown), and isolate data were combined for further analysis.
15 °C
The mean number of oozing pycnidia at 15 °C was 74.3/15 mm lesion. There was no significant effect of RH on the number of oozing pycnidia (P = 0.316). The effect of species was significant (P = 0.002, LSD = 24.06) with the highest mean number of oozing pycnidia (Table 1) from N. parvum (95.8/15 mm lesion) followed by N. australe (76.5/15 mm lesion) which were not significantly different from each other but were significantly greater than for N. ribis (50.5/15 mm lesion). The interaction between RH and species was not significant (P = 0.642).
The mean number of conidia released at 15 °C was 1.4 × 105/mL. There was no significant effect of RH on the number of conidia (P = 0.293). There was a significant effect of species on conidial production (P = 0.004, LSD = 1.14). The greatest mean number of conidia was from N. parvum (2.5 × 105/ mL) which was significantly different (Table 1) from N. australe (1.2 × 105/ mL) and N. ribis (0.5 × 105/ mL), which were not significantly different from each other. There was no significant effect of RH on conidial production (P = 0.293) nor a significant interaction between RH and species (P = 0.363).
20 °C
The mean number of oozing pycnidia at 20 °C was 84.2 /15 mm lesion. There was no significant effect of RH on the number of oozing pycnidia (P = 0.679). The effect of species was significant (P < 0.001, LSD = 23.78). The highest mean number of oozing pycnidia was from N. parvum (118.7/15 mm lesion) which was significantly different from N. ribis (63.8/15 mm lesion) and N. australe (70.0/15 mm lesion), which were not significantly different from each other (Table 1). There was a significant interaction (P < 0.001, LSD = 41.19) between RH and species. The highest number of oozing pycnidia was from N. parvum at 92–96% RH (154.9/15 mm lesion), which was significantly different from other treatments. This was followed by N. australe at 100% RH and, N. parvum at 80–81% and 100% (111.1, 103.5 and 97.8/15 mm lesion, respectively). The lowest number of oozing pycnidia was for N. australe at 92–96% RH (35.3/15 mm lesion).
The mean number of conidia released at 20 °C was 0.7 × 105/mL. There was no significant effect of RH on the number of conidia (P = 0.129). The effect of species on number of conidia was significant (P < 0.001, LSD = 0.82), with highest mean number from N. parvum (1.7 × 105/mL) compared with both N. australe (0.2 × 105/mL) and N. ribis (0.3 × 105/mL), which were not significantly different from each other (Table 2). There was no significant interaction between RH and species (P = 0.552).
25 °C
The mean number of oozing pycnidia at 25 °C was 93.6/15 mm lesion. There was a significant effect of RH on the number of oozing pycnidia (P < 0.001, LSD = 27.93) being significantly higher at 100% compared with both 92–96% and 80–81%. There was a significant effect of species (P < 0.001, LSD = 27.93) on the number of oozing pycnidia. The highest number of oozing pycnidia was from N. parvum (147.3/15 mm lesion), followed by N. ribis (81.9/15 mm lesion) which were significantly different (Table 1). The lowest number was produced by N. australe (51.8/15 mm lesion) which was significantly different from the other species. There was a significant interaction (P < 0.001, LSD = 48.38) between RH and species, for both N. australe and N. ribis there were significantly higher number of oozing pycnidia at 100% RH, whilst for N. parvum the highest number of oozing pycnidia was at a RH of 80–81% which was significantly higher than 92–96% but not 100%.
The mean number of conidia released at 25 °C was 1.5 × 105/mL. There was a significant effect of RH on the number of conidia (P = 0.005, LSD = 0.65), being significantly higher at 100% compared with both 92–96% and 80–81%. The effect of species was significant (P = 0.005, LSD = 0.65), with significantly higher mean number of conidia produced by N. parvum (2.8 × 105/mL) compared with N. ribis (1.1 × 105/mL) and N. australe (0.7 × 105/mL), which were not significantly different from each other (Table 1). There was a significant interaction between RH and species (P = 0.017, LSD = 1.12). For N. ribis there were significantly more conidia at 100% RH compared with at the other RH’s, whilst for N. parvum the most conidia was at a RH of 80–81% which was significantly higher than 92–96% but not 100%. For N. australe there was no significant effect of RH on the number of conidia.
