Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease that affects the central nervous system. MS is causing progressive and relapsing neurological disability, due to demyelination and axonal damage. The etiopathogenesis of MS is poorly understood. A number of environmental factors have been previously suggested, including: month of birth, vitamin D levels, smoking and viral infections. Previous studies assessing seasonal variation of relapses in multiple sclerosis have had conflicting results. The aim of this review is to assess the association between seasonal factors and MS, in terms of disease onset, relapses and activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a T-cell mediated autoimmune disease, causing central nervous system (CNS) damage and a progressive decline in neurological function. The etiopathogenesis of MS is poorly understood, but it is widely accepted to be a multifactorial disease. A genome wide study reported in 2007 confirmed that HLA class II DRB1*1501 is associated with the disease, as well as two other genetic associations—to Interleukin-7 receptor-α chain (IL7RA 5p13) and interleukin-2 receptor-α chain (IL2RA 10p15) [1]; although the relatively low odds ratio for this association, as well as only 24 % concordance rate between monozygotic twins [2] suggest a crucial role of environmental factors in the development of MS.
A number of environmental factors have been suggested, including: month of birth, vitamin D levels, smoking and viral infections [3], but the most influential environmental factor that was found is geographic latitude—at birth and early adulthood [4, 5]. This gives rise to the hypothesis that climate, and perhaps seasonality, play an important role in the pathogenesis of MS.
The purpose of this review is to assess this association in terms of disease onset and activity, and to evaluate which season-dependent factors play a role in it (Table 1).
Month of birth
The effect of month of birth was first proposed in 1987, as a part of a study examining different neurological diseases. It suggests that those who were born in winter months have a reduced risk of developing MS, whilst people born in spring are at a higher risk [6]. An association between month of birth and MS was also found in a large-scale population based study conducted in 2005; the association was stronger in familial cases of MS, indicating a possible epigenetic influence during gestation [7]. This finding was supported by a case-control study, that found the effect of month of birth to be a more pronounced in MS patients with HLA-DRB1 genotype [8]. The month of birth also had an effect on the phenotype of the disease (primary progressive MS vs relapsing–remitting MS) [9].
It has been hypothesized that low maternal vitamin D levels, due to reduced sun exposure in the winter months, plays a significant role in this phenomenon. Reduced vitamin D levels may affect the immunological development of the fetus in a critical time during pregnancy, resulting in an increased risk of MS [10]. A recent meta-analysis and systematic review found that the month of birth effect was latitude dependent, and only significant in places that were over 52°N [11]. It was previously suggested that at this latitude, insufficient ultraviolet B (UVB) radiation reaches the skin between October and March, leading to inadequate vitamin D synthesis during this time [12, 13]. One significant limitation of the previously discussed meta-analysis was that it only included studies in the northern hemisphere. Demonstration of an inverse relationship between month of birth and MS risk in similar latitudes of the southern hemisphere would significantly strengthen its conclusion [11].
If the month of birth effect is related to maternal vitamin D levels as mentioned above, it is plausible that the effect would be eliminated by the use of vitamin D supplementation during pregnancy. A retrospective study is needed to investigate the impact of maternal vitamin D supplementation the effect of the month of birth on MS incidence, and to establish the underlying mechanism. This could potentially lead to the use of antenatal vitamin D supplementation in those with a strong family history of MS, as the effect of month of birth is strongest in familial cases [8].
Whilst maternal vitamin D levels are the most widely accepted explanation of the month of birth effect, other factors that may play a role, include: perinatal infections, climate and variations in lifestyle.
Not all investigators agree with this conclusion. A study by Fiddes et al. [14] suggests that the association between MS and the month of birth is only mediated by several confounding variables, such a year and place of birth.
Vitamin D levels
Vitamin D is synthesized in the human body on response to exposure to sunlight, and specifically UV radiation [15]; therefore, it is easy to infer that vitamin D levels are higher during the summer months, as mentioned above [13].
