Abstract
To investigate the association of serum 25-hydroxyvitamin D (25(OH)D3) with survival in a large prospective cohort study of colorectal cancer (CRC) patients. The study population consisted of 2,910 patients diagnosed with CRC between 2003 and 2010 who participated in the DACHS study, a multicenter study from Germany with comprehensive long-term follow-up. 25(OH)D3 was determined in serum samples collected shortly after cancer diagnosis by High Performance Liquid Chromatography-Electro Spray Ionization-Mass Spectrometry. Analyses of survival outcomes were performed using Cox regression with comprehensive adjustment for relevant confounders. The majority (59%) of CRC patients were vitamin D deficient (serum 25(OH)D3 levels <30 nmol/L). During a median follow-up of 4.8 years, 787 deaths occurred, 573 of which were due to CRC. Compared to patients in the highest 25(OH)D3 quintile (>45.20 nmol/L), those in the lowest 25(OH)D3 quintile (<11.83 nmol/L) had a strongly increased mortality. Adjusted hazard ratios (95% Confidence Interval) were 1.78 (1.39–2.27), 1.65 (1.24–2.21), 1.32 (1.03–1.71) and 1.48 (1.18–1.85) for all-cause mortality, CRC-specific mortality, recurrence-free and disease-free survival, respectively. Subgroup analyses did not show any significant effect modification across strata defined by sex, age, stage, body mass index, or the late entry. Dose–response analyses showed a strong inverse relationship between serum 25(OH)D3 levels and survival endpoints at 25(OH)D3 levels <30 nmol/L, and no association with mortality at higher 25(OH)D3 levels. Vitamin D deficiency is highly prevalent in CRC patients and a strong independent predictor of poor prognosis. The possibility of enhancing CRC prognosis by vitamin D supplementation, ideally combined with outdoor physical activity, should be evaluated by randomized controlled trials focusing on patients with vitamin D deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related deaths globally, with more than 2.2 million new cases and 1.1 million cancer deaths expected by 2030 [1]. Despite gradual progress in prognosis, a large number of patients still die from the disease, especially if diagnosed in advanced stages. Identification of modifiable factors that may enhance the prognosis of CRC patients is highly desirable.
In the last decades, vitamin D obtained from food and skin synthesis after sunlight exposure, has received increasing attention as a potentially promising modifiable factor. Findings from both ecological and individual-level epidemiological studies have shown positive associations of CRC prognosis with higher ultraviolet-B exposure [2,3,4,5] and higher concentrations of circulating 25-hydroxyvitamin D (25(OH)D), the most reliable indicator of vitamin D status in blood reflecting both dietary intake and skin synthesis [6,7,8]. We previously summarized results of five prospective cohort studies investigating the relationship between measured 25(OH)D levels and survival in CRC patients [9]. Pooled estimates showed that, compared to those with low levels, CRC patients with high 25(OH)D levels had 29% and 35% lower all-cause and CRC-specific mortality, respectively. More recent studies [10,11,12,13] likewise mostly reported inverse associations between serum 25(OH)D levels and mortality in CRC patients.
However, despite promising results from epidemiological studies supporting a potential role of vitamin D in enhancing CRC patients’ prognosis, evidence is still limited by the small number of studies (n = 9), their small sample sizes (mostly <1000), and their heterogeneous consideration of important clinical factors which may confound or modify the association, such as stage at diagnosis or disease recurrence. Furthermore, while an inverse association between 25(OH)D levels and mortality seems likely, data on the underlying exact dose–response relationship have remained very limited [14, 15].
We therefore assessed the association between 25(OH)D3 and prognosis in a very large cohort of CRC patients from Germany, particularly focusing on the comprehensive consideration of tumor and therapy-related factors and the dose–response relationship of vitamin D status with specific survival outcomes.
Methodology
Study design and study population
Since 2003, patients with incident CRC (International Classification of Diseases, 10th Revision [ICD-10] codes C18–C20), aged ≥30 years (no upper age limit) have been recruited in the DACHS-study (Darmkrebs: Chancen der Verhütung durch S creening), an ongoing population-based study with prospective, long-term follow-up of cases conducted in the southwest of Germany (Rhine-Neckar-Region). The study was approved by the ethics committees of the University of Heidelberg and the state medical boards of Baden-Württemberg and Rhineland-Palatinate, and written informed consent was obtained from each participant. Details of the study design, recruitment, data collection and follow-up procedures have been reported elsewhere [16,17,18,19].
