Abstract
Tobacco bacterial wilt (TBW) is caused by Ralstonia solanacearum (R. solanacearum), a severe pathogenic agent with a wide host range. In this study, lime + ammonium bicarbonate (L + AB), organic fertilizer (OF), bio-organic fertilizer (BOF), and integrated treatment (L + AB + BOF) were assessed for the ability to control TBW and to influence the composition of native soil bacterial communities. The results showed that disease incidence of L + AB + BOF for two growth seasons in pot experiment was the lowest, with only 15.56 and 11.11 % at seasons 1 and 2, respectively. The integrated treatment could also significantly suppress TBW in the field, with a disease incidence of only 14.27 % compared with 35.41, 50.03, and 31.32 % in L + AB, OF, and BOF treatments, respectively. With application of the integrated treatment in pot and field experiments, the abundances of R. solanacearum were both significantly lower than those with other treatments. Denaturing gradient gel electrophoresis (DGGE) patterns showed that application of BOF significantly affected composition of bacterial communities of rhizosphere. The analysis of 454 sequencing data showed that application of integrated treatment recruited more beneficial bacteria than other treatments, such as Bacillus, Paenibacillus, Arthrobacter, and Streptomyces, while the abundance of Ralstonia with the integrated treatment was decreased. Overall, these results suggested that application of integrated agricultural management could effectively suppress bacterial wilt by affecting the composition of bacterial community and reducing the population of R. solanacearum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial wilt is a serious crop disease caused by Ralstonia solanacearum. This vascular pathogen can infect many economically important crops, such as potato, tomato, banana, and pepper plants, and causes large economic losses every year (Hayward 1991). Resistant cultivars and chemical pesticides are usually used to control bacterial wilt. However, most resistant tobacco cultivars also have low product quality. Use of chemical pesticides not only causes bacterial resistance but is also harmful to the environment (Gamliel et al. 2000; Goldman et al. 1994). There is no effective chemical available to control bacterial wilt to date.
Currently, the bio-control of soil-borne disease using plant-growth-promoting rhizobacteria (PGPR) is a promising research area (Kloepper 1993). Both beneficial Bacillus (Guo et al. 2004) and Pseudomonas (Vanitha et al. 2009) have been widely used in the control of bacterial wilt. Guo et al. (2004) found that the bio-control efficacy of bacterial wilt by beneficial agents was inconsistent in different field trials and was influenced by environmental conditions. Schönfeld et al. (2003) found that application of compost could significantly change the composition of rhizosphere microbial communities and reduce the populations of R. solanacearum. Soil pH was considered to be the major factor for controlling soil-borne plant pathogens (Niwa et al. 2007). Application of lime could reduce the severity of Fusarium wilt by elevating the soil pH (Woltz and Engelhard 1973). Jayaraj and Radhakrishnan (2008) also found that the suppression efficacy of tomato damping-off could be enhanced with the application of bio-control agents in solarized soils. Thus, integrated control approaches, including physiochemical suppressor and bio-control agents, should be taken into consideration for controlling soil-borne pathogens more effectively.
Several studies found that changes in the microbial community structure could affect the incidence of soil-borne wilt (Bernard et al. 2012; Lang et al. 2011; Luo et al. 2010). The microbial population and its composition were found to be responsible for specific soil suppression of plant pathogens (Weller et al. 2002). Xue et al. (2012) demonstrated that the composition of microbial communities of rhizosphere soil changed after inoculation of beneficial bacteria into soil, using a denaturing gradient gel electrophoresis (DGGE) analysis. Huang et al. (2012) found that bacillus-inoculated fertilizer could increase the bacterial diversity and suppress Rhizoctonia solani. However, only a few studies have focused on relationships between composition of the microbial community and the suppression of R. solanacearum under the influence of bio-organic fertilizer.
Biolog, phospholipid fatty acid (PLFA) analysis, and PCR-DGGE (Borrero et al. 2006; Insam 1997; Xue et al. 2012; Yao and Wu 2010) are typically used to analyze microbial community structure in soil. However, more detailed taxonomic information should be determined to identify the specific microbial groups that respond to changes in environmental factors. The application of bar-coded pyrosequencing (Hartman et al. 2008; Qiu et al. 2012) allows the determination of the variation of a large number of microbial groups in the bio-control of soil-borne plant diseases.
We hypothesized that application of different treatments could reduce the population of R. solanacearum and change the composition of bacterial communities of the rhizosphere soil with an increase of some beneficial bacteria in rhizosphere, resulting in decrease of disease incidence of tobacco bacterial wilt. Therefore, the objectives of this study were to examine the suppressive efficacy on tobacco bacterial wilt and changing of the composition of bacterial communities by integrated control measures. Bacterial communities were analyzed by DGGE and high-throughput 454 pyrosequencing.
