Abstract
Cucurbits are often cultivated in rotation with Solanaceae in double-cropping systems. Most cucurbits have been described as susceptible to root-knot nematodes (RKN) but little is known on their relative levels of susceptibility. Because RKN species differ in rates of root invasion and reproductive traits, isolates of M. arenaria, M. incognita and M. javanica were compared on five cucurbit hosts in experiments run in a climate growth chamber. They included zucchini squash cv Amalthee, cucumber cv Dasher II, melon cv Pistolero, pumpkin cv Totanera and watermelon cv Sugar Baby. All cucurbits were susceptible to the three RKN isolates although M. javanica showed higher invasion rates, faster development and higher egg production than M. arenaria on the selected cucurbits. Apparent differences among cucurbits were primarily due to root invasion rates and formation of egg masses. Both Cucumis species (cucumber and melon) were better hosts for nematode invasion and reproduction than zucchini squash, followed by watermelon. Large invasion rates followed by small reproduction traits were linked to M. incognita on zucchini squash. Reduced invasion rates and egg mass formation along with delayed early development were shown on watermelon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Root-knot nematodes (RKN) are important pests for many vegetable crops worldwide (Karssen 2002). The second-stage juveniles (J2) penetrate the roots and migrate through the intercellular space to the vascular cylinder to initiate and develop a permanent feeding site. Once established, J2 moult three times to become adults. Mature females lay eggs into a gelatinous matrix attached to the posterior end of the female.
Cucurbits are often cultivated in rotation with Solanaceae in double-cropping systems in several vegetable production areas. For instance, pepper is rotated with squash or cucumber (Thies et al. 2004) and tomato with melon or watermelon (Talavera et al. 2012). Most edible cucurbits are hosts of the most widespread root-knot nematodes M. arenaria, M. incognita and M. javanica, but comparative studies on their pathogenic effects on cucurbits are limited. Meloidogyne spp. differ in rates of root penetration (Arens et al. 1981; Khan and Khan 1991a; Ehwaeti et al. 1999; Dutta et al. 2011) and reproduction (Roberts and Thomason 1989) on different hosts (Carneiro et al. 2000); furthermore various plants species differ on their ability to withstand nematode damage (Ehwaeti et al. 1999). Non-host plants do not allow nematode attack, often preventing root penetration and thereby nematode development and reproduction. Resistance is used to describe the ability of a plant to suppress development or reproduction of the nematode. Susceptible plants allow normal nematode development and the expression of any associated disease (Cook and Evans 1987; Roberts 2002). Susceptible plants facilitating the building up of high nematode densities are considered good hosts (Seinhorst 1967), and this ability is generally referred as the reproduction factor (Rf) that is measured as the final population density (Pf) divided by the initial population density (Pi). On the contrary, poor host plant often show low Rf. Host plants to which the nematode multiplies but suffer little damage are termed tolerant (Cook and Evans 1987). Large soil infestations may result in high invasion rates that may cause great tissue injury of meristematic cells affecting initial plant growth. This situation can also be detrimental for nematode development, as nematodes will compete for available feeding sites resulting in reduced multiplication rates (Arens et al. 1981). Conversely, high nematode multiplication rates with no plant damage may be achieved with slight soil infestations (Di Vito et al. 1985). Therefore, information on the host-parasite relationship in rotational crops like members of the cucurbit family will be valuable for sustainable management of RKN in double cropping systems.
The objectives of this study were to compare penetration and reproduction of Meloidogyne arenaria, M. incognita and M. javanica isolates on a diversity of cucurbits including zucchini squash, cucumber, melon, pumpkin, and watermelon, and to select the most useful cultivar of cucurbits to include in double-cropping systems with Solanaceae.