30 °C
The mean number of oozing pycnidia at 30 °C was 104.6/15 mm lesion. There was a significant effect of RH on the number of oozing pycnidia (P < 0.001, LSD = 16.86), being significantly higher at 100% compared with both 92–96% and 80–81% (Table 1). The effect of species on the number of oozing pycnidia was significant (P < 0.001, LSD = 16.86). The highest mean number of oozing pycnidia was from N. parvum (149.9/15 mm lesion) which was followed by N. australe (108.8/15 mm lesion) which were significantly different (Table 1). The lowest mean number was produced by N. ribis (55.3/15 mm lesion) which was significantly different from the other species. The interaction between RH and species was significant (P < 0.001, LSD = 29.21). For N. parvum there were significantly higher number of oozing pycnidia at 100% RH compared with at the other RH’s, whilst for N. ribis the most oozing pycnidia was at a RH of 80–81% which was significantly higher compared with at the other RH’s. For N. australe there was no significant effect of RH on the number of oozing pycnidia.
The mean number of conidia released at 30 °C was 2.5 × 105/mL. There was no significant effect of RH on the number of conidia (P = 0.443). There was a significant effect of species (P < 0.001, LSD = 0.92) on conidial production. The highest mean number of conidia was from N. parvum (3.8 × 105/mL) which was significantly different from both N. australe (2.2 × 105/mL) and N. ribis (1.4 × 105/mL), which were not significantly different from each other (Table 1). There was no significant interaction between RH and species (P = 0.246).
In general, the greatest numbers of oozing pycnidia and conidial release occurred at higher temperatures (25–30 °C) and RHs (92–100%). Overall, there was a significant positive linear correlation (R2 = 0.472; P < 0.001) between the number of oozing pycnidia and the number of conidia. However, N. parvum incubated at 30 °C and 100% RH produced less conidia than predicted based on the number of oozing pycnidia (linear regression equation y = 0.0178×-0.0314, where y = number of conidia (105/mL) and x = number of oozing pycnidia).
Effects of different conidial numbers on wound infection
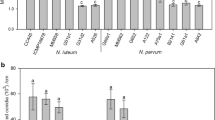
On soft green shoots, there was a significant effect of species (P = 0.002) on lesion lengths, with N. ribis (61.8 mm) producing significantly longer lesions than N. parvum (44.7 mm) (Table 2). There was a significant effect of conidial concentrations (P < 0.001, LSD = 14.59) on lesion length, with lesions produced on shoots inoculated with the highest concentrations of 5 × 106 conidia/mL (91.2 mm) being significantly longer compared with inoculation with any of the other concentrations (31.3–51.6 mm). The significant interaction (P = 0.021, LSD = 20.63) between species and conidial concentration was associated with greater increase in lesion length by N. ribis than N. parvum with increasing conidial concentration (Table 2). At the highest concentration of 5 × 106 conidia/mL N. ribis produced significantly longer lesions compared with N. parvum.
On hard green shoots there was a significant effect of species (P < 0.001) on lesion development on the outer bark, with N. ribis (37.7 mm) producing significantly longer lesions than N. parvum (29.3 mm) (Table 2). There was a significant effect of conidial concentrations (P < 0.001, LSD = 4.90) on lesion length with lesions produced on shoots inoculated with the highest concentrations of 5 × 106 conidia/mL (40.3 mm) being significantly longer compared with inoculation with either 5 × 104 or 1 × 105 (23.1 and 32.0 mm, respectively), but not compared with 5 × 105 conidia /mL (38.7 mm). The interaction between species and conidial concentration was not significant (P = 0.605). When the bark was removed from the hard green shoots light brown discolouration was observed in the wood due to the infection. There was a significant effect of species (P < 0.001) on lesion length, with lesion length produced by N. ribis (46.6 mm) significantly longer than for N. parvum (30.7 mm). There was a significant effect of conidial concentrations (P = 0.003, LSD = 6.17) on lesion length with lesions produced on shoots inoculated with the lowest concentration of 5 × 104 conidia /mL (31.1 mm) being significantly shorter compared with inoculation with all other concentrations (43.2–40.0 mm) which did not differ significantly from each other. The interaction between species and conidial concentration was not significant (P = 0.968). Control plants inoculated with sterile water did not produce any lesions and pathogen isolation yielded no colonies characteristics of N. parvum and N. ribis.