Materials and methods
Bacterial strains
The tobacco bacterial wilt pathogen R. solanacearum (GenBank accession number KC888020) was isolated from diseased tobacco plants in Fuquan, Guizhou province, China. Its strong pathogenicity was confirmed with a pot experiment in our laboratory.
Bacillus amyloliquefaciens SQY 162 (SQY162, CGMCC accession no. 7500, China General Microbiology Culture Collection Center) was isolated from the rhizosphere soil of healthy tobacco surviving in a diseased field in Fuquan, Guizhou province, China, according to its strong antagonistic ability against the tobacco bacterial wilt pathogen R. solanacearum in vitro (Wang et al. 2013). Briefly, the isolates were spotted on nutrient medium with sterile toothpicks. After 24-h incubation at 30 °C, the plates were sprayed with R. solanacearum (107 cfu/ml) and subsequently incubated at 30 °C for an additional 24 h to observe the inhibition zones.
Organic fertilizer and bio-organic fertilizer
The organic fertilizer was composed of cattle manure compost and amino acid fertilizer (1:1 w/w). Cattle manure compost, containing 35 % organic matter, 2.51 % N, 2.4 % P2O5, 1.13 % K2O, and 32.3 % water, was kindly supplied by Jiangyin Lianye Organic Fertilizers Ltd., China. The amino acid fertilizer, containing 44.2 % organic matter, 12.93 % total amino acids, 4.4 % N, 3.5 % P2O5, 0.67 % K2O, and 28.5 % water, was supplied by Jiangsu Xintiandi Amino Acid Fertilizers Ltd., China. The bio-organic fertilizer was produced as follows: nearly 10 % (v/w) SQY 162 culture (107 cfu/ml) was inoculated into organic fertilizer and fermented at 40–45 % moisture for 6 days, and the fertilizer was turned over two times per day. After solid fermentation, the number of antagonistic strain SQY 162 could reach to 2.5 × 107 cfu/g fertilizer. The population of the antagonistic agent was measured with selective media as described below.
Pot design
To evaluate the bio-control efficacy of integrated measures, pot experiments were conducted for two continuous growth seasons in a greenhouse. Soil was used in the second growth season after tobacco harvest in season 1. In each season, three replicates were performed, and each replicate contained ten tobacco plants.
Tobacco K326 seeds were sown in floating polystyrene trays. After growth for 45 days, seedlings were transplanted into the pots (7.5-kg soil). The pot treatments were designed as follows: (1) L + AB, soil pretreated only with 100 g of lime and 50 g of ammonium bicarbonate in each pot (7.5-kg soil); (2) OF, 100 g of organic fertilizer was supplied for each tobacco plant; (3) BOF, 100 g of bio-organic fertilizer was used in each tobacco plant; and (4) L + AB + BOF, soil was pretreated with 100 g of lime and 50 g of ammonium bicarbonate in each pot. Then, 100 g of bio-organic fertilizers was supplied for each tobacco plant. The same nutrient levels in all treatments were adjusted by application of chemical fertilizers.
Tobacco bacterial wilt disease incidence was recorded at the harvest time of season 1 and season 2 by disease index (di) according to the method of Scherf et al. (2010), where 0, no wilting; 1, 1 to 25 % of leaves wilted; 2, 26 to 50 % of leaves wilted; 3, 51 to 75 % of leaves wilted; and 4, 76 to 100 % of leaves wilted or died. The DI was calculated as DI = [∑ (number of diseased plants in this index × di) / (total number of plants investigated × highest di)] × 100 %.
Field design
A field experiment was conducted from May 6 to August 15, 2011, in Sansui, Guizhou province, China. The tobacco K326 seeds were sown in floating polystyrene trays. After growth for nearly 2.5 months, seedlings were transplanted into the field. Each treatment plot was approximately 39 m2 (length × width = 13 × 3 m). Each treatment was replicated three times. Each replicate had 60 tobacco plants that were randomly distributed in the experiment field. Pits were dug in the field, and the fertilizers were applied in each pit and then covered with soil. After that, one tobacco seedling was transplanted into each pit. Lime and ammonium bicarbonate were purchased from a local agriculture materials company in Sansui.
The field treatments were designed as follows: (1) L + AB, soil pretreated only with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate; (2) OF, 50 g of organic fertilizer was used for each tobacco plant; (3) BOF, 50 g of bio-organic fertilizer was used for each tobacco plant; and (4) L + AB + BOF, soil was pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate. Then, 50 g of bio-organic fertilizer was supplied for each tobacco plant. The same nutrient levels in all treatments were adjusted by application of chemical fertilizers.
Disease incidence and agronomic characteristic analysis in the field
The disease incidence of tobacco bacterial wilt was recorded at the end of the experiment and was calculated as described above in each replicate.
The plant height, stem perimeter, and maximal leaf area were measured on July 15 when tobacco was at topping time.