Materials and methods
Nematode isolates
The nematode isolates used were M. arenaria (code Ma 68), M. incognita (code Mi PM26) and M. javanica (code Mj 05) from the nematode collection at IRTA, Centre of Cabrils. Nematode cultures were started from the progeny of one single female and they were maintained on susceptible tomato cv Roma in spring-summer and on celery cv D’Elne in autumn-winter in a greenhouse. Juveniles were obtained from infected tomato roots cv Roma by the Baermann tray method (Whitehead and Hemming 1965).
Inoculation process
Cucurbit seeds were soaked overnight and germinated in vermiculite trays for 3 days. Seedlings were transplanted at the cotyledon stage to 20-cm3 capacity clay pots filled with sterilized river sand. Seedlings were allowed to growth 2 weeks for watermelon and 1 week for the others species before nematode inoculation. Individual seedlings were inoculated with 200 freshly hatched J2 (less than 72 h-old, Pi) of each isolate in approximately 0.5 ml of water.
Plants were maintained in a growth chamber at 26 ± 1 °C with a 16 h light photoperiod, watered as needed and fertilized with a slow-release fertilizer (Osmocote ® Scotts Company, Netherlands, 15 % N + 10 % P2O5 + 12 % K2O + 2 % MgO2 + microelements) at the beginning of the test.
Nematode root penetration
A time course experiment was conducted to compare root penetration by three RKN isolates on five cucurbit species. Each RKN isolate and cucurbit combination was replicated seven times. Tests were run separately and repeated two times. The cucurbits included zucchini squash (Cucurbita pepo L.) cv Amalthee, cucumber (Cucumis sativus L.) cv Dasher II, melon (C. melo L.) cv Pistolero, pumkin (Cucurbita maxima Duschesne) cv Totanera and watermelon [Citrullus lanatus (Thunb), Matsum & Nakai] cv Sugar Baby. For each RKN isolate, seedlings were grouped into three sets, one set per harvest date at 4, 7, and 11 days post-inoculation - dpi. At each harvesting time, plants were carefully removed from the pots and the root system washed free of soil. Roots were stained with acid fuchsin 0.05 % (Bridge and Page 1982), and examined under a stereo microscope to count the number of infection sites, and nematodes inside them. Infection sites were recognized because the root tissue was swollen and contained at least one nematode inside. Nematodes were categorized according to their post-embryonic developmental stages as J2 (vermiform), third-stage juveniles (J3, sausage-like) and fourth-stage juvenile (J4, sac-shape) (Taylor and Sasser 1978).
Nematode reproduction
A comparison of RKN reproduction on cucurbit hosts was conducted using the same nematode isolates, cucurbit cultivars except pumpkin that was not included, and experimental conditions. Seedlings were obtained and inoculated as referred before to penetration test. The experimental design was a completely randomized block that included all possible combinations corresponding to 12 treatments (four cucurbit species × three RKN species) with 12 replicates per treatment. Five plants from each treatment were harvested 7 dpi to determine J2 penetration following the methodology previously described. The remaining seven plants/treatment were uprooted, the roots gently rinsed in water and transplanted into new pots filled with 500 cm3 of sterilized river sand to remove all J2 from root surface and prevent any further root penetration. The experiment was conducted twice.
At 45 dpi (728°-days, basal temperature of 10 °C), the root systems were washed free of soil and weighed. Egg masses (EMs) were stained with a 0.1 g l−1 erioglaucine solution (Aldrich Chemical Company) for 2 h (Omwega et al. 1988) and recorded. Eggs from the entire root system were extracted by maceration in a blender containing a 0.5 % NaOCl solution for 10 min (Hussey and Barker 1973) and counted to determine Pf. Both non-hatched eggs and empty eggs (egg shells) were recorded and the hatching rate was estimated. The fecundity of the females was estimated by dividing Pf by EMs and the Rf (Rf = Pf/Pi) was calculated.
