Abstract
The colonization of rhizosphere by microorganisms is directly associated with bacterial growth, chemotaxis, biofilm formation, and the interaction with host plant root exudates. In this study, the responses of Ralstonia solanacearum to the root exudates from two tobacco cultivars (Hongda, susceptible; K326, resistant) were determined. The results showed that the population of R. solanacearum was much higher in the rhizosphere soil of Hongda than in the rhizosphere soil of K326, resulting in a higher disease index for the Hongda treatments (92.73 %). The attraction of R. solanacearum to Hongda root exudates (HRE) was stronger than the response to K326 root exudates (KRE). Four organic acids, oxalic acid, malic acid, citric acid, and succinic acid, from the root exudates were identified and subsequently evaluated. The amount of oxalic acid from HRE was significantly higher than that from KRE. The results also showed that oxalic acid could both significantly induce the chemotactic response and increase the biofilm biomass of R. solanacearum. Both malic acid and citric acid could significantly increase the chemotaxis ability in vitro and the recruitment of R. solanacearum to tobacco root under gnotobiotic conditions. Overall, these data suggested that the colonization of tobacco rhizosphere by pathogenic bacterial strains was influenced by the organic acids secreted from the roots. The results expand our understanding of the roles of root exudates in plant-microbe interactions and will be useful for screening and applying beneficial bacteria for better control of plant wilt diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root exudates can not only serve as a nutrient source but also serve as signals for rhizosphere microbes to influence their behaviours and gene expression (Mark et al. 2005). It is well known that plant species can influence the structure and function of a soil microbial community (Grayston et al. 1998; Kourtev et al. 2002). Smalla et al. (2001) suggested that different plant species selected different bacterial communities with their roots and that these plant-specific enrichments could be increased by repeated cultivation of the plant species in the same field. Broeckling et al. (2008) also showed that the root exudates from Arabidopsis thaliana and Medicago truncatula could cultivate their resident soil fungal communities but were unable to maintain nonresident soil fungal populations. Rhizosphere bacteria are affected not only by the plant species but also by the cultivar (Germida and Siciliano 2001). Yao and Wu (2010) suggested that in cucumber cultivars, the level of resistance to Fusarium wilt had a significant effect on the soil microbial community and activity. These findings indicate that plants dramatically select their microflora (Hartmann et al. 2009).
Successful root colonization in the rhizosphere is a prerequisite for the infection of plants by pathogens (Walker et al. 2004). A well-colonized microorganism in the rhizosphere could show good adaptation to the selection exerted by root exudates. Chemotaxis and biofilm formation are regarded as two primary parts of colonization (Bais 2004; Yao and Allen 2006). Chemotaxis is the process by which bacteria respond to particular chemicals from root exudates and is suggested to be the first step in colonization (Gupta Sood 2003; Yao and Allen 2006). Ralstonia solanacearum (R. solanacearum) was found to be specifically attracted to diverse amino acids (glycine), sugars (galactose) and organic acid (citric acid). Meanwhile, R. solanacearum was more strongly attracted to the root exudates from tomato (host) than to those from rice (nonhost) (Yao and Allen 2006).
Organic acids are the main components of root exudates. Recent studies showed the involvement of malic acid in the Bacillus biofilm formation on the root surface of plants (Rudrappa et al. 2008; Tan et al. 2013). Zhang et al. (2014) also found that citric acid could stimulate the biofilm formation and colonization of plant growth promoting bacteria on the root surface of cucumber. Moreover, oxalic acid was found to act as a pathogenicity factor during fungal infection (Schoonbeek et al. 2007). Thus, the influence of organic acids on the biofilm formation and colonization of this pathogen should be determined.
Tobacco bacterial wilt is caused by R. solanacearum which is one of the most important soil-borne pathogens threatening the production of tobacco in the world. Therefore, we hypothesized that the response of R. solanacearum to the root exudates from two cultivars affects its rhizosphere colonization ability, which directly influences the tobacco bacterial wilt disease index of two cultivars. In the present study, the disease indexes of tobacco bacterial wilt were evaluated for two cultivars. Organic acids from the root exudates of two cultivars were identified. The effects of root exudates and organic acids on the chemotactic response and biofilm formation of R. solanacearum were assessed. The ability of organic acids to recruit R. solanacearum was also evaluated under gnotobiotic conditions. This study could be helpful for understanding bacteria-plant interactions for future plant protection.