Effect of wound age on susceptibility of different tissue types
On soft green shoots there was a significant effect of wound age (visible lesions only developed on 0–7 day old wounds) on lesion length (P < 0.001, LSD = 31.0) (Table 3). The longest lesions were produced after inoculation of fresh wounds (103.3 mm) which were significantly longer than lesions which developed on 7 and 4 day old wounds (24.3 and 57.4 mm, respectively) but not compared with lesions which developed on 1 day old wounds (88.3 mm). On soft green shoots there was a significant effect of wound age on colonisation length (P < 0.001) as indicated by the mean length of tissue colonised by the pathogens. The mean colonisation length from inoculated fresh wounds (96.7 mm) and 1-day-old wounds (96.7 mm) were significantly greater than for all the other wound ages (16.7–61.7 mm). The lowest mean colonisation length (16.7 mm) observed in 28 day old wounds was significantly different from all other times except 7 and 14 day old wounds.
On hard green shoots, there was a significant effect of wound age (visible lesions only developed on 0–4 days old wounds) on lesion lengths (P = 0.002), with the mean lesion length produced after inoculation of fresh wounds (28.3 mm) being significantly longer than those produced with inoculation of 1and 4 day old wounds (20.5 and 15.4 mm, respectively), which were not significantly different from each other (Table 3). There was also a significant effect of wound age on colonisation length (P < 0.001). Mean colonisation length was not significantly different between the inoculation of fresh wounds (90.0 mm) and 1-day-old wounds (85.0 mm) which were significantly greater than for all the other wound ages (33.3–63.3 mm). The lowest mean colonisation length (33.3 mm) was observed after inoculation of 28-day-old wounds, which was significantly different from other treatments.
On woody trunks, there was a significant effect of wound age (visible lesions only developed on 0–4 days old wounds) on lesion length (P = 0.003). Mean lesion length after inoculation of fresh wounds (22.0 mm) was significantly longer than those produced after inoculation of 4-day-old wounds (11.6 mm), but not compared with 1-day-old wounds (Table 3). There was also a significant effect of wound age on colonisation length (P < 0.001). Mean colonisation length after inoculation of fresh wounds (81.7 mm) was not significantly different from 1-day-old wounds (73.3 mm) and significantly greater than for all the other wound ages (35.0–63.3 mm).
Infection incidence for the pathogen inoculated plants, irrespective of tissue type, was 100% and 0% for the uninoculated controls.
Effect of wounding and environmental factors on infection
In wounded inoculated shoots dark brown lesions developed irrespective of the environmental factors used to incubate the plants after inoculation. In the non-wounded tissues brown spot-like lesions appeared which did not develop into clear lesions irrespective of the environmental factors. Therefore the lesion lengths were measured and analysed for only the wounded shoots. At 20 °C there was a significant effect of RH (P < 0.001) on lesion lengths produced, with significantly longer mean lesions at 90% RH (51.5 mm) than at 100% RH (26.7 mm). At 25 °C there was no significant effect of RH (P = 0.379) on lesion lengths produced, at 90 and 100% RH (32.0 and 25.7 mm, respectively). At 20 °C, there was a significant effect of wounding (P < 0.001) on colonisation length, with mean colonisation length being significantly greater for wounded shoots (86.7 mm) than non-wounded shoots (69.2 mm). There was a significant effect of RH on colonisation length (P < 0.001), with significantly greater colonisation length at 90% RH (91.7 mm) compared with 100% RH (64.2 mm). There was also a significant interaction (P = 0.002) between RH and wounding on the colonisation length, which was associated with differences in the effect of the wounding treatments for the two RHs. Mean colonisation length at 90% RH for wounded shoots (95.0 mm) was significantly longer than for non-wounded shoots (88.3 mm), but not at 100% RH, with means of 78.0 and 50.0 mm, respectively. For plants incubated at 25 °C, there was a significant effect of wounding (P = 0.002) on colonisation length, with mean colonisation length being significantly greater for wounded shoots (70.0 mm) than non-wounded shoots (52.5 mm). There was a significant effect of RH on colonisation length (P < 0.001), with significantly greater colonisation length at 90% RH (70.8 mm) compared with 100% RH (51.7 mm). There was no significant interaction (P = 0.865) between wounding and RH on the colonisation length. The infection incidences were 100% for all the pathogen-inoculated treatments and 0% for the uninoculated controls. No Botryosphaeriaceae species were recovered from the uninoculated control plants.