Statistical analyses
The SAS system V8 (SAS Institute Inc., Cary, NC) was used for statistical analyses. Prior to the analyses, when needed, nematode data were log transformed [log10 (x + 1)] to homogenize the variances. Data from the root penetration and nematode reproduction experiments were combined because there was no significant difference between the repeated experiments and they were analyzed using analysis of variance (ANOVA). Comparisons were conducted within cucurbit host and within nematode isolate (n = 14; seven replications × two experiments). In addition, data were grouped by nematode isolate (four cucurbits × 14 replications) and the new set of data subjected to ANOVA. Data from cucurbit species were pooled (three RKN isolates × 14 replications) and subjected to ANOVA. When the analyses showed statistical differences (P = 0.05), the means were separated according to Tukey HSD (Honestly Significant Difference) Test.
Results
Nematode root penetration
Significantly more (P < 0.05) infection sites were found on zucchini squash infected by M. incognita (94 ± 12, mean ± standard deviation) at 11 dpi than M. arenaria (68 ± 7) followed by M. javanica (49 ± 16). The number of infection sites on watermelon roots (25 ± 7) at 11 dpi was significantly fewer (P < 0.05) than on cucumber (44 ± 6), melon (44 ± 7) and pumpkin (59 ± 12) regardless of the RKN isolates.
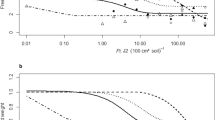
Root penetration followed a similar pattern on both Cucurbita species (zucchini squash and pumpkin) although M. incognita J2 invaded zucchini squash roots more rapidly and in numbers significantly highest (P < 0.05) than the other two isolates at all harvesting times (Fig. 1a). Final penetration rates on zucchini squash were 98 % for M. incognita, 46 % for M. arenaria and 48 % for M. javanica (Fig. 1a); on pumpkin were 99 %, 70 % and 68 %, respectively (Fig. 1b). On cucumber, M. incognita and M. javanica showed significantly higher (P < 0.05) penetration rates than M. arenaria at all harvesting times, and M. javanica was higher (P < 0.05) than M. incognita at 11 dpi (Fig. 1c). The final penetration rates were 96 %, 78 %, and 59 %, for M. javanica, M. incognita and M. arenaria, respectively (Fig. 1c). On melon, M. javanica invaded more rapidly and in numbers significantly highest (P < 0.05) than M. incognita followed by M. arenaria, and final penetration rates were 100 %, 84 % and 54 %, respectively (Fig. 1d). On watermelon, root penetration by M. javanica (51 %) was significantly higher (P < 0.05) than that of the other two RKN isolates (41 % to 42 %) (Fig. 1e).
Number of second-stage juveniles of Meloidogyne arenaria, M. incognita and M. javanica inside the roots of zucchini squash (Cucurbita pepo) cv Amalthee (a), pumpkin (Cucurbita maxima) cv Totanera (b), cucumber (Cucumis sativus) cv Dasher II (c), melon (Cucumis melo) cv Pistolero (d), and watermelon (Citrullus lanatus) cv Sugar Baby (e) 4, 7, and 11 days post-inoculation with 200 juveniles per plant. Values are means of 14 replicates. At each harvesting date, values sharing the same letter do not differ significantly according to the Tukey HDS Test (P = 0.05)
At 4 dpi only vermiform J2 were found in roots of cucurbit species (data not shown). At 7 dpi, most penetrating J2 were at the J3-stage on zucchini squash, cucumber, and melon, with the exception of M. javanica on zucchini squash. RKN development was delayed on pumpkin and watermelon (Fig. 2a). At 11 dpi most nematodes were at J4-stage in zucchini squash, cucumber, and melon roots except for M. arenaria on melon (Fig. 2b). A mixture of J3 and J4 stages occurred on pumpkin, whereas on watermelon were found the three juveniles stages (J2, J3 and J4) with a dominance of the J3-stage (Fig. 2b).