Materials and methods
Bacterial strains
R. solanacearum was isolated from diseased tobacco plants in Sansui, Guizhou province, China (Wu et al. 2014). The strain was grown in Casamino acid-Peptone-Glucose (CPG) medium (Hendrick and Sequeira 1984). If necessary, antibiotics were used in following concentration: ampicillin, 100 mg/l; kanamycin, 25 mg/l; tetracycline, 15 mg/l; and gentamicin, 12.5 mg/l.
Pot experiment design
To evaluate the disease index of two different tobacco cultivars infected by R. solanacearum, pot experiments were conducted in a greenhouse. Seeds of Hongda (susceptible) and K326 (resistant) supplied by the Institute of Guizhou Tobacco Research Center were surface-sterilized with gentle shaking in 70 % ethanol for 1 min, rinsed with sterile water, immersed in 2 % NaClO for 2 min, and washed three times in sterile water. The seeds were then transferred onto steam-sterilized vermiculite. After 30 days of incubation, the plants were gently transferred to a pot containing 7.5 kg of soil. The diseased soil was collected from a field suffering from severe tobacco bacterial wilt in Sansui, China. The pot treatments were designed as follows: (1) Hongda - the transplanted cultivar was Hongda - and (2) K326 - the transplanted cultivar was K326. Each treatment was performed with 3 replicates. Each replicate contained 10 plants.
The tobacco bacterial wilt disease index (di) was recorded at the harvest time according to a method described elsewhere (Scherf et al. 2010), where: 0 indicated no wilting, 1 indicated that 1 to 25 % of the leaves were wilted, 2 indicated that 26 to 50 % of the leaves were wilted, 3 indicated that 51 to 75 % of the leaves were wilted, and 4 indicated that 76 to 100 % of the leaves were wilted or dead. The DI was calculated as DI = [∑ (number of diseased plants in this index × di) / (total number of plants investigated × highest di)] × 100 %.
The R. solanacearum in the rhizosphere soil at the harvest time in the different pot treatments were counted on semi-selective medium from South Afrcia (SMSA) medium (Elphinstone et al. 1996). SMSA medium (1 l): casamino acid 1 g, peptone 10 g, glycerol 5 ml, agar 20 g, crystal violet 5 mg, polymyxin B sulphate 100 mg, bacitracin 25 mg, chloromycetin 5 mg, penicillin 0.5 mg, and cycloheximide 100 mg.
Collection of root exudates
Seedlings were cultivated as described above. After 30 days of incubation, the plants were gently transferred to a plastic pot (volume of 700 ml) with sterilized vermiculite. Each treatment was performed with three replicates. Each replicate contained four plants.
Plants that were transplanted for 30 days were used to collect the root exudates. The procedure for collecting the tobacco root exudates was performed as described previously with minor modifications (Hao et al. 2010). Briefly, plants were gently washed three times with sterilized, double-distilled water and transplanted into plastic cups containing 300 ml of sterilized, double-distilled water. Each treatment was conducted with four replicates. Each cup contained three tobacco plants. After incubation of the plant growth chamber at 28 °C for 24 h (16 h light/8 h dark), the root exudates were collected, lyophilized and sterilized by passing through a 0.2-μm filter and then stored at −80 °C. To normalize the concentrations of concentrated root exudates, the total protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (ComWin Biotech Co., Ltd., Beijing, China) according to the manufacturer’s protocol.
Identification of the organic acids in the root exudates
Organic acids were identified from the collected root exudates by high performance liquid chromatography (HPLC, Agilent 1200 series, Agilent Technologies, Santa Clara, CA, USA). The procedure for identifying the organic acids in the root exudates was conducted as described previously (Tan et al. 2013). Briefly, an XDB-C18 column (4.6 × 250 mm) was used at 30 °C with a mobile phase consisting of 0.5 % (NH4)2HPO4-H3PO4 buffer (pH 2.5). Each sample (10 μl) was injected and eluted for 10 min at a flow rate of 0.8 ml min−1 and was detected at 214 nm with a UV detector. Five commercial standard compounds, malic acid, citric acid, oxalic acid, succinic acid, and fumaric acid, were purchased from Sigma-Aldrich and chromatographed alone and in a mixture.