Briefly, eligible patients in all of the 22 participating clinics in the study region were informed about the study, usually a few days after their surgery. Patients who could not be recruited during their inpatient stay were contacted after discharge by clinicians and clinical cancer registries by mail. Baseline information on patients’ sociodemographic, medical, and lifestyle history was obtained by personal interviews with trained interviewers using a standardized questionnaire. Medical data including tumor stage and location and details of therapy were extracted from hospital charts. In addition, blood samples were drawn at a median of 3 days after time of the interview, from which serum aliquots were obtained and stored at −80 °C until analysis. Recruited patients were followed up with respect to therapy, the course of the disease, and survival. Briefly, the vital status of participants was determined through population registries at 3 and 5 years after CRC diagnosis, and death certificates were obtained from health authorities to determine the cause of death. Information about CRC treatment and recurrence was collected from the patients’ attending physicians about 3 years after diagnosis using a standardized questionnaire. Further information about medical, lifestyle and recurrence history was collected from a standardized follow-up questionnaire filled out by the patients approximatively 5 years after diagnosis. If the patient died before the follow-up or did not complete the follow-up questionnaire, information on cancer recurrence before death was collected from the last attending physicians.
The current analysis includes CRC patients recruited between 2003 and 2010. From 3,146 patients, we excluded 2 patients lost to follow-up and 234 patients with missing serum 25(OH)D3 measurements, leaving 2,910 patients for analysis.
Serum 25-hydroxyvitamin D3 measurements
We used High Performance Liquid Chromatography-Electro Spray Ionization-Mass Spectrometry (HPLC–ESI–MS) in positive-ion mode to measure 25(OH)D3 in 70 µL of serum. All measurements were conducted in the Division of Preventive Oncology at the German Cancer Research Center over a period of 6 months. For statistical analysis, 25(OH)D3 values below the lower detection limit (0.25 nmol/L) were assigned the lowest concentration of serum 25(OH)D3 in our sample [0.5 nmol/L, n = 19 (0.65%)]. The HPLC–ESI–MS method was standardized using pooled human serum, purchased from a local blood bank, and the Standard Reference Material (SRM) 972a developed by the National Institute of Standards and Technology (NIST) [20]. The study population was divided into five groups by serum 25(OH)D3 level quintiles and into three groups by serum 25(OH)D3 clinical categories as proposed by the American Institute of Medicine (IOM), defining vitamin D deficiency as serum 25(OH)D3 levels <30 nmol/L (<12 ng/mL), insufficiency as 30–<50 nmol/L (12–<20 ng/mL), and sufficiency as ≥50 nmol/L (≥ 20 ng/mL) [21, 22].
Outcomes
Follow-up time for overall, CRC-specific, non-CRC, recurrence-free and disease-free survival endpoints was calculated in days starting from the date of CRC diagnosis and ending at the date of having the event (death from any cause, death from CRC, death from causes other than CRC, recurrence or death from CRC, recurrence or death from any cause, respectively). Patients not reaching a specific endpoint were censored at a point in time when they were last known to have been alive or free of recurrence. In analyses of CRC-specific and recurrence-free survival, patients dying from causes other than CRC were censored at the time of their death. In analyses of non-CRC survival patients dying from CRC were censored at the time of their death. Non-CRC survival results will only be reported in the text, not in tables.
Covariates assessment
Information on relevant prognostic and clinical factors such as tumor detection mode, cancer stage at diagnosis, tumor location, surgery, chemotherapy, history of cardiovascular diseases (heart failure, myocardial infarction, angina pectoris and stroke), history of diabetes and history of hypertension were extracted from medical charts, and information on demographic, environmental and lifestyle factors, known or suspected to be associated with serum 25(OH)D3 concentrations or prognosis were extracted from the baseline questionnaire, including: sex, age, smoking, season of blood draw, body mass index (BMI), and physical activity.