Enumeration of R. solanacearum and antagonistic bacteria
Ten-gram rhizosphere soils from different treatments were serial 10-fold diluted with sterile water. To measure the population of R. solanacearum in the rhizosphere soil, 0.1-ml dilution was spread onto semi-selective medium from South Africa (SMSA) medium. After 2-day incubation at 28 °C, the typical colonies of R. solanacearum were then counted (Elphinstone et al. 1996). The SMSA medium (1 l) is composed of casamino acid 1 g, peptone 10 g, glycerol 5 ml, agar 20 g, crystal violet 5 mg, polymyxin B sulfate 100 mg, bacitracin 25 mg, chloromycetin 5 mg, penicillin 0.5 mg, and cycloheximide 100 mg. The population of SQY 162 in the rhizosphere soil was counted on nutrient medium supplemented with polymyxin B (35 μg/ml), lincomycin (5 μg/ml), and cycloheximide (50 μg/ml).
Sampling soil in the field and soil property analysis
Rhizosphere soils from three tobacco plants were collected individually from each replicate in the field as described by Smalla et al. (2001). Each rhizosphere sample per replicate was collected from roots of three randomly selected plants. The roots were shaken vigorously to separate soil not tightly adhering to the roots. Then, soil still tightly adhering to the roots was harvested as rhizosphere soil. Samples collected were analyzed for their chemical and physical properties as already described (Sun et al. 2011). Briefly, soil pH (soil/water = 1:2.5) was measured with a Sartorius PB-10 basic pH meter. Total C and N were determined with an elemental analyzer according to the manufacturer’s instructions (Elementar vario EL III, Germany). Ammonium-N and NO3 −-N were determined using an AutoAnalyzer 3 (Bran + Luebbe AutoAnalyzer 3, Germany). The available phosphate (P) and potassium (K) were extracted with sodium bicarbonate and ammonium acetate, respectively, and were measured using the molybdenum blue method and flame photometry, respectively.
Soil DNA extraction
Soil total DNA was extracted from 1 g of each sample collected from the pots or field using the PowerSoil DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions; 100 of extract contained 25 ng DNA/μl as determined with a spectrophotometer (NanoDrop, ND2000, Thermo Scientific, Wilmington, DE). The extracted DNA was then visualized on a 1.0 % agarose gel electrophoresis (1× Tris-acetate-EDTA buffer; 120 V; 45 min) stained with ethidium bromide (Ascher et al. 2009) and DNA mass ladder mix (λ-Hind III digest DNA marker, 125 ~ 23,130 bp) for comparison.
DGGE analysis of the soil samples from pot experiments
DGGE profiles were generated using the DCode system (Bio-Rad Laboratories, Hercules, CA, USA). The primers (338 F-GC and 518R) targeted on V3 region of the 16S ribosome RNA (rRNA) gene were used (Lopez et al. 2003). The PCR products were loaded onto polyacrylamide gels containing 8 % (w/v) acrylamide in 1× Tris-acetate-EDTA (TAE) buffer and run on 45 to 65 % denaturant gradient gels at 200 V for 10 min and subsequently at 80 V for 16 h at 60 °C. All gels were silver-stained. After air drying gels for 2 days according to the procedure, gels were digitized for further analysis.
Shannon’s diversity index (H) was calculated using the following equations previously described by Lang et al. (2011): H = −∑P i ln(P i ) = −∑(n i / N)ln(n i / N), where P i expresses the proportional number in a specific group relative to the total number, n i is the intensities of a band, and N is the sum of all band intensities in the DGGE profile.
454 Pyrosequencing of the samples from field experiments
The procedure of PCR amplification before 454 pyrosequencing has been already described (Qiu et al. 2012). Briefly, a pair of primers, 124-f (5′-χχχ CACGGATCCGGACGGGTGAGTAACACG-3′) and 515-r (5′-χχχ ATCGTATTACCGCGGCTGCTGCTGGCA-3′), as described by Lane (1991), was used for the amplification of bacterial 16S rRNA genes. Here, “χχχ” was designed as a bar-coded key for sample identification. Then, PCR mixtures with an approximate size of 400 bp were purified using a PCR purification kit (Axygen Bio, USA), and purified PCR products were sequenced by Majorbio Biotech Co., Ltd. (Shanghai, China). Twelve thousand sequences were read for each sample. The sequence data generated by 454 pyrosequencing were deposited in the NCBI Sequence Read Archive (SRA) database with accession number SRA072879. Sequence analysis was performed using Mothur (ver. 1.25.1) as described by Schloss et al. (2009). The unique sequences were aligned with the Silva 106 database. The operational taxonomic units (OTUs) analyzed were all classified at a dissimilarity of 0.03. Sequences that could not be assigned to any division were labeled as unclassified. Richness (Ace and Chao 1) and Shannon diversity indices were generated. Finally, the heat map format was digitized with Bray-Curtis indices in “R” (ver. 2.15.0; http://www.r-project.org/) with the Vegan package (http://vegan.r-forge.r-project.org) to depict the similarity among the five treatments.