Chemotaxis experiments
A capillary assay was performed as previously described with minor modifications (Rudrappa et al. 2008). Cells of the R. solanacearum strain were grown in 3 ml of CPG broth overnight. Then, a 1 ml culture was transferred to 100 ml of fresh CPG broth with shaking at 170 r/min and 30 °C until the OD600 reached a value from 0.3 to 0.7. The cells were then collected by centrifugation, washed twice in sterile phosphate buffer (10 mM potassium phosphate, pH 7.0, 0.1 mM EDTA, and 1 mM MgSO4) (Yao and Allen 2006), and diluted to an OD600 of 0.1 for future testing. A 200-μl pipette tip was used as a chamber for holding 100 μl of the R. solanacearum suspension collected above in phosphate buffer, and a 4-cm, 25-gauge needle (Becton-Dickinson) was used as chemotaxis capillary and attached to a 1-ml tuberculin syringe containing 100 μl of the serial concentrations of the concentrated tobacco exudates or 100 μl of organic acid (100 μg/ml final concentration). The tuberculin syringe containing 100 μl of the chemotaxis buffer was regarded as the control treatment. The tuberculin syringe containing 100 μl of 0.1 % yeast extract was regarded as the positive control treatment. After 2 h of incubation at 30 °C in the incubation chamber, the syringe needle was removed, and the contents were serially diluted in sterile phosphate buffer and plated on CPG medium. The number of bacteria in the capillaries was calculated as the average from the colony forming units (cfu)/ml of triplicate plates. Each treatment was tested with three separate capillary assays.
Biofilm formation assay
The biofilm formation was quantified with a modified version of the polyvinylchloride (PVC) microtitre plate assay, as described previously (Hamon and Lazazzera 2001). Cells of the R. solanacearum strain growing in CPG medium with shaking to mid-exponential growth stage were collected and diluted to an OD600 of 0.01. R. solanacearum suspensions (100 μl) containing the 15 × tobacco exudates or organic acid (100 μg/ml final concentration) or oxalic acid (0, 10, 50, 100, 200, 250, and 500 μg/ml final concentration) were then added to each well of a 96-well PVC microtitre plate, and the plates were incubated under stationary conditions at 30 °C for 48 h. Medium- and non-adherent cells were removed, and the adherent cells were washed twice with the phosphate buffer described above and air-dried. The adherent cells were then stained with 1 % crystal violet (CV) in phosphate buffer at room temperature for 20 min. Excess CV was then removed, and the wells were rinsed with water. The bound CV was solubilized in 200 μl of 80 % ethanol and 20 % acetone. The biofilm formation was measured at A530 for each well. The assays were repeated four times.
Recruitment ability assay
A recruitment ability assay was performed in culture bottles containing 100 ml of sterile solid nutrient medium, as described previously (Tan et al. 2013). The seeds of two tobacco cultivars were surface sterilized and germinated under the conditions described above. Five days later, 10 μl of R. solanacearum suspensions (OD600 = 0.4) was injected into the nutrient medium 2-mm away from the plant roots, and 20 μl of the different organic acid solutions (100 μg/ml) was dropped onto the plant roots, while the control treatment was performed with 20 μl of sterile phosphate buffer. The cell numbers of colonized R. solanacearum were quantified with SMSA medium, as described above, 30 days after inoculation. Each treatment consisted of nine replicates.
Hydroponic experiments
The effects of oxalic acid at different concentrations on the disease index of tobacco bacterial wilt were evaluated in the hydroponic system. Seedlings were cultivated as described above. After 30 days of incubation, the plants were gently transferred to a 50 ml-triangle flask containing 50 ml Hoagland solution. The population of R. solanacearum (107 cfu/ml) was inoculated to the flask. After 24 h, oxalic acid was added into the solution at different final concentrations of 0, 50, 100, and 150 μg/ml. Each treatment was performed with four replicates. Each replicate contained two plants. After the plants transplanted for 14 days, the disease index was recorded according to the method as described above.
Data analysis
The data were analyzed with Microsoft Excel 2007 and SPSS software (ver. 13.0; SPSS, Chicago, IL, USA). The data were subjected to a one-way ANOVA analysis, and the means were subjected to Duncan’s multiple range test at p < 0.05.