Vitamin D has been previously associated with numerous autoimmune diseases, such as systemic lupus erythematosus, type I diabetes, inflammatory bowel diseases, rheumatoid arthritis, and of course, with MS [16–19].
The BENEFIT trial showed an inverse relationship between vitamin D levels and MS disease activity, progression, new active lesions, yearly increase in T2 lesion volume and yearly loss in brain volume. This study was performed in patients presenting with their first MS event, although the safety and efficacy of vitamin D therapy for relapsing remitting MS patients is still under clinical trial [20, 21]. Similar results, in regard to low levels of vitamin D and high disease activity and progression were found in another study [20]. Munger et al. [22] have demonstrated a protective effect of vitamin D intake on the risk for developing MS. Norway has low UV radiation exposure, since its latitude is 58°–71°N, which should expose its residence to high risk for MS. In a research done there, reduced incidence of MS was found in coastal fishing villages compared to inland farming areas. The interpretation of this finding was based on the assumption that coastal inhabitants have increased dietary vitamin D intake [23, 24]. This shows that dietary vitamin D can modulate the latitude-dependent risk of MS in areas with low exposure to the sunlight.
It could be concluded that vitamin D plays a crucial role as a seasonal factor of MS, although it seems that the seasonality effect could not be attributed to vitamin D alone.
Infections
Many infectious diseases have seasonal pattern of outbreaks [25, 26]; these patterns are due to seasonal changes in both the pathogens and in the host [26].
Infections have been noted to increase levels of cytokine IFN-γ [27, 28], which has a significant role in MS disease exacerbation [29–31]. Sibley et al. showed minor infections, particularly those of viral origin, frequently encountered in the winter, precipitated disease exacerbation. Nearly 30 % of MS attacks were associated with either preceding or concurrent infection. Infections were mainly of the upper respiratory and gastrointestinal systems, although no particularly antigen was isolated. Bacterial infections, in particular urinary tract infections, were not significantly documented as a disease trigger [32]. Other investigators have demonstrated the relationship between infection and exacerbation since [33–35].
Edwards et al. [35] detected a significant increase in the volume of gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) enhancing lesions in MRI scans at the time of serologically confirmed viral infections compared to non-infection periods. The area and volume of Gd-DPTA enhancing lesions has been associated with MS disease exacerbation although the quantitation of such lesions remains undetermined [35–38]. Such findings suggest a positive connection between viral infection and MRI evidence of the destruction of the blood-brain-barrier. This could result from a viral-induced inflammatory response affecting the CNS [35].
Melatonin
Melatonin is a hormone made in the pineal gland, and it is responsible for the circadian rhythms of physiological functions. Its secretion changes according to daylight time and night length, and therefore, it changes with the seasons [39]. In recent years, there is increasing evidence that Melatonin has a great influence on the immune system, and more specifically, it seems to enhance the release of T-helper type 1 (Th1) cytokines, and might also influence Th2 cells [40, 41]. Melatonin’s influence on the pathogenesis of autoimmune diseases in general, and of MS in particular, is still controversial [42]. Polymorphisms in genes related to the melatonin pathways were found to be associated with progressive subtypes and higher disability status in Finnish MS patients [43]. Evidence for the role of melatonin as an immunologic enhancer, and its adverse effect on MS can also be found in a study that tested the impact of treatment with luzindole, a melatonin receptor antagonist; Luzindole treatment prevented the development of experimental autoimmune encephalomyelitis (EAE) in mice [44].
Evidence for the ameliorating effect of melatonin on MS can be found in a study by Farez et al. [45] in which investigators showed melatonin levels were inversely correlated with MS relapses in humans. In animal models of EAE, it was shown that melatonin treatment could improve the disease’s clinical symptoms. In another study, investigators demonstrated that oral administration of melatonin reduced the clinical severity and spinal cord inflammatory infiltration in mice with EAE [46].