Effect of wounding at different times of the year on susceptibility
Inoculation of wounded shoots during summer resulted in the development of visible external lesions in hard green shoots and trunks, with means of 50.0 and 70.8 mm, respectively, whilst no external lesions were observed after inoculation in autumn and winter. No lesions were observed and no isolates of Botryosphaeriaceae species were recovered from any of the non-inoculated control shoots or trunks. In hard green shoots, there were no significant effects of tissue type (bark or wood) on infection incidence in all seasons (P = 0.08 for both summer and autumn and P = 0.07 for winter; Table 4). There was a significant effect of tissue type on pathogen movement in summer (P < 0.011) and winter (P = 0.003) but not in autumn (P = 0.195), with consistently greater pathogen progression in bark than wood (Table 4). In trunks there was no significant effect of tissue type on infection incidence (P = 0.33 for both summer and autumn and P = 1.00 for winter) or on pathogen movement (P = 1.00, P = 0.823 and P = 0.467 for summer, autumn and winter, respectively). No Botryosphaeriaceae like isolates were recovered from the non-inoculated controls.
Discussion
The current study is the first in blueberries to show the effect of humidity and temperature on the release of Neofusicoccum spp. conidia from pycnidia under laboratory conditions, and so the influence of environmental factors on abundance of conidia in the environment. The results showed that all the species were capable of oozing conidia but that there were some differences between species. Overall, N. parvum produced the greatest numbers of oozing pycnidia and conidia under the relative humidities and temperatures used, followed by N. australe and N. ribis. This does not seem to be associated with the relative virulence of the species with respect to lesion production on the initial stem tissue. For the more virulent species the central 15 mm of the lesion used as the experimental tissue to assess sporulation would potentially represent older more mature lesioned tissue that is more ready to sporulate compared to lesions caused by less virulent species. However, this was not the case since N. ribis was shown in the current study to produce longer lesions than N. parvum, with the study of Tennakoon et al. (2017b) reporting N. ribis to be more virulent than N. australe. In contrast, pycnidial production in Zymoseptoria tritici (as Septoria tritici) was shown to correlate with leaf necrotic area (Eyal 1971). Overall, the greatest numbers of oozing pycnidia and conidia were observed at higher temperatures (25–30 °C) and RHs (92–100% RH), although all species sporulated to some extent across all temperature (15–30 °C) and RH (80–100%) tested. Whether pycnidia ooze conidia at temperatures below 15 °C was not tested in the current study, however, recent work in New Zealand vineyards naturally infected with Botryosphaeriaceae reported that cirrhi of conidia were observed on infected grapevine stem tissue when the temperature was as low as 8.4 °C (Shafi et al. 2017). Spore production under controlled conditions may, however, differ from that under field conditions. For example Gough (1978) reported that for Zymoseptoria tritici (as Septoria tritici) twice as many spores were released from pycnidia on wheat in a growth chamber compared with from pycnidia in a greenhouse, with this difference being suggested to be due to one or more environmental factors such as light quality, humidity, leaf water potential and temperature. Further work is needed to determine the effect of these factors on sporulation under controlled conditions, and under field conditions where the effect of temperature and relative humidity within blueberry canopies on the production of pycnidia and oozing conidia can be investigated. Although further work is required to validate the results under field conditions, the current study indicate that relative humidity and temperature conditions are unlikely to restrict spore production and indicate conidia will be present to cause infection. However, the factors that are favourable for pycnidial oozing of conidia may not be the same as for the infection processes of the pathogen.