Results
Pot traits
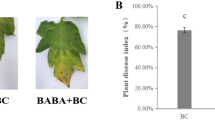
The disease index at harvest time differed for the various cultivars. The index of the Hongda treatments was 92.73 ± 2.26 %, which was significantly higher than that for the K326 treatments (53.33 ± 6.67 %) in the pot experiments (Table S1). The populations of R. solanacearum were measured at harvest time with the selected medium, as described above. The results showed that the population of R. solanacearum in the rhizosphere soil was clearly related to the tobacco cultivar. The population of R. solanacearum in the Hongda rhizosphere was 6.99 ± 0.10 cfu/g dry weight soil (cfu/g dw), whereas that in the K326 rhizosphere was 6.64 ± 0.06 log cfu/g dw. A simple correlation analysis also showed a positive correlation (r = 0.952, p < 0.05) between the population of R. solanacearum and the disease index of the two cultivars.
Identification of the organic acids in the root exudates
HPLC analysis and comparison to the retention times of standard samples identified the organic acids from the two different tobacco cultivars to be oxalic acid, malic acid, citric acid, and succinic acid (Fig. 1). The concentration of oxalic acid from Hongda was 140.34 ± 34.22 μg/g dry weight root (μg/g dw root), which was significantly higher than that from K326 (27.23 ± 5.25 μg/g dw root). However, there were no significant differences between the amount of malic acid from Hongda and that from K326.
Evaluation of the chemotaxis of R. solanacearum in response to the root exudates and different organic acids
Root exudates are a significant source of substrate for rhizosphere microbes and affect their functions. The chemotaxis assay showed that R. solanacearum could be attracted by root exudates and a variety of organic acids. As shown in Fig 2, R. solanacearum cells demonstrated greater attraction to the root exudates from Hongda than to the root exudates from K326. The highest populations of cells were found in both root exudates at exudate protein concentrations of 1000 μg/ml, and these populations were as high as 6.37 log cfu/ml in the Hongda root exudates (HRE) and 6.22 log cfu/ml in the K326 root exudates (KRE), respectively. The attraction to these two root exudates at exudates protein concentrations of 1000 μg/ml was even stronger than the attraction to 0.1 % yeast extract (positive control). The differences disappeared at exudates protein concentrations of 1 μg/ml.
Chemotactic responses of R. solanacearum towards the root exudates from Hongda and K326. Data are shown as the means and standard errors among the samples. The different letters above each bar refer to the Duncan test, p < 0.05. Control, chemotaxis buffer; PC, positive control (0.1 % yeast extract); H, root exudates from the Hongda cultivar; and K, root exudates from the K326 cultivar
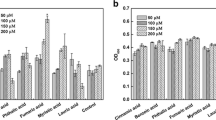
All the organic acids tested were found to be capable of attracting R. solanacearum. The population of R. solanacearum attracted by oxalic acid was significantly higher than that in the control. The most attracting organic acid was citric acid, with a concentration of 5.89 log cfu/ml (Fig. 3).
Chemotactic responses of R. solanacearum towards various organic acids. Data are shown as the means and standard errors among the samples. The different letters above each bar refer to the Duncan test, p < 0.05. Control: chemotaxis buffer; Ox, oxalic acid; Ma, malic acid; Ci, citric acid; and Su, succinic acid
Assessment of the biofilm formation of R. solanacearum in response to the root exudates and different organic acids
A biofilm formation assay was performed to investigate the response to root exudates from two different tobacco cultivars and to organic acids. The results showed that the root exudates from two different tobacco cultivars could stimulate the biofilm formation of R. solanacearum (Fig. 4). The biofilm biomass of R. solanacearum in the presence of root exudates from Hongda was nearly 1.14-fold higher than that in the root exudates from K326.
Effects of the root exudates from Hongda and K326 and the different organic acids on the biofilm formation of R. solanacearum. The assays were repeated four times and quantified by measuring the A530 of crystal violet-stained wells that were rinsed with 80 % ethanol and 20 % acetone. Data are shown as the means and standard errors among the samples. The different letters above each bar refer to the Duncan test, p < 0.05. Control: CPG medium; H, CPG medium inoculated with 15 × concentrated Hongda root exudates; K, CPG medium inoculated with 15 × concentrated K326 root exudates; Ox, CPG medium inoculated with oxalic acid; Ma, CPG medium inoculated with malic acid; Ci, CPG medium inoculated with citric acid; and Su, CPG medium inoculated with succinic acid
Forty-eight hours after the inoculation of R. solanacearum (Fig. 4), the oxalic acid concentration was found to significantly enhance the biofilm formation, with a biomass increase of 74.60 % compared with the control. The other four acids only had a slight effect on the biofilm formation of R. solanacearum.