Post-embryonic development of Meloidogyne arenaria, M. incognita and M. javanica in roots of zucchini squash (Cucurbita pepo) cv Amalthee, pumpkin cv Totanera (Cucurbita maxima), cucumber (Cucumis sativus) cv Dasher II, melon (Cucumis melo) cv Pistolero, and watermelon (Citrullus lanatus) cv Sugar Baby at 7 (a) and 11 (b) days post-inoculation of 200 juveniles per plant. Values are means of 14 replicates. J2 - second-stage juveniles; J3 - third-stage juveniles; J4 - fourth-stage juveniles
Nematode reproduction
The Pi for this experiment was the number of nematodes that penetrated the roots at 7 dpi. Therefore, differences in Pi values were due to different penetration rates of the isolates on the cucurbit hosts (Table 1).
Within cucurbit host, M. incognita showed higher Pi values on zucchini squash than M. arenaria followed by M. javanica but there was no difference in EMs among the isolates (Table 1). However, M. incognita Pf was significantly lower (P < 0.05) than that of the other two isolates. On cucumber, M. javanica showed significantly higher (P < 0.05) Pi values than the other two isolates; but only M. javanica Pf significantly differed (P < 0.05) from M. arenaria Pf (Table 1). On melon, the Pi values of M. javanica were significantly higher (P < 0.05) than those of M. incognita followed by M. arenaria. Besides, M. javanica and M. incognita showed significantly higher (P < 0.05) Pf than M. arenaria. On watermelon, M. javanica showed significantly higher (P < 0.05) Pi values than the other two isolates, but only M. incognita Pf was higher (P < 0.05) than M. arenaria Pf (Table 1).
The combined analysis indicated that root weight was higher in M. incognita than M. arenaria infected plants (Table 2). Meloidogyne incognita and M. javanica formed significantly more (P < 0.05) EMs than M. arenaria, but M. javanica Pf was significantly higher (P < 0.05) than M. incognita Pf (Table 2). However, the fecundity of the females did not differ among the three RKN isolates (Table 2). The hatching rate of M. arenaria (20 ± 2) was lower (P < 0.05) than that of M. incognita (32 ± 2) or M. javanica (30 ± 2). Both Cucumis species (cucumber and melon) showed significantly higher (P = 0.05) root weight than zucchini squash, followed by watermelon (Table 3). Significantly higher (P = 0.05) EMs were observed on cucumber than on melon or zucchini squash followed by watermelon (Table 3). Statistical differences in Pf, and Rf among the cucurbits consistently corresponded with those observed for EMs. There was no difference in the fecundity of the females among the cucurbit hosts (Table 3). Hatching rates on cucumber (34 ± 2), melon (30 ± 2) and watermelon (28 + 2) were comparable and significantly higher than on zucchini squash (22 ± 2).
Discussion
All cucurbits were susceptible to the three Meloidogyne isolates but significant differences in root penetration and nematode reproduction that persisted through the experimental period were detected. Cucurbits also differed in root galling severity of M. javanica and M. incognita (Edelstein et al. 2010). The present study confirms that the genetic background of the host as well as that of the nematode affect the host-nematode interaction in good hosts and poor/resistant hosts (Ehwaeti et al. 1999; Fournet et al. 2012; Verdejo–Lucas et al. 2013). Root invasion and formation of egg masses were the primary factors explaining differences among cucurbits and the observed differences were thereafter consistently shown in final population densities and reproduction factors. Nevertheless, females that reached maturity laid similar numbers of eggs regardless the RKN isolate or the cucurbit host which corroborates that female fecundity is not a major factor in the response of the host to the nematode (Arens et al. 1981; Faske 2013). The exception to this trend was M. incognita on zucchini squash that showed reduced fecundity (236 eggs/female) in comparison to the other isolates (603 eggs/female). In general, M. javanica showed greater root penetration, faster development and reproduction than M. arenaria on the selected cucurbits. Seemingly, M. javanica invaded tobacco roots more rapidly and in greater numbers than M. arenaria or M. incognita (Arens et al. 1981). The lessen reproductive ability of M. arenaria in comparison to M. javanica on cucurbits is consistent with similar observations on tomato (Verdejo–Lucas et al. 2013) and could explain the restricted distribution and detection of M. arenaria in some vegetable areas (Giné et al. 2012; Talavera et al. 2012).