Overall, there was a correlation between the number of oozing pycnidia and the number of conidia indicating that, irrespective of the environmental conditions, each pycnidium oozed a similar number of conidia, although the number of pycnidia triggered to ooze was affected by the environmental conditions. However, for N. parvum isolates although there was an increasing number of oozing pycnidia at 100% RH at 30 °C, this did not result in an increased total number of conidia. The reason for this is unclear, but indicates that for N. parvum incubation at this temperature and RH combination results in each cirrhus containing on average fewer conidia. Similar results were obtained for Guignardia bidwelli (Onesti et al. 2017) with the number of conidia per cirrhus from pycnidia on leaf lesions incubated under 100% RH at 30 °C being lower compared with incubation at 15–20 °C. However, in contrast to the current study where the effect of temperature and RH on production of cirrhi from pycnidia only was assessed, in the study of Onesti et al. (2017) the leaf lesions were incubated at these temperatures to also induce pycnidial production and this may have also influenced the development of conidia within the pycnidia.
In contrast to the majority of published studies on Botryosphaeriaceae disease in blueberry where artificial inoculation using mycelial plugs were used to evaluate pathogenicity, cultivar susceptibility and control methods (Milholland 1972b; Creswell and Milholland 1987; Espinoza et al. 2009; Smith 2009 and Latorre et al. 2013) the current studies used conidia to simulate natural infection conditions. All conidial concentrations used caused 100% incidence but lesion lengths increased with increasing concentrations. Also lesion lengths were longer in soft green shoots than in the hard green shoots, and there was a trend for longer lesions in the wood than in the bark of the hard green shoots. Similar effects were observed by Creswell and Milholland (1987) where inoculation of 2-year-old blueberry plants of three cultivars (‘Bluechip’, ‘Powderblue’ and ‘Murphy’) with increasing concentrations of B. dothidea conidia (1 × 103 to 5 × 104 conidia per inoculation site) resulted in lesions of increasing length. Inoculation of detached 2 year old blueberry stems with N. parvum at a similar inoculation concentrations (1.5 × 104 conidia per wound site) to the current study (2.5 × 104 conidia per wound site) was reported by Espinoza et al. (2009) to result in lesions of 44–96 mm after 25 days which was approximately 1.3 to 3.0 times the lesion lengths found in the current study with attached hard green shoots after 30 days. The differences are likely to be due to the lack of defence response in detached shoots compared to those in attached shoots. Further, incubation of stems in a humid chamber at 20 °C may have increased pathogen development within the tissue in the study of Espinoza et al. (2009), compared with the outdoor environment used in the current study.
External lesions developed in soft green shoots only when wounds were inoculated at up to 7 days old and in hard green shoots and trunks only in wounds up to 4 days old. A decrease in the colonisation length was also observed with increasing wound age. A similar effect of decreasing infection of shoots by B. dothidea with increasing wound age from 0, 1, 7, 14 and 28 day old, was also reported by Creswell and Milholland (1987). However, in contrast to the current study where inoculation of older wounds (>4 days) in soft green stems caused lesser colonisation length than in similar aged wounds of hard green shoots and trunks, Creswell and Milholland (1987) reported the infection proportions of older wounds were higher in succulent stems than in the woody stems. This is probably due to differences in the incubation period since soft green shoots were harvested 14 days after inoculation in the current study while hard green shoots and trunks were harvested after 30 days compared with after 4 weeks for both tissue types by Creswell and Milholland (1987). Differences in the cultivar and inoculum type between the current study and the study of Creswell and Milholland (1987) might also have caused this variation. The decrease in infection incidence with increasing age of wounds is probably associated with wound healing processes. Wound healing processes was reported to be associated with the decreased infection of peach bark with wound age by Leucostoma persoonnii (as Cytospora leucostoma), with lignification and formation of lignosuberized tissues decreasing the rate of colonization rather than preventing colonization (Biggs 1986). In the current study, an improved understanding of the susceptibility period of blueberry wounds will help to identify the most effective application timing of fungicides. Based on the results of the current study regarding the length of time wounds on blueberry stems and trunks remain susceptible to infection, it seems that fungicides should be applied soon after the pruning and trimming of blueberry shoots and would need to be reapplied at least once to protect wounds from Botryosphaeriaceae infection.