Statistical analysis
Characteristics of the study population as well as the distribution and seasonal variation of serum 25(OH)D3 levels were analyzed using descriptive statistics.
Cox proportional hazards regression models were fitted to evaluate the association of serum 25(OH)D3 concentrations (categorized by quintiles or clinical categories) with the various survival endpoints. Different adjustment settings were defined a priori. In model 1, analyses were adjusted for sex (male, female), age (30–59, 60–69, 70–79, 80+), and season of blood draw (winter: December–February, spring: March–May, summer: June–August, autumn: September–November). In model 2, analyses were additionally adjusted for cancer stage at diagnosis (I-IV according to the International Union Against Cancer (UICC) classification), tumor location (colon, rectum), tumor detection mode (screening, other), surgery (yes, no), chemotherapy (yes, no), history of cardiovascular diseases (heart failure, myocardial infarction, angina pectoris and stroke) (yes, no), history of diabetes (yes, no), history of hypertension (yes, no), smoking history (lifetime pack-years; never, <10, 10–19, 20–29, ≥30), BMI (Kg/m2; <25, 25–<30, 30+), and physical activity (quartiles of average lifetime Metabolic Equivalent of Task hours per week (MET-h/week); 3.3–<130.9, 130.9–<192.7, 192.7–<278.4, 278.4. Since the participants were enrolled into the study at varying time intervals after CRC diagnosis, we adjusted all survival analyses for late entry in days. Survival analyses were performed with complete cases after exclusion of participants with missing covariate data dependent on adjustment setting (exclusions <7% in all cases). The proportional hazards assumption was tested using interaction terms of covariates with time. Furthermore, we explored associations after stratifying by sex, age (<65, ≥65y), tumor detection mode (screening, other), stage (I–II, III, IV), obesity (BMI <30, ≥30 kg/m2), late entry (≤1, >1 month). Potential interactions between covariates and 25(OH)D3 levels were investigated by adding pertinent product terms to the full model and evaluation of the corresponding Wald test. Adjusted survival curves [23] were used to visualize patients’ survival according to serum 25(OH)D3 categories.
In addition to analyses with 25(OH)D3 as categorical variable, dose–response relationships between serum 25(OH)D3 levels (continuous variable) and survival were assessed using restricted cubic splines [24] with predefined knots at the upper limit of each 25(OH)D3 quintile and the lowest limit of the 5th quintile as the reference.
All statistical tests were 2-sided with an α level of 0.05, and all analyses were carried out using SAS statistical software (version 9.3; SAS Institute, Inc., Cary, Nc).
Results
Patient characteristics
Characteristics of the 2,910 patients included in the analysis are shown in Table 1. Forty percent of patients were female. Median age at diagnosis was 69 years (range: 30–96 years). Slightly more than half of the patients were diagnosed in stages I or II, 15% in stage IV. Median BMI was 26.1 kg/m2 (range: 15.4–56.1). More than half (53%) of the patients were recruited into the study during the first month after CRC diagnosis.
Serum 25(OH)D3 levels
Vitamin D concentrations were assessed in blood samples drawn at a median of 23 days after CRC diagnosis. The median level (interquartile range) of serum 25(OH)D3 was 25.2 nmol/L (0.5–234.1) and the mean (standard deviation) was 30.3 nmol/L (22.7). More than half (59%) of the patients were vitamin D deficient (<30 nmol/L), 25% were vitamin D insufficient (30–50 nmol/L), and only 16% were vitamin D sufficient (≥50 nmol/L). The distribution of serum 25(OH)D3 levels by month of blood draw showed strong seasonal variation with lowest values in February/March and highest values in July/August (Fig. 1).