Data analysis
The data were analyzed with Microsoft Excel 2007 and SPSS software (ver. 13.0; SPSS, Chicago, IL, USA). Duncan’s multiple tests were used with analysis of variance (ANOVA). Principal component analysis was performed using SPSS.
Results
Pot experiment
As is shown in Fig. 1a, the isolate showed strong inhibitory effects against the pathogen. The bio-control efficacy of TBW in pot experiments varied with treatment (Fig. 1b). The disease incidence of season 2 was significantly lower than that of season 1 in each treatment. Application of BOF could significantly reduce the disease incidence by 45.79 and 53.73 % in seasons 1 and 2, respectively, compared to OF treatment. Disease incidences were both suppressed with application of lime and ammonium bicarbonate by 32.62 and 36.59 % in seasons 1 and 2, respectively, compared to OF treatment. The results also showed that the disease incidence of L + AB + BOF for two growth seasons was the lowest, with only 15.56 and 11.11 % at seasons 1 and 2, respectively.
Inhibitory effects of antagonistic strain SQY 162 on the pathogen in vitro and the suppression of tobacco bacterial wilt in pot experiments for two growth seasons. Inhibitory effect of SQY 162 on the pathogen was determined in vitro (a), disease incidence (b), and abundance of SQY 162 (c) and R. solanacearum (d) at the harvest time of seasons 1 and 2. The means and standard errors are also shown. Different letters above each bar refer to the Duncan’s test, p < 0.05. L + AB (soil pretreated with 100 g of lime and 50 g of ammonium bicarbonate in each pot), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 100 g of lime and 50 g of ammonium bicarbonate in each pot and then amended with bio-organic fertilizers)
The number of the antagonistic strain SQY 162 in the rhizosphere soil at seasons 1 and 2 in L + AB + BOF treatment increased by 66.06 and 142 % compared with BOF treatment, indicating that soil pretreatment with lime and ammonium bicarbonate increased SQY 162 colonization of the rhizosphere soil (Fig. 1c).
The population of R. solanacearum was calculated at the harvest time of seasons 1 and 2 with selection medium as described above. The population of R. solanacearum in each treatment at season 2 significantly decreased compared to that at season 1 (Fig. 1d). The populations of R. solanacearum in the OF treatment for two growth seasons were the highest, with 7.15 and 6.78 log cfu/g dry weight (log cfu/g dw) soils at seasons 1 and 2, respectively. There were no significant differences between the numbers of R. solanacearum in BOF and L + AB treatments for the two tobacco growth seasons. The numbers of R. solanacearum in the L + AB + BOF treatment for the two growth seasons were the lowest, with only 5.92 and 5.38 log cfu/g dw soil at seasons 1 and 2, respectively.
Bacterial diversity of soil samples from each treatment at the harvest time of season 2 was investigated by DGGE (Fig. S1a), whose bands were statistically analyzed by Shannon’s diversity index (H). The bacterial diversity (H) of the BOF treatment was 3.269 (Fig. S1c), which was the highest value compared to that of the other three treatment values. The unweighted pair group method with arithmetic mean (UPGMA) dendrogram (Fig. S1b) also showed that samples from BOF were clearly distinguishable than those of the other three treatments.
Field experiment
The disease incidence was recorded when the experiment ended. The incidence of bacterial wilt was 35.41, 50.03, and 31.32 % in the L + AB, OF, and BOF treatments, respectively, but it was only 14.27 % in L + AB + BOF treatment (Fig. 2a). Therefore, the “integrated treatment,” which included pretreatment with lime and ammonium bicarbonate and then application of bio-organic fertilizer, was most effective in controlling tobacco bacterial wilt among these four treatments.
Effects of agronomic measures on the suppression of tobacco bacterial wilt in the field. Disease incidence (a) and abundance of R. solanacearum (b) at harvest time. The means and standard errors are also shown. Different letters above each bar refer to the Duncan’s test, p < 0.05. L + AB (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate and then amended with bio-organic fertilizers)
After soil pretreatment with lime and ammonium bicarbonate in the field, the population of R. solanacearum decreased to 3.48 log cfu/g dw soil, which is lower than that of the untreated soil (4.18 log cfu/g dw soil). Furthermore, at the end of the experiment, the abundance of R. solanacearum was still lower in the L + AB and L + AB + BOF treatments than that of the BOF treatment with decreases of 50.00 and 63.04 % (Fig. 2b). Application of BOF could also reduce the population of R. solanacearum by 39.07 % compared to the OF treatment. Furthermore, the abundance of SQY 162 in L + AB + BOF treatment was higher than that in BOF treatment, resulting in higher suppression efficacies of bacterial wilt. The number of the antagonistic strain SQY 162 in the rhizosphere soil in L + AB + BOF treatment (5.57 log cfu/g dw soil) increased by 85 % compared with that in BOF treatment (5.29 log cfu/g dw soil).