Lesions developed on N. ribis inoculated wounded shoots irrespective of whether the shoots were incubated at 20 or 25 °C and 90 or 100% RH during the early infection processes. However, in non-wounded shoots only spot-like lesions were observed, also irrespective of environmental conditions. Similar results were reported by Milholland (1972b) where inoculation of wounded stems with B. dothidea resulted in development of stem blight, but only small slightly raised lesions developed on non-wounded stems, similar to those in the current study. Although no external lesions developed on the inoculated non-wounded stems, N. ribis was recovered from the stem tissue beyond the inoculation point. Since the surface sterilisation procedure is likely to kill any superficial external infection, this indicates the ability of N. ribis to infect and colonise the stem tissue in the absence of wounding, at least for green shoots. Further, for inoculation of wounded hard blueberry shoots in different seasons disease progression was higher in the bark compared to the wood. This indicates the potential for conidial infection or saprophytic colonisation in the plant bark at any time of the year, with penetration of the wood later when conditions were favourable. This is supported by the studies of Billones-Baaijens et al. (2015) who found fewer Botryosphaeriaceae spp. isolates were sited within the wood and most in the bark of surface sterilised grapevine canes, which suggested that they were latent in surface tissues. Conidia of B. dothidea have been reported to germinate and penetrate blueberry stems through stomata (Milholland 1972b). Similarly, in other woody hosts such as apple, peach and pistachio Botryosphaeriaceae pathogens have also been shown to infect through natural openings such as stomata and lenticels, or penetrate the host tissue directly (Pusey 1993; Michailides 1991; Kim et al. 2001). These findings indicate N. ribis is able to infect non-wounded green shoots and saprophytically colonise the bark of woody blueberry shoots and trunks subsequently infecting the underlying wood when wounds are produced, and have major implications for disease control strategies for these pathogens.
The results of the current study indicated that plants could be infected by N. ribis in all the seasons tested, with an overall mean incidence of 77.8%. However, external lesions were observed only in summer and no lesions were observed in the shoots that were wounded and inoculated in the autumn and winter even though N. ribis isolates were recovered. Overall colonisation length was also lower in winter-inoculated plants compared to other seasons. Similar observations were reported by Creswell and Milholland (1988) who showed that the majority of blueberry plants inoculated with B. dothidea became symptomatic when inoculated during March to April (spring) when conditions were warm. They also stated that some naturally infected plants were symptomless, possibly due to the infections having occurred earlier in the year or at the time of pruning in late fall or winter. Van Niekerk et al. (2011) reported that for grapevines, pruning wounds created in late winter were more susceptible to trunk pathogens (Eutypa lata, N. australe, Phaeomoniella chlamydospora and Diporthe neoviticola (as Phomopsis viticola)) than early winter wounds. Ferreira (1999) reported that carbohydrate and nitrogen concentrations were greater in South African grapevines in the winter period of June to August, and higher growth was shown by E. lata in the extract obtained from the shoots in August that in June. Although such experiments have not been conducted in blueberry to evaluate factors such as a nutrient availability which may have an effect on pathogen penetration in the plant, similar effects may have occurred in the current study since N. ribis progression was higher in spring and autumn pruned shoots than in winter pruned shoots. In the current study, infection incidence and disease progression were also higher in the bark compared to wood of the hard shoots throughout the seasons investigated. This indicated the potential for conidial infection or saprophytic survival in the plant bark at any time of the year, with penetration of the wood later when conditions were favorable. This hypothesis was supported by the studies of Billones-Baaijens et al. (2015), who isolated from bark and wood separately when they conducted sequential isolations along an entire grapevine cane of several meters. Further, their genotyping studies showed that multiple Botryosphaeriaceae species and genotypes were distributed along the cane bark, with a few adjacent wood and bark infections being caused by the same genotypes, indicating that wood infection may have originated from the bark.