The result above clearly revealed the composition of root exudates from two cultivars and the role of oxalic acid on the biofilm formation of R. solanacearum. Therefore, the effect of the concentrations of oxalic acids on the biofilm formation of R. solanacearum was evaluated. The stimulation of biofilm formation of R. solanacearum was observed with oxalic acid concentrations ranging from 50 to 250 μg/ml (Fig. 5). In contrast, the biofilm formation of R. solanacearum was inhibited when oxalic acid concentration over 250 μg/ml.
Effects of different concentrations of oxalic acids on the biofilm formation of R. solanacearum. The assays were repeated four times and quantified by measuring the A530 of crystal violet-stained wells that were rinsed with 80 % ethanol and 20 % acetone. Data are shown as the means and standard errors among the samples
Recruitment ability assay
To confirm the effects of organic acids on R. solanacearum (chemotaxis and biofilm formation, as described above), a recruitment assay was also conducted in the rhizoplane of two tobacco cultivars. The population of R. solanacearum was much higher on the Hongda root surface (5.82 log cfu/g root) (Fig. 6) than on K326 (5.26 log cfu/g root) (Fig. 7). All the organic acids also increased the number of R. solanacearum on the root surface of both tobacco cultivars. The populations of R. solanacearum were significantly higher in malic acid and citric acid than in the other organic acids for both tobacco cultivars.
Effects of different organic acids on the colonization of Hongda cultivars by R. solanacearum. Data are shown as the means and standard errors among the samples. The different letters above each bar refer to the Duncan test, p < 0.05. Control: chemotaxis buffer; Ox, oxalic acid; Ma, malic acid; Ci, citric acid; and Su, succinic acid
Effects of different organic acids on the colonization of K326 cultivars by R. solanacearum. Data are shown as the means and standard errors among the samples. The different letters above each bar refer to the Duncan test, p < 0.05. Control: chemotaxis buffer; Ox, oxalic acid; Ma, malic acid; Ci, citric acid; and Su, succinic acid
Effects of oxalic acid at different concentration on the disease index
The influence of oxalic acid at different concentration on the disease index of tobacco bacterial wilt was evaluated in the hydroponic system. The stimulation of the disease index was observed from oxalic acid concentrations from 50 to 150 μg/ml (Fig. 8). The results showed that the stimulation rate of the disease index with the inoculation of oxalic acid in K326 was higher than in Hongda. The highest disease indices of Hongda and K326 cultivars were both found in the treatment inoculated with oxalic acid at the final concentrations of 150 μg/ml. However, there was no significant difference between the treatment amended with 100 μg/ml oxalic acid and the treatment inoculated with 150 μg/ml oxalic acid.
Discussion
Plants root exudates can provide carbon compounds to activate specific microbial populations in the rhizosphere and enhance bio-control efficiency (Morgan et al. 2005). However, plants root exudates may also cause the development of plant pathogens, resulting in disease (Whipps 2001). The Hongda rhizosphere harboured a much higher number of R. solanacearum than did the K326 rhizosphere, suggesting that R. solanacearum could be more strongly attracted by the Hongda root exudates than by the K326 root exudates. The colonization ability of R. solanacearum on the root affected its ability to infect the host (Yao and Allen 2006). Herein, the higher number of R. solanacearum that colonized the rhizosphere soil of Hongda resulted in the higher disease index.
Chemotaxis plays an important role in efficient bacterial colonization in rhizospheres (Van de Broek et al. 1998). Plants can secrete a variety of sugars, organic acids, and secondary metabolites, all of which could act as chemotactic compounds for plant-associated bacteria (Brencic and Winans 2005). Bacilio-Jiménez et al. (2003) found that the root exudates from rice could induce a higher chemotactic response from endophytic bacteria than from the other bacteria present in the rice rhizosphere. Zhang et al. (2014) also found that the root exudates from banana were better able to attract B. subtilis N11 than B. amyloliquefaciens SQR 9. In the present research, R. solanacearum was more attracted by the root exudates from Hongda than those from K326 in vitro, resulting in higher populations of colonized R. solanacearum at the Hongda rhizosphere, as described above. Actually, the root exudates from K326 seem to repel R. solanacearum (Fig. 2). All the organic acids tested were found to attract R. solanacearum. The chemotactic response could be attributed to the nutritional and signaling characteristics of the organic acids, which are involved in the tricarboxylic acid cycle (Jones 1998).