The tobacco agronomic characteristics at 70 days after transplanting are listed in Table S1. The three agronomic characteristics in BOF and L + AB + BOF treatments were significantly higher than those in L + AB and OF treatments, indicating that strain SQY 162 could promote tobacco growth to increase the yields in the field. In addition, the characteristics of tobacco in the L + AB + BOF treatment were the highest, suggesting that integrated management may be better than only the application of BOF treatment under field conditions.
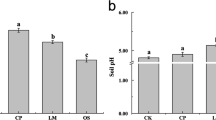
The soil properties of the different treatments are summarized in Table S2. The soil to which lime was applied (L + AB and L + AB + BOF) had a higher pH than that of the OF- or BOF-treated soil. All soils that received the lime and ammonium bicarbonate and bio-organic fertilizer had a significant higher amount of available K than those of the other three treatments. There were significant differences in the C/N ratios among treatments, but little difference in total C or total N was observed.
Bacterial diversity in differently treated soils
The bacterial community composition of soil as determined by pyrosequencing analysis is summarized in Table 1. The number of detected OTUs showed the following trend: L + AB + BOF > L + AB > BOF > OF. The number of OTUs of the L + AB + BOF rhizosphere soil increased by 6.33, 22.52, and 18.52 % compared to that of L + AB, OF, and BOF rhizosphere soils. The bacterial communities of L + AB + BOF-treated rhizosphere soil were more diverse than those of the other treated soils. Both the average values of abundance-based coverage estimator (ACE) and Chao 1 reflected the same trend as the OTUs. The heat map (Fig. S2) showed that the samples from BOF and L + AB + BOF treatments were clearly distinguishable than those of the L + AB or OF treatment, which was mainly due to the application of bio-organic fertilizer.
Bacterial diversity at the phylum and family levels
The pyrosequencing results showed that there was no difference in phylum diversity, but the relative abundance of each phylum did differ in all the treated soils. The majority of bacteria from the four samples were classified as Acidobacteria, Actinobacteria, Bacteroidetes, and Proteobacteria. Actinobacteria and Proteobacteria were the dominant bacterial phyla in all treated soils (Fig. 3). Reads detected as Bacteroidetes were significantly lower in the L + AB + BOF treatment than in L + AB, OF, and BOF treatments. On the other hand, the reads of Firmicutes, which were lower in the total reads, increased with the BOF and L + AB + BOF treatments compared to those with the L + AB or OF treatment.
Bacterial community structure of the four treated rhizosphere soils at the phylum level. Others and unclassified: others contain the phyla representing <1 % of the total reads; unclassified means all of the unclassified reads. L + AB (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate and then amended with bio-organic fertilizers)
The lower family taxonomic analysis demonstrated that Streptomycetaceae was the most dominant family with the L + AB, OF, BOF, and L + AB + BOF treatments (Fig. 4). The abundances of the family in BOF and L + AB + BOF treatments were significantly higher than those in L + AB or OF treatment.
Percentages of Actinobacteria families. Others and unclassified: others contain the families representing <1 % of the total reads; unclassified means all of the unclassified reads. L + AB (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate and then amended with bio-organic fertilizers)
The bacteria belonging to Alphaproteobacteria represented 50.28, 53.95, 67.38, and 69.19 % of the Proteobacteria in the L + AB-, OF- BOF-, and L + AB + BOF-treated soils, respectively (Fig. 5). The percentages of Proteobacteria present as Gammaproteobacteria were 7.5, 8.1, 17.1, and 20.2 % in the BOF, L + AB + BOF, L + AB, and OF rhizosphere soils, respectively. There were no significant differences in the bacterial numbers of Betaproteobacteria among four treatments. Both Bradyrhizobiaceae and Rhizobiaceae families were the dominant ones in all treatments. The relative abundances of Alcaligenaceae in Betaproteobacteria and Xanthomonadaceae in Gammaproteobacteria were lower in the BOF- and L + AB-treated rhizosphere soils than the respective ones of the OF or L + AB + BOF treatment.