This study has provided valuable information on host and environmental factors that affect inoculum production and disease development that is essential for the development of effective management of Botryosphaeria stem blight of blueberry. Knowledge regarding the risk period for wound infection will enable the appropriate timing for application of control products such as fungicide and biocontrol products. Removal of infected tissue with pycnidia should also be conducted to reduce inoculum sources within the blueberry fields. However, as has been reported for grapevines (Billones-Baaijens et al. 2013) N. ribis was recovered from the blueberry stem tissue beyond the visible lesions. For woody trunks, the pathogen was recovered at a distance five times the length of the visible lesion indicating that for effective elimination of infected tissue pruning need to remove infected tissue beyond the visible lesion.
References
Amponsah, N. T., Jones, E. E., Ridgway, H. J., & Jaspers, M. V. (2014). Factors affecting Neofusicoccum luteum infection and disease progression in grapevines. Australasian Plant Pathology, 43(5), 547–556.
Biggs, A. B. (1986). Wound age and infection of peach bark by Cytospora leucostoma. Canadian Journal of Botany, 64, 2319–2321.
Billones-Baaijens, R., Jones, E. E., Ridgway, H. J., & Jaspers, M. V. (2013). Virulence affected by assay parameters grapevine pathogenicity studies with Botryosphaeriaceae nursery isolates. Plant Pathology, 62, 1214–1225.
Billones-Baaijens, R., Jones, E. E., Ridgway, H. J., & Jaspers, M. V. (2015). Spatial distribution of Neofusicoccum species within a rootstock mother vine indicates potential infection pathways. European Journal of Plant Pathology, 141, 267–269.
Cline, O. (2013). Stem Blight of Blueberry. http://www.ces.ncsu.edu/depts/pp/notes/Fruit/fdin009/fdin009.htm. Accessed 20 Nov 2013.
Creswell, T. C., & Milholland, R. D. (1987). Responses of blueberry genotypes to infection by Botryosphaeria dothidea. Plant Disease, 71, 710–713.
Creswell, T. C., & Milholland, R. D. (1988). Spore release and infection periods of Botryosphaeria dothidea on blueberry in North Carolina. Plant Disease, 72, 342–346.
Espinoza, J. G., Braceno, E. X., Chavez, E. R., Urbez-Torres, J. R., & Latorre, B. A. (2009). Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Disease, 93(11), 1187–1194.
Eyal, Z. (1971). The kinetics of pycnidiospore liberation in Septoria tritici. Canadian Journal of Botany, 49, 1095–1099.
Ferreira, J. H. S. (1999). Growth of Eutypa lata in grapevine wood extracts. South African Journal of Ecology and Viticulture, 20, 54–56.
Gough, F. J. (1978). Effect of wheat host cultivars on pycnidiospore production by Septoria tritici. Phytopathology, 68, 1343–1345.
Greenspan, L. (1977). Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards A. Physics and Chemistry, 18 A(1), 89–96.
Kim, K. W., Park, E. W., Kim, Y. H., Ahn, K. K., Kim, P. G., & Kim, K. S. (2001). Latency-and defense-related ultrastructural characteristics of apple fruit tissues infected with Botryosphaeria dothidea. Phytopathology, 91, 165–172.
Latorre, B. A., Torres, R., Silva, T., & Elfar, K. (2013). Evaluation of the use of wound-protectant fungicides and biological control agents against stem canker (Neofusicoccum parvum) of blueberry. Ciencia e Investigación Agraria, 40(3), 537–545.
Michailides, T. J. (1991). Pathogenicity, distribution, sources of inoculum and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology, 81, 566–573.
Michailides, T. J., & Morgan, D. P. (2004). Panicle and shoot blight of pistachio: A major threat to the California pistachio industry. http://www.apsnet.org/publications/apsnetfeatures/Pages/Pistachio.aspx. Accessed 9 Mar 2013.
Milholland, R. D. (1972a). Factors affecting sporulation and infection by the blueberry stem canker fungus, Botryosphaeria cortices. Phytopathology, 62, 137–139.