Box plot of the distribution of serum 25(OH)D3 levels across months of blood collection in 2,910 CRC patients. Box plots = median with 25th percentile and 75th percentile, upper boxplot whiskers represent the last data point within the range of 75% quantile + 1.5 IQR, lower boxplot whiskers represent the last data point within the range of 25% quantile–1.5 IQR, empty circles = maximum observation in the group. 25(OH)D 3 25-hydroxyvitamin D3, Jan january, Feb february, Mar march, Apr april, Jun june, Jul july, Aug august, Sep september, Oct october, Nov november, Dec december
Vitamin D and survival
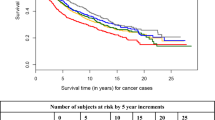
During a median follow-up duration of 4.8 years for overall survival and 3.9 years for recurrence-free survival, a total of 787 deaths occurred, of which 573 were due to CRC, and 817 patients recurred. Adjusted survival curves are shown according to serum 25(OH)D3 quintiles in Fig. 2 and according to vitamin D clinical categories in Supplementary Fig. 1. Patients in the lowest quintile of serum 25(OH)D3 levels and those with vitamin D deficiency had much poorer survival than those in the highest quintiles of serum 25(OH)D3 levels.
Adjusted survival curves for overall survival (a), CRC-specific survival (b), recurrence-free survival (c), and disease-free survival (d) according to serum 25(OH)D3 quintiles. Survival curves were adjusted for: sex, age at diagnosis, season of blood draw, BMI, cancer stage at diagnosis, tumor location, tumor detection mode, surgery, chemotherapy, history of cardiovascular diseases (heart failure, myocardial infarction, angina pectoris and stroke), history of diabetes, history of hypertension, smoking, physical activity and late entry. BMI body mass index, CRC colorectal cancer, Q quintiles, 25(OH)D 3 25-hydroxyvitamin D3
Hazard ratios (HRs) according to serum 25(OH)D3 quintiles are shown in Table 2. After adjustment for sex, age and season of blood draw, lower serum 25(OH)D3 levels were associated with a significantly poorer survival (P-trend <.0001 for all outcomes). Associations were only slightly attenuated but remained clearly statistically significant for all outcomes after comprehensive adjustment for all covariates of interest of which stage was the most influential one. Fully adjusted HRs (95% CI) for the lowest (25(OH)D3 <11.83 nmol/L) versus highest (25(OH)D3 >45.20 nmol/L) quintile were 1.78 (1.39–2.27), 1.65 (1.24–2.21), 1.32 (1.03–1.71) and 1.48 (1.18–1.85) for all-cause, CRC-specific, recurrence-free and disease-free survival, respectively. Besides the above endpoints reported in Table 2, we also found an inverse association between serum 25(OH)D3 levels and non-CRC mortality (HR = 1.96, 95% CI: 1.24–3.10) for the lowest vs highest quintile.
Analyses using vitamin D clinical categories showed a weaker association but with a strong trend for survival outcomes in CRC patients with vitamin D deficient or insufficient levels compared to those with vitamin D sufficient levels (Supplementary Table 1). Subgroup analyses did not show any significant effect modification across strata of sex, age, stage, BMI, or late entry for any of the outcomes (Table 3).
Restricted cubic spline curves showed a strong inverse relationship between serum 25(OH)D3 levels and survival endpoints in the range of <30 nmol/L, and no associations at higher levels (Fig. 3).
Dose-response relationship plots of the association between serum 25-hydroxyvitamin D3 levels and overall survival (a), CRC-specific survival (b), recurrence-free survival (c), and disease-free survival (d). All plots were adjusted for: sex, age at diagnosis, season of blood draw, BMI, cancer stage at diagnosis, tumor location, tumor detection mode, surgery, chemotherapy, history of cardiovascular diseases (heart failure, myocardial infarction, angina pectoris and stroke), history of diabetes, history of hypertension, smoking, physical activity and late entry. Curves were assessed by restricted cubic splines with knots at 11.83, 20.35, and 30.18 nmol/L. The reference was at 45.20 nmol/L (quintile 5). BMI body mass index, CRC colorectal cancer, CI confidence interval
Discussion
In this large multi-center cohort of CRC patients from Germany, more than half of the patients (59%) were found to be vitamin D deficient (25(OH)D3 <30 nmol/L), and there was a steep increase in various measures of mortality (all-cause and CRC specific mortality, recurrence-free and disease-free survival) with decreasing 25(OH)D3 levels within this group of vitamin D deficient patients which persisted after control for a large variety of potential confounding factors. Differences in 25(OH)D3 levels above 30 nmol/L were unrelated to the survival outcomes.