Rudrappa demonstrated that 5 μM L-malic acid could increase the expression of the yqxM operon of Bacillus, which directly affected the matrix production for biofilm formation (Rudrappa et al. 2008). Malic acid and citric acid both increased the R. solanacearum biofilm formation. Moreover, the biofilm biomass of R. solanacearum was greater in the presence of oxalic acid than in the presence of the other tested organic acids. Citric acid was also found to stimulate the biofilm formation of Bacillus amyloliquefaciens SQR9 (Zhang et al. 2014). Unfortunately, few studies have illustrated the effect of oxalic acid on bacterial biofilm formation. Due to the high amounts of oxalic acid in the Hongda root exudates, the R. solanacearum biofilm biomass was higher in the root exudates from Hongda than in those from K326. On the other hand, oxalic acid is thought to be a key factor in the early pathogenic stage of a wide range of necrotrophic fungi (Cessna et al. 2000). It has been suggested to suppress the oxidative burst (Cessna et al. 2000) and is involved in triggering the plant pathways responsible for programmed cell death (Kim et al. 2008). Thus, whether R. solanacearum could secrete oxalic acid or induce oxalic acid secretion from the plant host when colonized at the root surface should be studied in the future.
The chemotaxis response and biofilm formation of R. solanacearum in the rhizosphere of two cultivars were further studied. The strain R. solanacearum could colonize well on the root of two cultivars but exhibited different colonization patterns with the application of different organic acids. All the organic acids that acted as chemo-attractants as described above were found to enhance the colonization of R. solanacearum on the two cultivars.
As described above, the rhizosphere is so complex that the colonization could be affected by many factors, such as antimicrobials within the exudates (Walker et al. 2004), oxygen concentration (Yao and Allen 2007), plant structure (Beauregard et al. 2013), and other variables. Indeed, the microbe could also provide feedback on the effects of stimulatory or inhibitory compounds from the root to change the rhizosphere niche (Hartmann et al. 2009).
In general, the data presented in this research highlight the organic acids from two different tobacco resistant cultivars that result in different behaviors by the pathogen agent. These findings increased our understanding of the response of the pathogen R. solanacearum to the root exudates, particularly the organic acids, from two cultivars and provide new traits to study the mechanisms of bio-control in the rhizosphere niche.
References
Bacilio-Jiménez, M., Aguilar-Flores, S., Ventura-Zapata, E., Pérez-Campos, E., Bouquelet, S., & Zenteno, E. (2003). Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil, 249, 271–277.
Bais, H. P. (2004). Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol, 134, 307–319.
Beauregard, P. B., Chai, Y., Vlamakis, H., Losick, R., & Kolter, R. (2013). Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A, 110, E1621–E1630.
Brencic, A., & Winans, S. C. (2005). Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol Mol Biol Rev, 69, 155–194.
Broeckling, C. D., Broz, A. K., Bergelson, J., Manter, D. K., & Vivanco, J. M. (2008). Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol, 74, 738–744.
Cessna, S. G., Sears, V. E., Dickman, M. B., & Low, P. S. (2000). Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell, 12, 2191–2199.
Elphinstone, J., Hennessy, J., Wilson, J., & Stead, D. (1996). Sensitivity of different methods for the detection of Ralstonia solanacearum in potato tuber extracts. EPPO Bull, 26, 663–678.
Germida, J., & Siciliano, S. (2001). Taxonomic diversity of bacteria associated with the roots of modern, recent and ancient wheat cultivars. Biol Fertil Soils, 33, 410–415.
Grayston, S. J., Wang, S., Campbell, C. D., & Edwards, A. C. (1998). Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem, 30, 369–378.
Gupta Sood, S. (2003). Chemotactic response of plant‐growth‐promoting bacteria towards roots of vesicular‐arbuscular mycorrhizal tomato plants. FEMS Microbiol Ecol, 45, 219–227.
Hamon, M. A., & Lazazzera, B. A. (2001). The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol, 42, 1199–1209.