Both Cucumis species (cucumber and melon) were better hosts for nematode invasion and reproduction than zucchini squash followed by watermelon. Meloidogyne incognita and M. javanica showed similar root penetration pattern, infection and reproduction on cucumber and melon.
The M. incognita isolate showed a remarkable interaction with zucchini squash cv Amalthee with significantly greater numbers of penetrating J2, similar egg mass production and lower Pf than the other two RKN isolates. Accordingly, zucchini squash was a poorer host of M. incognita than M. arenaria or M. javanica. The specificity of this interaction for the crop cultivar or RKN isolate is not presently known but deserves further exploration since it resulted in increased root penetration and reduced Pf. This is an interesting combination of effects that might be of utility for the sustainable management of nematode infestations. Zucchini squash has been described as RKN susceptible (Thies et al. 2004) but whether squash cultivars differ in susceptibility levels is unknown. Species-specific and even race or population specific, non-host or resistance responses to Meloidogyne spp. were found on cultivars of cauliflower, tomato rootstocks and several wild plants (Khan and Khan 1991b; Ehwaeti et al. 1999; Cortada et al. 2009).
Apparently, the zucchini squash cv Amalthee hindered the development of the nematode from the J4-stage to mature egg-laying-female with no effect on penetrating J2 since they progressively developed into immotile J3 and J4 stages. J4-stage juveniles may have died or stop developing as occurred on Cucumis sativus infected by M. hapla (Stephan and Trudgill 1982). Faske (2013) found empty galls on Cucumis melo var. texanus and suggested that juveniles developed to males and had left the roots. An increase in the male/female ratio on Cucumis myriocarpus, a non-host for M. incognita, was reported by Pofu and Mashela (2011). Egression of juveniles after penetration of the roots occurred on resistant Cucumis genotypes (Faske 2013) although it seems little likely to be the case here, since large numbers of penetrating J2 developed into J4-stages. Sub-optimal development of feeding sites unable to supply sufficient nutrients for the nematode could possibly explain the M. incognita-zucchini squash interaction. Such resistance mechanism has been suggested on RKN resistant Cucumis (Walters et al. 2006).
The response of watermelon cv Sugar Baby was differentiated by a great reduction in J2 penetration which suggested a pre-infectional mechanism that was not related to the RKN isolate. Allelochemicals released into the rizhosphere could affect nematode behavior, and thus modify the host recognition process (Dutta et al. 2011). Other mechanisms were retardation in juvenile development and low rates of penetrating J2 becoming egg-laying females. All these mechanisms together make watermelon cv Sugar Baby the less suitable host to the three RKN isolates among the tested cucurbits. Montalvo and Esnard (1994) found that Sugar baby supported the lowest M. incognita root galling and Rf among ten watermelon cultivars and all Rf values were significantly lower on watermelon than tomato. Other cucurbits also showed reduced Rf values in comparison to tomato or eggplants (Anwar and McKenry 2010). These results support the worth of searching for less suitable RKN host since resistance to M. arenaria, M. incognita and M. javanica is not commercially available on Cucumis, Cucurbita or Citrullus. Resistance to M. hapla has been reported on some cultivars of squash and melon (Carneiro et al. 2000).