Sandyk et al. investigated the association between nocturnal melatonin levels and the presence of pineal gland calcification during MS exacerbations. Abnormal melatonin levels, mostly low nocturnal levels, were found in 52.0 % of patients. Melatonin levels were found to be positively correlated with the age of onset of symptoms of the disease (p < 0.0001) and negatively correlated with the disease’s duration (p < 0.05). Pineal gland calcification was found in 96 % of subjects, suggesting a possible role of pineal failure in the disease [47].
Thereby, melatonin could be one of the factors that mediated the association between MS and seasonality, and its role in the disease should be studied further.
Seasonality and disease activity
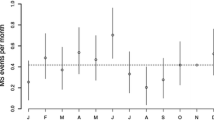
Previous studies have demonstrated seasonal variations in disease activity (Table 1). These variations have been investigated using three main parameters of disease activity: clinical relapses, magnetic resonance imaging (MRI) findings and immunoregulatory cytokine production.
Seasonality and clinical MS relapses
Several studies have examined the seasonal variation in relapse rates, and have reported conflicting results. In 2000, Jin et al. performed a meta-analysis examining the relationship between season and mono-symptomatic optic neuritis (a known symptom of MS [48]), MS onset and MS exacerbations. The researchers concluded that there is a peak in incidence during spring and a nadir during winter months in the northern hemisphere in all three parameters, which was highest with respect to MS onset (45 % with 95 % CI 36–55 %), and lowest with respect to its relapses (10 % with 95 % CI 7–13 %) [49]. A large, global multi-center study conducted in 2014 by Spelman et al. further demonstrated a latitude dependent effect. This study involved 8411 patients in 31 centers in the northern hemisphere, and 1400 patients in 15 centers in the southern one, with a total of 32,762 MS relapses analyzed. They found a significant difference of 7.62 % between peak and trough relapse difference per 100 patients, and the mean lag time between UV radiation trough and relapse onset peak was 2.72 months, for both northern and southern hemispheres. The relationship between lag time and latitude was also assessed, and it was found that with each change of 10° in latitude (away from the equator) the lag time was reduced by a mean of 28.5 days [50].
The variation according to latitude may be mediated through the UVR exposure, and its immunomodulatory effect and it is most likely due to vitamin D synthesis. However, this raises concerns because does not fully explain the high rate of exacerbations during the spring months, as vitamin D levels are lowest during winter time [13]. Inhabitants of countries further from the equator are known to have lower vitamin D levels in general [51], and thus are more likely to reach a vitamin D deficiency sooner after the UVR deprivation. In 2010, Simpson et al. reported an inverse relationship between vitamin D status and the likelihood of MS relapse [52]. An Australian study concluded that vitamin D levels and sun exposure are independent risk factors for CNS demyelination [53].
Seasonality and brain lesions in MRI
MRI is a useful technique to evaluate the effect of seasonal factors on MS, as it is an objective measure of sub-clinical disease activity in the CNS. Unfortunately, its use is limited by its high cost and therefore studies using MRI data are scant and typically have small sample sizes.
In 2000, Auer et al. [54] demonstrated a sinusoidal variation in the number of new contrast enhancing lesions (CEL), similar to the patterns described in clinical relapse rates. However, two studies published the following year independently concluded that no seasonal variation was observed using MRI, one followed 120 MS patients for 10 months while the other followed 13 patients for 9 months [55, 56]. The lack of an acceptable inclusion criteria and poor longitudinal follow up has cast doubt over the validity of the results of previous studies.
More recently, an observational cohort study concluded that there was a strong seasonal pattern of MS activity observed using non-contrast brain MRI. Although only 44 patients were included, its design and control for confounding variables, such as treatment, strengthened its validity. A total of 939 scans were used, with a median of 22 scans per patient, and it concluded there was a two-three-fold increase in likelihood of new lesions as well as increased disease activity during the spring-summer months [57].