Percentages of Proteobacteria families. Others and unclassified: others contain the families representing <1 % of the total reads; unclassified means all of the unclassified reads. L + AB (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate and then amended with bio-organic fertilizers)
Bacterial diversity at the genus level
At the genus level, Streptomyces was the most abundant, comprising 6.76, 5.06, 12.18, and 13.25 % of the bacterial population in the L + AB-, OF-, BOF-, and L + AB + BOF-treated rhizosphere soils, respectively (Fig. 6). The relative abundance of Bacillus with the L + AB + BOF treatment (0.472 %) was significantly higher than that of the L + AB-treated (0.179 %), OF-treated (0.196 %), and BOF-treated (0.166 %) soils. The relative abundance of Paenibacillus in the L + AB + BOF treatment (0.039 %) was also significantly higher than that in L + AB (0.018 %), OF (0.030 %), and BOF (0.019 %) treatments. Arthrobacter of the Micrococcaceae family represented 7.68 % of the total reads in the L + AB + BOF-treated rhizosphere soil, while it was 4.2, 0.8, and 4.8 % in the L + AB-, OF-, and BOF-treated rhizosphere soils, respectively. There were no significant differences among the relative abundances of Pseudomonas of the different treated rhizosphere soils. Furthermore, Ralstonia comprised 0.61 % of the population of the L + AB + BOF-treated rhizosphere soil, and this was significantly lower than that of the L + AB-treated (1.8 %), OF-treated (1.8 %), and BOF-treated (1.2 %) rhizosphere soils, indicating that integrated management could significantly reduce the abundance of the pathogen.
Microbial composition of the four soil treatments: L + AB (blue), OF (wine), BOF (green), and L + AB + BOF (violet) at the genus level. Others and unclassified: others contain the genus representing <1 % of the total reads; unclassified means all of the unclassified reads. Bars represent the averages of the genus proportion of three replicates. L + AB (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate), OF (soil amended with organic fertilizers), BOF (soil amended with bio-organic fertilizers), L + AB + BOF (soil pretreated with 750 kg/ha of lime and 325 kg/ha of ammonium bicarbonate and then amended with bio-organic fertilizers)
Discussion
A strong antagonistic activity in vitro by beneficial agents seems to result in high potential suppression efficacy of bacterial diseases in the field (Xue et al. 2012). Strains which strongly colonized the rhizosphere soil in pot and field experiments could efficiently reduce the bacterial diseases with a directly defense niche formed against pathogen agents (Yuan et al. 2014). The antagonistic strain SQY 162, applied with organic fertilizer, could colonize the rhizosphere soil and significantly suppress TBW in pot and field experiments. This result indicated that bio-organic fertilizer was a good alternative for controlling soil-borne pathogens. A positive correlation between the population of R. solanacearum and disease incidence was observed (r = 0.919, p < 0.001) confirming what reported by Wei et al. (2011). Furthermore, the integrated treatment (L + AB + BOF) significantly suppressed the population of R. solanacearum in pot and field experiments. Thus, it was not surprising that the disease incidences of the integrated treatments were the lowest compared to those of the other treatments, indicating that integrated agriculture management was a more effective way to suppress the TBW. Additionally, the plant biomass increased due to the plant-growth-promoting effects and strong colonization ability of SQY 162 and the successful bio-control of the pathogen.
The bacterial diversity and the number of bacterial ribotypes in the soil can be improved significantly with long-term manure application (Parham et al. 2003; Sun et al. 2004). With manipulation of rhizosphere microbial community, suppression of specific soil-borne pathogens can be enhanced (Chaparro et al. 2012; Larkin and Honeycutt 2006; Mazzola 2007; Qiu et al. 2012). The PCR-DGGE patterns showed that the bacterial diversity increased in the BOF treatment compared with respect to that in other treatments after two continuous growth seasons, in agreement with a previous report (Wang et al. 2013). The UPGMA dendrogram also showed that application of BOF could significantly affect the composition of bacterial community in the rhizosphere soil after two continuous growth seasons. These results basically matched with those of the analysis of 454 sequencing data in the field.
Analysis of the microbial taxonomic distribution showed that Actinobacteria (r = −0.728) and Proteobacteria (r = 0.753) were associated with disease incidence. Both Actinobacteria and Micrococcaceae exhibited more abundance in the L + AB + BOF-treated rhizosphere soil than in the other treated rhizosphere soils. Arthrobacter, some of which have been identified as antagonistic agents against Ralstonia (Huang et al. 2011), was detected as a dominant genus in this study, and the relative abundance was the highest in the integrated management. The results showed that the abundance of Arthrobacter (r = −0.994, p < 0.001) was negatively associated with disease incidence. Additionally, application of bio-organic fertilizers enhanced the abundance of Streptomyces, which can produce antibiotics to control the diseases of tomato (El-Abyad et al. 1993). Bottomley et al. (2006) suggested that a high-quality soil exhibited higher bacterial and Actinomycetes diversity than a low-quality soil. Firmicutes, Bacillus, and Paenibacillus, which are involved both in suppressing plant pathogens and promoting plant growth (Algam et al. 2010; Aliye et al. 2008), were significantly enriched following treatments with L + AB + BOF compared with other treatments. However, the number of Pseudomonas showed no significant difference among the treatments. Xue et al. (2012) demonstrated that inoculation of Serratia XY21 into the soil to suppress tomato bacterial wilt could significantly affect the abundance of Pseudomonas, but not that of Bacillus. These results may be explained by different plants and different bio-control agents with different effects on different microbial species.