In summary, the main results from this comparative study were: i) high root penetration and reproduction on cucumber and melon irrespective of the RKN isolate, ii) high penetration rates linked to low Pf values on M. incognita-infected zucchini squash, and iii) reduced invasion and delay in post-embryonic development on watermelon. These mechanisms operate on resistant germplasm (Khan and Khan 1991a) and poor host plants (Ehwaeti et al. 1999). Although the tested cucurbits were all susceptible to the three RKN isolates, differences in susceptibility levels were significant; cucumber cv Dasher II followed by melon cv Pistolero were the most susceptible hosts, and watermelon cv Sugar Baby the least. The host status affects not only the damage a crop is likely to suffer but also the residual populations left in the soil which are the inoculum for the next crop in the rotation. Thus, watermelon cv. Sugar Baby could be used for the sustainable management of the disease in double-cropping systems with Solanaceae since Rf were a third lower of that on cucumber. Similarly, zucchini squash cv. Amalthee could be used in M. incognita infested fields.
References
Anwar, S. A., & McKenry, M. V. (2010). Incidence and reproduction of Meloidogyne incognita on vegetable crops genotypes. Pakistan Journal of Zoology, 42, 135–141.
Arens, M. L., Rich, J. R., & Dickson, D. W. (1981). Comparative studies on root invasion, root galling, and fecundity of three Meloidogyne spp. on a susceptible tobacco cultivar. Journal of Nematology, 13, 201–205.
Bridge, J., & Page, L. J. (1982). The rice root-knot nematode, Meloidogyne graminicola, on deep water rice (Oryza sativa subsp. indica). Revue Nematology, 5, 225–232.
Carneiro, R. M. D. G., Randig, O., Almeida, M., & Diniz Campos, A. (2000). Resistance of vegetable crops to Meloidogyne spp.: suggestion for a crop rotation system. Nematologia Brasileira, 24, 49–54.
Cook, R., & Evans, K. (1987). Resistance and tolerance. In R. H. Brown & B. R. Kerry (Eds.), Principles and Practice of nematode control in crops (pp. 179–232). Sidney: Academic Press.
Cortada, L., Sorribas, F. J., Ornat, C., Andrés, M. F., & Verdejo-Lucas, S. (2009). Response of tomato rootstocks carrying the Mi-resistance gene to populations of Meloidogyne arenaria, M. incognita and M. javanica. European Journal of Plant Pathology, 124, 337–343.
Di Vito, M., Greco, N., & Carella, A. (1985). Population densities of Meloidogyne incognita and yield of Capsicum annum. Journal of Nematology, 17, 45–49.
Dutta, T. K., Powers, S. J., Kerry, B. R., Gaur, H. S., & Curtis, R. H. C. (2011). Comparison of host recognition, invasion, development and reproduction of Meloidogyne graminicola and M. incognita on rice and tomato. Nematology, 13, 509–520.
Edelstein, M., Oka, Y., Burger, Y., Eizenberg, H., & Cohen, R. (2010). Variation in the response of cucurbits to Meloidogyne incognita and M. javanica. Israel Journal of Plant Sciences, 58, 77–84.
Ehwaeti, M. E., Fargette, M., Phillips, M. S., & Trudgill, D. L. (1999). Host status differences and their relevance by Meloidogyne incognita. Nematology, 1, 421–432.
Faske, T. R. (2013). Penetration, post-penetration development and reproduction of Meloidogyne incognita on Cucumis melo var. texanus. Journal of Nematology, 45, 58–65.
Fournet, S., Kerlan, M. C., Renault, J. P., Rouaux, C., & Montarry, J. (2012). Selection of nematodes by resistant plants has implications for local adaptation and cross-virulence. Plant Pathology, 62, 184–193.
Giné, A., Bonmatí, M., Sarro, A., Stchigel, A., Valero, J., Ornat, C., Fernández, C., & Sorribas, F. J. (2012). Natural occurrence of fungal egg parasites of root-knot nematodes, Meloidogyne spp. in organic and integrated vegetable production systems in Spain. BioControl, 58, 407–416.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease, 57, 1025–1028.
Karssen, G. (2002). The plant- parasitic nematode genus Meloidogyne Göldi, 1892 (Tylenchida) in Europe. The Netherlands: Leiden.