Our results are in agreement with previous studies that have shown an inverse relationship between 25(OH)D levels and prognosis of CRC patients [6,7,8, 11,12,13]. However, most of these studies were much smaller in size, and none of them assessed within-study dose–response relationships in detail. Furthermore, none of the previous studies comprehensively assessed all of the four major survival outcomes in parallel, and sample size limitations mostly precluded or strongly limited subgroup-specific analyses, such as stage-specific analyses, as well as assessment of potential interactions by important covariates.
Several plausible explanations may explain the inverse relationship between 25(OH)D levels and prognosis of CRC patients. In cancer cells, vitamin D regulates transcription of genes involved in inhibition of proliferation and angiogenesis, and stimulation of differentiation, apoptosis, and DNA repair mechanisms [25]. Due to its angiogenesis inhibitory actions, vitamin D at adequate levels may prevent cancer recurrence after treatment by inhibition of immortal cancer cells’ growth. Vitamin D could furthermore enhance CRC patient survival through increasing muscle strength and mass [26], attenuation of fatigue symptoms [27], and a decrease of mood disorders or depression [28]. Apart from reducing the patient’s disease burden, adherence to treatment might also improves [29, 30]. Further research is needed to better understand the role of vitamin D and vitamin D deficiency in CRC prognosis.
Dose–response relationships were assessed in two previous meta-analyses by pooling aggregated data from different studies [14, 15]. These studies likewise found an inverse relationship between 25(OH)D levels and prognosis of CRC patients, but the heterogeneity of study populations, vitamin D measurement methods, and vitamin D levels across study populations included and the need of pooling aggregated data made it difficult to reliably establish the specific shape of the dose–response relationship. In particular, no critical threshold could be determined below which variation of 25(OH)D levels is most relevant for prognosis. Our detailed dose–response analyses based on individual-level data from a large cohort of CRC patients suggest a strong inverse relationship between vitamin D and the various mortality outcomes at 25(OH)D3 levels below 30 nmol/L, i.e., among the majority of patients with vitamin D deficiency, but essentially no further decrease in mortality risk with higher levels of 25(OH)D3. Hence, hazard ratios for patients with 25(OH)D3 levels below 30 nmol/L would, for this study population, essentially be the same if patients with higher 25(OH)D3 values than those suggested by IOM were used as “normal value” reference group.
This particular dose–response-relationship may have important implications for potential intervention studies, to enhance prognosis of CRC patients by vitamin D supplementation which might be considered assuming causality of the observed associations. Our results suggest that patients with 25(OH)D3 levels below 30 nmol/L may potentially strongly benefit from such intervention, whereas patients with higher 25(OH)D3 levels might have only little if any benefit. Pre-testing of 25(OH)D3 levels and focusing interventions on patients with low 25(OH)D3 levels might, therefore, be essential to increase the power of intervention studies and the cost-effectiveness of interventions in clinical practice.
Vitamin D supplementation is one way to increase 25(OH)D levels among cancer patients. However, the most important source of vitamin D is its production by the skin during sunlight exposure [31]. Even modest levels of exposure far below critical levels for sunburn and other potential adverse health effects, such as spending as little as 15 min outside (without specific sun protection) around noon two to three times a week appear to be sufficient to maintain adequate vitamin D levels in the general population [32]. Nevertheless, a somewhat more extended exposure may be necessary among CRC patients due to less effective vitamin D synthesis of the skin at advanced age [33] and, combining such exposure with outdoors physical activity may be a particularly promising approach to enhance the prognosis of CRC patients. There is rapidly increasing evidence on the beneficial effects of physical activity on the prognosis of patients with colorectal and other cancers from both observational studies [34, 35] and randomized trials [36,37,38,39], and conducting physical activity outdoors may not just enhance survival but also quality of life of CRC patients.