Among Proteobacteria, the abundance of Betaproteobacteria was the same among the four treatments, confirming a previous report (Schönfeld et al. 2003). Mendes et al. (2011) suggested that Xanthomonadales, a family of Gammaproteobacteria, was more abundant in soil suppressive against a fungal pathogen than in a nonsuppressive soil. Furthermore, the relative abundance of Xanthomonadaceae was also significantly higher in soil treated with L + AB + BOF than in soil treated with other amendments. These results showed that Xanthomonadaceae might also play an important role in the control of bacterial pathogen. These results suggested that beneficial microbes could be enriched with the amendment of chemicals followed by application of BOF fortified with the antagonist. This was in agreement with recent findings that decoupling of root–microbiome associations followed by antagonist inoculation can improve rhizosphere soil suppressiveness to cucumber Fusarium wilt (Qiu et al. 2014). Thus, with the health rhizosphere niche formed, it was not surprising to find that the abundance of the pathogen species (Ralstonia) with the integrated treatment was lower than that with other amendments, resulting in a lower disease incidence of bacterial wilt.
In conclusion, our results demonstrated that an integrated agricultural management (the pretreatment of lime + ammonium bicarbonate and then application of BOF) was more effective in suppressing tobacco bacterial wilt than in the single treatment of acid soils. Specific beneficial bacterial groups were enriched, while the abundance of Ralstonia was decreased as it was the suppression of tobacco bacterial wilt. The integrated management probably favored the synergistic action of microbial consortia to suppress bacterial wilt; research is, however, needed to better understand the relative mechanisms.
References
Algam SAE, Xie G, Li B, Yu S, Su T, Larsen J (2010) Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J Plant Pathol 92(3):593–600
Aliye N, Fininsa C, Hiskias Y (2008) Evaluation of rhizosphere bacterial antagonists for their potential to bioprotect potato (Solanum tuberosum) against bacterial wilt (Ralstonia solanacearum). Biol Control 47(3):282–288
Ascher J, Ceccherini M, Pantani O, Agnelli A, Borgogni F, Guerri G, Nannipieri P, Pietramellara G (2009) Sequential extraction and genetic fingerprinting of a forest soil metagenome. Appl Soil Ecol 42(2):176–181
Bernard E, Larkin RP, Tavantzis S, Erich MS, Alyokhin A, Sewell G, Lannan A, Gross SD (2012) Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl Soil Ecol 52:29–41
Borrero C, Ordovás J, Trillas MI, Avilés M (2006) Tomato Fusarium wilt suppressiveness. The relationship between the organic plant growth media and their microbial communities as characterised by Biolog®. Soil Biol Biochem 38(7):1631–1637
Bottomley P, Yarwood R, Kageyama S, Waterstripe K, Williams M, Cromack K Jr, Myrold D (2006) Responses of soil bacterial and fungal communities to reciprocal transfers of soil between adjacent coniferous forest and meadow vegetation in the Cascade Mountains of Oregon. Plant Soil 289(1–2):35–45
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fert Soils 48(5):489–499
El-Abyad M, El-Sayed M, El-Shanshoury A, El-Sabbagh SM (1993) Towards the biological control of fungal and bacterial diseases of tomato using antagonistic Streptomyces spp. Plant Soil 149(2):185–195
Elphinstone J, Hennessy J, Wilson J, Stead D (1996) Sensitivity of different methods for the detection of Ralstonia solanacearum in potato tuber extracts. EPPO B 26(3–4):663–678
Gamliel A, Austerweil M, Kritzman G (2000) Non-chemical approach to soilborne pest management-organic amendments. Crop Prot 19(8–10):847–853
Goldman GH, Hayes C, Harman GE (1994) Molecular and cellular biology of biocontrol by Trichoderma spp. Trends Biotechnol 12(12):478–482
Guo JH, Qi HY, Guo YH, Ge HL, Gong LY, Zhang LX, Sun PH (2004) Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biol Control 29(1):66–72
Hartman WH, Richardson CJ, Vilgalys R, Bruland G (2008) Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc Natl Acad Sci U S A 105(46):17842–17847
Hayward A (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29(1):65–87
Huang MY, Gu WJ, Zhang FB, Xu PZ, Yang SH, Wang LQ, Xie KZ (2011) Identification and fermentation of antagonistic bacterium against Ralstonia solanacearum. Microbiol China 38(2):214–220 (in Chinese)
Huang X, Zhang N, Yong X, Yang X, Shen Q (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res 167(3):135–143
Insam H (1997) A new set of substrates proposed for community characterization in environmental samples. In: Insam H, Rangger A (eds) Microbial communities: functional versus structural approaches. Springer-Verlag, Berlin, pp 259–260