Khan, A. A., & Khan, M. W. (1991a). Penetration and development of Meloidogyne incognita race 1 and Meloidogyne javanica in susceptible and resistant vegetables. Nematropica, 21, 71–77.
Khan, A. A., & Khan, M. W. (1991b). Reaction of cauliflower cultivars to Meloidogyne javanica and races of Meloidogyne incognita. Nematropica, 21, 161–166.
Montalvo, A. E., & Esnard, J. (1994). Reactions of ten cultivars of watermelon (Citrullus lanatus) to a Puerto Rican population of Meloidogyne incognita. Journal of Nematology, 26(Suppl.), 640–643.
Omwega, C., Thomason, I. J., & Roberts, P. A. (1988). A non-destructive technique for screening bean germplasm for resistance to Meloidogyne incognita. Plant Disease, 72, 970–972.
Pofu, K. M., & Mashela, P. W. (2011). Using relative penetration and maleness indices in Meloidogyne incognita to establish resistance type in Cucumis myriocarpus. African Journal of Biotechnology, 10, 390–393.
Roberts, P. A. (2002). Concepts and consequences of resistance. In J. L. Starr, R. Cook, & J. Bridge (Eds.), Plant resistance to parasitic nematodes (pp. 23–40). New York: CAB International UK.
Roberts, P. A., & Thomason, I. J. (1989). A review of variability in four Meloidogyne spp. measured by reproduction on several hosts including Lycopersicon. Agricultural Zoology Reviews, 3, 225–252.
Seinhorst, J. W. (1967). The relationships between population increase and population density in plant parasitic nematode. Nematologica, 13, 429–442.
Stephan, Z. A., & Trudgill, D. L. (1982). Development of four populations of Meloidogyne hapla on two cultivars of cucumber at different temperatures. Journal of Nematology, 14, 545–549.
Talavera, M., Sayadi, S., Chirosa-Rios, M., Salmerón, T., Flor-Peregrin, E., & Verdejo-Lucas, S. (2012). Perception of the impact of root-knot nematode induced diseases in horticultural protected crops of south-eastern Spain. Nematology, 14, 517–527.
Taylor, A. L., & Sasser, J. N. (1978). Biology, identification, and control of root-knot nematodes (Meloidogyne species). North Carolina State University Graphics, The genus Meloidogyne (Root-Knot Nematodes) (pp. 1–13). NC: Raleigh.
Thies, J. A., Davis, R. F., Mueller, J. D., Fery, R. L., Langston, D. B., & Miller, G. (2004). Double-cropping cucumbers and squash after bell pepper for root-knot nematode management. Plant Disease, 88, 589–593.
Verdejo–Lucas, S., Blanco, M., Cortada, L., & Sorribas, F. J. (2013). Resistance of tomato rootstocks to Meloidogyne arenaria and M. javanica under intermittent elevated soil temperatures above 28°C. Crop Rotation, 46, 57–62.
Walters, S. A., Wehner, T. C., Daykin, M. E., & Barker, K. R. (2006). Penetration rates of root-knot nematodes into Cucumis sativus and C. metuliferus roots and subsequent histological changes. Nematropica, 36, 231–242.
Whitehead, A. G., & Hemming, J. R. (1965). A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals Applied Biology, 55, 25–38.
Acknowledgments
The authors acknowledge the financial support provided by Intituto Nacional de Investigaciones Agrarias (INIA), project RTA2010-00017-C02-01 and the European Union through the Regional Development Funds. M. López-Gómez acknowledges INIA for support through a pre-doctoral grant. Thanks are given to Dr. Sorribas for statistical advice. The seeds were kindly provided by Gautier Seeds, Intersemillas S.A., Ramiro Arnedo S. A. and Sakata Vegetables Europe S.A.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Gómez, M., Verdejo-Lucas, S. Penetration and reproduction of root-knot nematodes on cucurbit species. Eur J Plant Pathol 138, 863–871 (2014). https://doi.org/10.1007/s10658-013-0359-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0359-4