Mowry et al. [58] reached a conclusion that vitamin D levels are inversely associated with MS activity on brain MRI, using scans of 469 subjects, and suggested this could possibly explain the effect of seasonality. Zivadinov et al. used MRI scans, serum vitamin D metabolite levels and questionnaires of 264 MS patients in order to explore the relationship between sun exposure, vitamin D and MRI documented disease activity. They concluded that sun exposure has a direct effect on MRI measures of neurodegeneration (measured by changes in brain volume and grey matter volume), independently of vitamin D levels [59]. Although this study does not examine seasonal variation of vitamin D or UVR, but rather sun exposure throughout the patient’s life, we have included it in this review, as it demonstrates the existence of an effect of UVR that is not mediated by vitamin D, and thus highlights a need for further research in order to identify this factor.
Seasonality and immunoregulatory cytokines
The CNS damage and progressive neurological decline characteristic of MS is thought to be mediated by the Th1 immune response, of which interferon γ (IFN-γ) is the hallmark cytokine. Levels of IFN-γ are naturally elevated during the inflammatory process of the nervous system via Interleukin-12 (IL-12), a pro-inflammatory cytokine. The CD40–CD40 ligand interaction between antigen presenting cells and T-cells leads to production of IL-12 and subsequently secretion of IFN-γ. IFN-γ and IL-12 are both also released by peripheral blood mononuclear (PBMN) cells [29]. In progressive MS patients, increased levels of IFN-γ have been found to precede clinical attacks and treatment of these patients with recombinant IFN-γ induces disease exacerbation [29–31].
Balashov et al. studied the relationship between levels of immunoregulatory cytokines in progressive MS patients and the time of year. They, amongst other studies such as Killestein et al., found significantly increased IFN-γ production in these patients during autumn and winter months when compared to spring and summer months, as well as when compared to IFN-γ levels of healthy patients during autumn and winter months [29, 30]. The apex of these increased levels occurred from October to December. Expanded Disability Status Scale (EDSS—validated evaluation of neurologic impairment in MS [60]) scores of progressive MS patients seen during this period worsened by 0.03 more compared to those patients seen during the March to August period. IL-12 was studied as well to determine its role in IFN-γ secretion. With neutralization of IL-12, IFN-γ production significantly decreased in progressive MS patients during the winter and autumn months. There was no change in IFN-γ levels with IL-12 neutralization during the spring and summer months for progressive MS patients or for healthy controls [29].
Although the mechanism for this seasonal effect is unknown, it is suggested that environmental factors, such as UVR, act as the inciting causes for disease onset and exacerbation. UVR exposure is positively correlated to vitamin D activation, and vitamin D inhibits IFN-γ -therefore increased UVR exposure leads to decreased IFN-γ levels [29, 61]. Vitamin D supplementation was also previously associated with increased levels of transforming growth factor beta 1 (TGF-β1) [62].
Conclusion
MS is an autoimmune multifactorial disease whose outbreak and course are influenced by genetic and environmental factors. One important environmental factor is seasonality. The effect of seasonality itself could be explained by various factors, including vitamin D levels, melatonin, infectious diseases and the month of birth, which is most widely explained by maternal vitamin D levels as described above.
Seasonal variation on MS disease’s course has been demonstrated in various studies, and seems to have an effect on clinical manifestations of the disease, MRI brain lesions, and inflammatory cytokine levels.
Low vitamin D levels are associated to most, but not all of the environmental factors mentioned. This review article supports the theory, past research and present research of vitamin D as an immunomodulator in MS, but also of seasonality as an independent factor in the disease.
References
Bhargava SK, Fall CHD, Osmond C. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357(9):851–62.
Hansen T, Skytthe A, Stenager E, Petersen HC, Brønnum-Hansen H, Kyvik KO. Concordance for multiple sclerosis in Danish twins: an update of a nationwide study. Multiple Scler. 2005;11(5):504–10.
Pugliatti M, Harbo HF, Holmøy T, Kampman MT, Myhr K, Riise T, et al. Environmental risk factors in multiple sclerosis. Acta Neurol Scand. 2008;117(s188):34–40.
Lauer K. Ecologic studies of multiple sclerosis. Neurology. 1997;49(2 Suppl 2):S18–26.
Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis. Brain. 2000;123(5):968–74.
Shimura MKT, Miura T. T (1987) Season of birth in some neurological disorders: multiple sclerosis, ALS senile dementia. Prog Biometeorol. 1987;6:163–8.
Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005;330(7483):120.
Ramagopalan SV, Link J, Byrnes JK, Dyment DA, Giovannoni G, Hintzen RQ, et al. HLA-DRB1 and month of birth in multiple sclerosis. Neurology. 2009;73(24):2107–11.
Sadovnick AD, Duquette P, Herrera B, Yee IM, Ebers GC. A timing-of-birth effect on multiple sclerosis clinical phenotype. Neurology. 2007;69(1):60–2.
Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7(3):268–77.
Dobson R, Giovannoni G, Ramagopalan S. The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84(4):427–32.
Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–8.
Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108–10.
Fiddes B, Wason J, Kemppinen A, Ban M, Compston A, Sawcer S. Confounding underlies the apparent month of birth effect in multiple sclerosis. Ann Neurol. 2013;73(6):714–20.
Holick MF. McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. Am J Clin Nutri. 1994;60(4):619–30.
Azrielant S, Shoenfeld Y. Eppur Si Muove: vitamin D is essential in preventing and modulating SLE. Lupus. 2016;25(6):563–72.
Watad A, G. Neumann S, Soriano A, Amital H, Shoenfeld Y. Vitamin D and systemic lupus erythematosus: myth or reality? Isr Med Assoc J. 2016;18(March–April):177–82.
Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–42.
Agmon-Levin N, Kopilov R, Selmi C, Nussinovitch U, Sánchez-Castañón M, López-Hoyos M, Amital H, Kivity S, Gershwin EM, Shoenfeld Y. Vitamin D in primary biliary cirrhosis, a plausible marker of advanced disease. Immunol Res. 2015;61(1–2):141–6.
Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung HP, Miller DH, Montalbán X, Edan G. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol Am Med Assoc. 2014;71(3):306–14.
Bhargava P, Cassard S, Steele SU, Azevedo C, Pelletier D, Sugar EA, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials. 2014;39(2):288–93.
Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Kampman MT, Brustad M. Vitamin D: a candidate for the environmental effect in multiple sclerosis—observations from Norway. Neuroepidemiology. 2008;30(3):140–6.
Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proc R Soc Lond B Biol Sci. 2006;273(1600):2541–50.
Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7(3):369.
Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. 1994;36:25–8.
Sibley WA, Bamford CR. Clark K. NY: Triggering factors in multiple sclerosis, diagnosis mult sclerosis thieme-stratton; 1984. p. 14–22.
Balashov KE, Olek MJ, Smith DR, Khoury SJ, Weiner HL. Seasonal variation of interferon-γ production in progressive multiple sclerosis. Ann Neurol. 1998;44(5):824–8.
Killestein J, Rep MHG, Meilof JF, Ader HJ, Uitdehaag BMJ, Barkhof F, et al. Seasonal variation in immune measurements and MRI markers of disease activity in MS. Neurology. 2002;58(7):1077–80.
Panitch H, Haley A, Hirsch R, Johnson K. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;329(8538):893–5.
Sibley W, Bamford C, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;325(8441):1313–5.
Andersen O, Lygner P-E, Bergström T, Andersson M, Vablne A. Viral infections trigger multiple sclerosis relapses: a prospective sero epidemiological study. J Neurol. 1993;240(7):417–22.
Tremlett H, Van Der Mei IAF, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31(4):271–9.
Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(6):736–41.
Filippi M, Horsfield MA, Campi A, Mammi S, Pereira C, Comi G. Resolution-dependent estimates of lesion volumes in magnetic resonance imaging studies of the brain in multiple sclerosis. Ann Neurol. 1995;38(5):749–54.
Van Walderveen MAA, Barkhof F, Hommes OR, Polman CH, Tobi H, Frequin S, et al. Correlating MRI and clinical disease activity in multiple sclerosis relevance of hypointense lesions on short-TR/short-TE (T1-weighted) spin-echo images. Neurology. 1995;45(9):1684–90.