Jayaraj J, Radhakrishnan N (2008) Enhanced activity of introduced biocontrol agents in solarized soils and its implications on the integrated control of tomato damping-off caused by Pythium spp. Plant Soil 304(1):189–197
Kloepper J (1993) Plant growth-promoting rhizobacteria as biological control agents. In: Metting JF (ed) Soil microbial ecology: applications in agricultural and environmental management. Marcel Dekker, New York, pp 255–274
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt ER, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Lang J, Hu J, Ran W, Xu Y, Shen Q (2011) Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol Fert Soils 48(2):191–203
Larkin RP, Honeycutt CW (2006) Effects of different 3-year cropping systems on soil microbial communities and Rhizoctonia diseases of potato. Phytopathology 96(1):68–79
Lopez I, Ruiz LF, Cocolin L, Orr E, Phister T, Marshall M, Vander GJ, Mills DA (2003) Design and evaluation of PCR primers for analysis of bacterial populations in wine by denaturing gradient gel electrophoresis. Appl Environ Microbiol 69(11):6801–6807
Luo J, Shen Q, Ran W, Yang X, Xu Y, Hu J (2010) Application of bio-organic fertilizer significantly affected fungal diversity of soils. Soil Sci Soc Am J 74(6):2039–2048
Mazzola M (2007) Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39(3):213–220
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Sci 332(6033):1097–1100
Niwa R, Kumei T, Nomura Y, Yoshida S, Osaki M, Ezawa T (2007) Increase in soil pH due to Ca-rich organic matter application causes suppression of the clubroot disease of crucifers. Soil Biol Biochem 39(3):778–785
Parham J, Deng S, Da H, Sun H, Raun W (2003) Long-term cattle manure application in soil. II. Effect on soil microbial populations and community structure. Biol Fert Soils 38(4):209–215
Qiu M, Zhang R, Xue C, Zhang S, Li S, Zhang N, Shen Q (2012) Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol Fert Soils 48(7):807–816
Qiu M, Li S, Zhou X, Cui X, Vivanco JM, Zhang N, Shen Q, Zhang R (2014) De-coupling of root–microbiome associations followed by antagonist inoculation improves rhizosphere soil suppressiveness. Biol Fert Soils 50(2):217–224
Scherf JM, Milling A, Allen C (2010) Moderate temperature fluctuations rapidly reduce the viability of Ralstonia solanacearum race 3, biovar 2, in infected geranium, tomato, and potato plants. Appl Environ Microbiol 76(21):7061–7067
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Schönfeld J, Gelsomino A, Overbeek LS, Gorissen A, Smalla K, Elsas JD (2003) Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2 and indigenous bacteria in soil. FEMS Microbiol Ecol 43(1):63–74
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67(10):4742–4751
Sun HY, Deng SP, Raun WR (2004) Bacterial community structure and diversity in a century-old manure-treated agroecosystem. Appl Environ Microbiol 70(10):5868–5874
Sun B, Dong ZX, Zhang XX, Li Y, Cao H, Cui ZL (2011) Rice to vegetables: short- versus long-term impact of land-use change on the indigenous soil microbial community. Microbial Ecol 62(2):474–485
Vanitha S, Niranjana S, Mortensen C, Umesha S (2009) Bacterial wilt of tomato in Karnataka and its management by Pseudomonas fluorescens. Biocontrol 54(5):685–695
Wang LL, Shi JX, Yuan SF, Wu K, Cai LT, Liu YX, Yang XM, Feng YG, Shen B, Shen QR (2013) Control of tobacco bacterial wilt with biomanure plus soil amendments. Acta Pedologica Sin 50(1):150–156, in Chinese
Wei Z, Yang X, Yin S, Shen Q, Ran W, Xu Y (2011) Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48(2):152–159
Weller DM, Raaijmakers JM, Gardener BBMS, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40(1):309–348
Woltz S, Engelhard AW (1973) Fusarium wilt of chrysanthemum: effect of nitrogen source and lime on disease development. Phytopathology 63(1):155–175
Xue QY, Ding GC, Li SM, Yang Y, Lan CZ, Guo JH, Smalla K (2012) Rhizocompetence and antagonistic activity towards genetically diverse Ralstonia solanacearum strains–an improved strategy for selecting biocontrol agents. Appl Microbiol Biotechnol 97(3):1361–1371
Yao H, Wu F (2010) Soil microbial community structure in cucumber rhizosphere of different resistance cultivars to fusarium wilt. FEMS Microbiol Ecol 72(3):456–463
Yuan S, Wang L, Wu K, Shi J, Wang M, Yang X, Shen Q, Shen B (2014) Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol 75:86–94
Acknowledgments
This research was financially supported by Programs of Study and Application of Key Technologies for Soil Bioremediation in Guizhou Province (110201002019) and by the 111 project (B12009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 152 kb)
Rights and permissions
About this article
Cite this article
Wu, K., Yuan, S., Wang, L. et al. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol Fertil Soils 50, 961–971 (2014). https://doi.org/10.1007/s00374-014-0916-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0916-9