In the interpretation of our study, specific strengths and limitations require careful consideration. Major strengths include the large size of our cohort and its comprehensive active follow-up which enabled parallel, detailed dose–response analyses not only of all-cause and CRC-specific mortality but also of recurrence-free and disease-free survival. A comprehensive collection of risk factor data by personal interviews and of medical data through medical records enabled comprehensive consideration of potential confounding factors and of potential effect modification in multivariate and subgroup-specific analyses. Vitamin D levels were measured by gold standard methods, i.e., HPLC–ESI–MS, with standardization to SRM 972a developed by the NIST[20].
A limitation of our study is that blood samples were taken at various time intervals after diagnosis and first-line treatment (median: 23 days after diagnosis). Potential changes of 25(OH)D3 levels during the early postoperative phase may thus have affected our results and would most likely have led to underestimation of associations with survival due to presumably non-differential measurement error compared to a 25(OH)D3 measurement taken at an uniform time-point. However, the most informative time-point for evaluation of long-term 25(OH)D3 levels and long-term prognosis is still to be found, which warrants further research with repeated measurements of 25(OH)D3 at multiple-time-points before and after first-line treatment.
Although we carefully adjusted for a large variety of possible confounding factors, we cannot rule out residual confounding by imperfect measurement of covariates or by unmeasured confounders, such as performance status. Data from population-based cohort studies which likewise found vitamin D deficiency to be associated with increased mortality [40] have suggested that this association may at least partly reflect vitamin D deficiency being an indicator of poor overall health status rather than a cause of increased mortality [41]. Future studies should, therefore, aim to include detailed comorbidity data in order to evaluate and overcome such potential confounding. In general, however, stage IV CRC patients are in a much worse overall health status than early stage patients, and the fact that the strong associations with survival persisted after control for stage and even tended to be stronger for the earlier stages, does not seem to support the fact that low vitamin D levels would primarily be an indicator of poor overall health status. Finally, data on vitamin D supplementation were not available. Other studies have shown, however, that Vitamin D supplements were not commonly used among older adults in Southern Germany during the period of recruitment [42].
Despite its limitations, our study, provides important evidence that low vitamin D levels in the range of vitamin D deficiency which are common among patients with CRC, are an important, independent prognostic factor. To what extent this prognostic value reflects a causal role of low vitamin D levels cannot be determined with certainty even from large observational studies with comprehensive confounder adjustment such as ours and will have to be clarified by randomized clinical trials (RCTs) aimed to increase vitamin D levels. Our results do provide, however, important information as to the design of such studies. For example, the absence of an association between 25(OH)D3 and survival at 25(OH)D3 levels above 30 nmol/L suggests that untargeted vitamin D supplementation to all patients is not warranted and may actually strongly compromise expected effect sizes and power of RCTs. Targeted supplementation following 25(OH)D3 measurements may, therefore, be a more prudent strategy. Ideally, such intervention should be combined with promotion of outdoor physical activity. Finally, our results also suggest that potential RCTs should not be preferentially focused on or even restricted to stage IV cases in whom ways to enhance prognosis are most desperately sought, because the observed associations were even tentatively stronger in patients with early stage cancers.
Conclusion
In conclusion, our study supports and extends evidence for an inverse relationship between serum 25(OH)D3 levels and CRC prognosis in the range of 25(OH)D3 levels <30 nmol/L, which are seen in the majority of CRC patients. The possibility of enhancing CRC prognosis by vitamin D supplementation, ideally combined with physical activity, should be evaluated by RCTs specifically targeting patients with vitamin D deficiency. If a causal relationship can be confirmed by such trials, vitamin D supplementation could be added to the standard treatment of CRC as an easy and cost-effective complementary therapy with a low burden for the patient.
References
Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016;. doi:10.1136/gutjnl-2015-310912.
Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9(3):227–31.
Gorham ED, Garland CF, Garland FC. Acid haze air pollution and breast and colon cancer mortality in 20 Canadian cities. Can J Public Health. 1989;80(2):96–100.
Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6):1867–75.
Moan J, Porojnicu AC, Robsahm TE, et al. Solar radiation, vitamin D and survival rate of colon cancer in Norway. J Photochem Photobiol, B. 2005;78(3):189–93.
Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–91.
Mezawa H, Sugiura T, Watanabe M, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347.
Fedirko V, Riboli E, Tjonneland A, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21(4):582–93.
Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50(8):1510–21.
Cooney RV, Chai W, Franke AA, et al. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1278–88.
Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32(23):2430–9.
Wesa KM, Segal NH, Cronin AM, et al. Serum 25-hydroxy vitamin D and survival in advanced colorectal cancer: a retrospective analysis. Nutr Cancer. 2015;67(3):424–30.
Facciorusso A, Del Prete V, Muscatiello N, Crucinio N, Barone M. Prognostic role of 25-hydroxyvitamin d in patients with liver metastases from colorectal cancer treated with radiofrequency ablation. J Gastroenterol Hepatol. 2016;. doi:10.1111/jgh.13326.
Ou B, Zhao J, Guan S, Lu A. Plasma 25-hydroxyvitamin D levels and survival of colorectal cancer patients: a meta-analysis. Eur J Cancer. 2015;51(6):786–8.
Wang B, Jing Z, Li C, Xu S, Wang Y. Blood 25-hydroxyvitamin D levels and overall mortality in patients with colorectal cancer: a dose-response meta-analysis. Eur J Cancer. 2014;50(12):2173–5.
Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30.
Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146(3):709–17.
Carr PR, Jansen L, Walter V, et al. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am J Clin Nutr. 2016;103(1):192–200.
Walter V, Jansen L, Ulrich A, et al. Alcohol consumption and survival of colorectal cancer patients: a population-based study from Germany. Am J Clin Nutr. 2016;103(6):1497–506.
Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88(2):511s–2s.
Slomski A. IOM endorses vitamin D, calcium only for bone health, dispels deficiency claims. Jama. 2011;305(5):453–6.
Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57.
Giammanco M, Di Majo D, La Guardia M, et al. Vitamin D in cancer chemoprevention. Pharm Biol. 2015;53(10):1399–434.
Kottler ML. Is vitamin D a key factor in muscle health? Endocrinology. 2013;154(11):3963–4.
Roy S, Sherman A, Monari-Sparks MJ, Schweiker O, Hunter K. Correction of Low Vitamin D Improves Fatigue: effect of Correction of Low Vitamin D in Fatigue Study (EViDiF Study). N Am J Med Sci. 2014;6(8):396–402.
Penckofer S, Kouba J, Byrn M, Estwing Ferrans C. Vitamin D and depression: where is all the sunshine? Issues Ment Health Nurs. 2010;31(6):385–93.
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, et al. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34(12):1339–44.
Paul CL, Cameron E, Doran C, et al. Experiences of colorectal cancer patients in the 2-years post-diagnosis and patient factors predicting poor outcome. Support Care Cancer. 2016;. doi:10.1007/s00520-016-3348-2.
Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–5.
Kaushal MJ, Magon N. Vitamin D in midlife: the sunrise vitamin in the sunset of life. J Midlife Health. 2012;3(2):97–9.
MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8.
Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–13.
Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–311.
Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):811–21.
Courneya KS, Friedenreich CM, Quinney HA, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl). 2003;12(4):347–57.
Devin JL, Sax AT, Hughes GI, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv. 2016;10(3):467–79.
Kim J, Choi WJ, Jeong SH. The effects of physical activity on breast cancer survivors after diagnosis. J Cancer Prev. 2013;18(3):193–200.
Schottker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656.
Schottker B, Brenner H. Vitamin D as a resilience factor, helpful for survival of potentially fatal conditions: a hypothesis emerging from recent findings of the esther Cohort study and the chances consortium. Nutrients. 2015;7(5):3264–78.
Schwab S, Heier M, Schneider A, et al. The use of dietary supplements among older persons in southern Germany - results from the KORA-age study. J Nutr Health Aging. 2014;18(5):510–9.
Acknowledgements
This work was supported by the German Research Council (BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, HO 5117/2-1), the German Federal Ministry of Education and Research (01KH0404 and 01ER0814), the Interdisciplinary Research Program of the National Center for Tumor Diseases, Germany, and the Klaus Tschira Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maalmi, H., Walter, V., Jansen, L. et al. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol 32, 961–971 (2017). https://doi.org/10.1007/s10654-017-0298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-017-0298-z