Hao, W. Y., Ren, L. X., Ran, W., & Shen, Q. R. (2010). Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil, 336, 485–497.
Hartmann, A., Schmid, M., van Tuinen, D., & Berg, G. (2009). Plant-driven selection of microbes. Plant Soil, 321, 235–257.
Hendrick, C. A., & Sequeira, L. (1984). Lipopolysaccharide-defective mutants of the wilt pathogen Pseudomonas solanacearum. Appl Environ Microbiol, 48, 94–101.
Jones, D. L. (1998). Organic acids in the rhizosphere–a critical review. Plant Soil, 205, 25–44.
Kim, K. S., Min, J. Y., & Dickman, M. B. (2008). Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Mol Plant-Microbe Interact, 21, 605–612.
Kourtev, P. S., Ehrenfeld, J. G., & Häggblom, M. (2002). Exotic plant species alter the microbial community structure and function in the soil. Ecology, 83, 3152–3166.
Mark, G. L., Dow, J. M., Kiely, P. D., Higgins, H., Haynes, J., Baysse, C., Abbas, A., Foley, T., Franks, A., & Morrissey, J. (2005). Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc Natl Acad Sci U S A, 102, 17454–17459.
Morgan, J., Bending, G., & White, P. (2005). Biological costs and benefits to plant–microbe interactions in the rhizosphere. J Exp Bot, 56, 1729–1739.
Rudrappa, T., Czymmek, K. J., Paré, P. W., & Bais, H. P. (2008). Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol, 148, 1547–1556.
Scherf, J. M., Milling, A., & Allen, C. (2010). Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum race 3, biovar 2, in infected geranium, tomato, and potato plants. Appl Environ Microbiol, 76, 7061–7067.
Schoonbeek, H. J., Jacquat-Bovet, A. C., Mascher, F., & Métraux, J. P. (2007). Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea. Mol Plant-Microbe Interact, 20, 1535–1544.
Smalla, K., Wieland, G., Buchner, A., Zock, A., Parzy, J., Kaiser, S., Roskot, N., Heuer, H., & Berg, G. (2001). Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol, 67, 4742–4751.
Tan, S., Yang, C., Mei, X., Shen, S., Raza, W., Shen, Q., & Xu, Y. (2013). The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol, 64, 15–22.
Van de Broek, A., Lambrecht, M., & Vanderleyden, J. (1998). Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology, 144, 2599–2606.
Walker, T. S., Bais, H. P., Déziel, E., Schweizer, H. P., Rahme, L. G., Fall, R., & Vivanco, J. M. (2004). Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol, 134, 320–331.
Whipps, J. M. (2001). Microbial interactions and biocontrol in the rhizosphere. Plant Physiol, 52, 487–511.
Wu, K., Yuan, S., Wang, L., Shi, J., Zhao, J., Shen, B., & Shen, Q. (2014). Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol Fertil Soils, 50, 961–971.
Yao, J., & Allen, C. (2006). Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J Bacteriol, 188, 3697–3708.
Yao, J., & Allen, C. (2007). The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J Bacteriol, 189, 6415–6424.
Yao, H., & Wu, F. (2010). Soil microbial community structure in cucumber rhizosphere of different resistance cultivars to fusarium wilt. FEMS Microbiol Ecol, 72, 456–463.
Zhang, N., Wang, D., Liu, Y., Li, S., Shen, Q., & Zhang, R. (2014). Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil, 374, 689–700.
Acknowledgments
This research was financially supported by the projects of the 111 project (B12009), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Chinese Ministry of Agriculture (201103004), the National Natural Science Foundation of China (41361075), and the Applied and Basic Research Foundation of Yunnan province (2013FA015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kai Wu and Saifei Yuan are equal to this work.
Highlights
>Root exudates affect chemotaxis and biofilm formation of Ralstonia. >Four organic acids were identified from both two tobacco cultivars. >Organic acids induced the chemotaxis and biofilm formation of Ralstonia. >Report on the stimulation of biofilm formation of Ralstonia by oxalic acid. >Organic acids affect the Ralstonia colonization at rhizosphere.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Wu, K., Yuan, S., Xun, G. et al. Root exudates from two tobacco cultivars affect colonization of Ralstonia solanacearum and the disease index. Eur J Plant Pathol 141, 667–677 (2015). https://doi.org/10.1007/s10658-014-0569-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0569-4