Frank JA, Stone LA, Smith ME, Albert PS, Maloni H, McFarland HF. Serial contrast-enhanced magnetic resonance imaging in patients with early relapsing–remitting multiple sclerosis: implications for treatment trials. Ann Neurol. 1994;36(S1):S86–90.
Bartness TJ, Goldman BD. Mammalian pineal melatonin: a clock for all seasons. Experientia. 1989;45(10):939–45.
Liebmann PM, Wölfler A, Felsner P, Hofer D, Schauenstein K. Melatonin and the immune system. Int Arch Allergy Immunol. 1997;112(3):203–11.
Maestroni GJM. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10(3):467–76.
Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189–200.
Natarajan R, Einarsdottir E, Riutta A, Hagman S, Raunio M, Mononen N, et al. Melatonin pathway genes are associated with progressive subtypes and disability status in multiple sclerosis among finnish patients. J Neuroimmunol. 2012;250(1):106–10.
Constantinescu CS, Hilliard B, Ventura E, Rostami A, Luzindole A. A melatonin receptor antagonist, suppresses experimental autoimmune encephalomyelitis. Pathobiology. 1997;65(4):190–4.
Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell. 2015;162(6):1338–52.
Kang J, Ahn M, Kim Y-S, Moon C, Lee Y, Wie M-B, et al. Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1. J Vet Sci Korean Soc Vet Sci. 2001;2(2):85–90.
Sandyk R, Awerbuch GI. Nocturnal plasma melatonin and alpha-melanocyte stimulating hormone levels during exacerbation of multiple sclerosis. Int J Neurosci. 1992;67(1–4):173–86.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Jin Y-P, de Pedro-Cuesta J, Söderström M, Stawiarz L, Link H. Seasonal patterns in optic neuritis and multiple sclerosis: a meta-analysis. J Neurol Sci. 2000;181(1):56–64.
Spelman T, Gray O, Trojano M, Petersen T, Izquierdo G, Lugaresi A, et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol. 2014;76(6):880–90.
Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20(1):133–40.
Simpson S, Taylor B, Blizzard L, Ponsonby A, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68(2):193–203.
Lucas RM, Ponsonby A-L, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76(6):540–8.
Auer DP, Schumann EM, Kümpfel T, Gössl C, Trenkwalder C. Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;47(2):276–7.
Rovaris M, Comi G, Sormani MP, Wolinsky JS, Ladkani D, Filippi M. Effects of seasons on magnetic resonance imaging-measured disease activity in patients with multiple sclerosis. Ann Neurol. 2001;49(3):415–6.
Killestein J, Rep MHG, Barkhof F, Roos MTL, Adèr HJ, van Lier RAW, et al. Active MRI lesion appearance in MS patients is preceded by fluctuations in circulating T-helper 1 and 2 cells. J Neuroimmunol. 2001;118(2):286–94.
Meier DS, Balashov KE, Healy B, Weiner HL, Guttmann CRG. Seasonal prevalence of MS disease activity. Neurology. 2010;75(9):799–806.
Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72(2):234–40.
Zivadinov R, Treu CN, Weinstock-Guttman B, Turner C, Bergsland N, O’Connor K, et al. Interdependence and contributions of sun exposure and vitamin D to MRI measures in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(10):1075–81.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444.
Stewart N, Taylor B, Ponsonby A-L, Pittas F, Van Der Mei I, Woods G, et al. The effect of season on cytokine expression in multiple sclerosis and healthy subjects. J Neuroimmunol. 2007;188(1):181–6.
Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134(1):128–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Abdulla Watad and Shir Azrielant have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Watad, A., Azrielant, S., Soriano, A. et al. Association between seasonal factors and multiple sclerosis. Eur J Epidemiol 31, 1081–1089 (2016). https://doi.org/10.1007/s10654-016-0165-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-016-0165-3