Milholland, R. D. (1972b). Histopathology and pathogenicity of Botryosphaeria dothidea on blueberry stems. Phytopathology, 62, 654–660.
van Niekerk, J. M., Fourie, P. H., Haleen, F., & Pedro, W. C. (2006). Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathologia Mediterranea, 45(Supplement), S43–S54.
van Niekerk, J. M., Haleen, F., & Fourie, P. H. (2011). Temporal susceptibility of grapevine pruning wounds to trunk pathogen infection in South African grapevines. Phytopathologia Mediterranea, 50(Supplement), S139–S150.
Onesti, G., Gonzalez-Dominguez, E., & Rossi, V. (2017). Production of pycnidia and conidia by Guignardia bidwellii, the causal agent of grape black rot, as affected by temperature and humidity. Phytopathology, 107, 173–183.
Phillips, A. J. L. (2002). Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathologia Mediterranea, 41, 3–18.
Poll, J. T. K., & Wood, F. H. (1985). Blueberries in New Zealand. Acta Horticulturae (ISHS), 165, 35–46.
Pusey, P. L. (1993). The role of Botryosphaeria species in peach-tree gummosis on the basis of differential isolation from outer and inner bark. Plant Disease, 77, 170–174.
Sammonds, J., Billones, R., Rocchetti, M., Ridgway, H. J., Walter, M., & Jaspers, M. V. (2009). Survey of blueberry farms for Botryosphaeria dieback and crown rot pathogens. New Zealand Plant Protection, 62, 238–242.
Shafi, A., Ridgway, H. J., Jaspers, M. V., & Jones, E. E. (2017). Conidial production by Botryosphaeriaceae species from grapevine shoot lesions in Marlborough vineyards. New Zealand Plant Protection, 70, 295–300.
Smith, B. J. (2009). Botryosphaeria stem blight of southern blueberries: cultivar susceptibility and effect of chemical treatments. Acta Horticulturae, 810, 385–394.
Smith, H., Wingfield, M. J Crous, P. W., & Coutinoho, T. A. (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. in South Africa. African Journal of Botany, S62 (2), 86-88.
Tennakoon, K. M. S., Jaspers, M. V., Ridgway, H. J., & Jones, E. E. (2015). Herbicide injuries on blueberry provide suitable infection sites for Neofusicoccum ribis. New Zealand Plant Protection, 68, 411–414.
Tennakoon, K. M. S., Jaspers, M. V., Ridgway, H. J., & Jones, E. E. (2017a). Botryosphaeriaceae species associated with blueberry dieback and sources of primary inoculum in propagation nurseries in New Zealand. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-017-1283-9.
Tennakoon, K. M. S., Ridgway, H. J., Jaspers, M. V., & Jones, E. E. (2017b). Production of Neofusicoccum species conidia and their pathogenicity on wounded and non-wounded blueberry shoots. New Zealand Plant Protection, 70, 209–214.
Wright, A. F., & Harmon, P. F. (2010). Identification of species in the Botryosphaeriaceae family causing stem blight on southern highbush blueberry in Florida. Plant Disease, 94, 966–971.
Xu, C., Zhang, H., Zhou, Z., Hu, T., Wang, S., Wang, Y., & Cao, K. (2015). Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. European Journal of Plant Pathology, 143(4), 737–752.
Acknowledgments
We thank Blueberries New Zealand and Lincoln University for funding this research, and Brent Richards and Leona Meachen for maintaining the plants in the nursery at Lincoln University. Statistical advice was provided by Dr. Dean O’Connell.
Funding
Funding was provided by Lincoln University (Postgraduate research scholarship awarded to the first author) and Blueberries New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declare a conflict of interest, with all authors consenting to publication.
Rights and permissions
About this article
Cite this article
Tennakoon, K.M.S., Ridgway, H.J., Jaspers, M.V. et al. Factors affecting Neofuscicoccum ribis infection and disease progression in blueberry. Eur J Plant Pathol 151, 87–99 (2018). https://doi.org/10.1007/s10658-017-1355-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1355-x