Abstract
The availability of potable drinking water is a tough challenge particularly in arid and semiarid regions as it is closely linked to human health. Fluoride and nitrate are widely reported concern in different districts of Rajasthan. Therefore, this study was engaged in the Churu District of Rajasthan to appraise the water quality especially in reference to fluoride and nitrate and health risk associated with its consumption. The overall potability of water was evaluated using water quality index and PCA indicated major sources responsible for water contamination. A total of 515 groundwater samples were collected from different locations of Churu District and16 water quality parameters were analyzed as per the standard protocol of APHA. The results showed that the values for all analyzed water quality parameters were greater than the prescribed limit of WHO and BIS. F− levels in 191 samples and nitrate levels in 147 samples were found to be over than BIS-acceptable limit. The results of the fluoride and nitrate risk assessment revealed that the Hazard Index value was greater than one of 393 groundwater samples for males, 403 groundwater samples for females, and 397 groundwater samples for children, indicating that drinking groundwater poses a significant health risk in the study area. Only 46.02 percent of groundwater samples may be utilized for drinking, according to the water quality index (WQI), while the remaining are unfit for drinking purpose without treatment. The huge number of variables impacting the overall quality and chemistry of groundwater were reduced using principal component analysis (PCA), which identified four key components that account for 69.11 percent of variance in the dataset. The PCA indicated that both geogenic and anthropogenic factors significantly influenced the water quality of the study region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In both urban and rural India, groundwater pollution is a major problem (Jandu et al., 2021; Reddy et al., 2016). Pure drinking water is the elixir of life and the most basic necessity of human civilization, but its quality is diminishing on a daily basis, threatening the supply of pure drinking water and posing other environmental concerns (Duvva et al., 2022). Anthropogenic issues such as over usage of fertilizer and pesticides, agricultural runoff, sewage mismanagement, city drain leakage, poultry and dairy animal waste, and personal waste have dramatically degraded groundwater supplies in recent decades (Rahman et al., 2021; Tanwer et al., 2022a). Groundwater and surface water are the two most important water resources for drinking, agricultural, and industrial needs. Because of its high quality, groundwater is ideally regarded to be a substantial asset used by a large portion of the population for drinking purposes (Kaur et al., 2019). Its use becomes more prevalent in arid and semiarid regions, where surface water resources are limited and of poor quality (Li & Qian, 2018). According to reports, groundwater provides 65 percent of the world's water need and nearly 80% of the drinking need of India's rural and urban populations. Groundwater use is far higher than the pace at which it can be replenished, particularly in Delhi, Rajasthan, Haryana, and Punjab (CWC, 2019; Jandu et al., 2021; Tanwer et al., 2022b). As a result, the ionic composition of water is altered. Further, the origin of F− in groundwater is from both environmental and geogenic sources, with geogenic sources accounting for the majority of inputs (Adimalla et al., 2020). F− levels in soil increased due to the addition of phosphatic fertilizers in the range of 217–454 mg/kg (Kashyap et al., 2020).
According to various reports, 200 million people rely on water supplies that contain excessive amounts of fluoride, which is having negative health effects on the consumers in more than 25 nations around the world (Ayoob & Gupta, 2006; Kimambo et al., 2019). Twenty percent of the fluoride-affected communities worldwide are in India (Gugulothu et al., 2022; Rao et al., 2021a; Subba Rao, 2011, 2018, 2021; Subba Rao et al., 2013, 2016, 2017, 2020, Tanwer et al., 2023). F− pollution (more than 1.5 mg/l, BIS permitted limit) has been found in the groundwater of 23 Indian states and in 85 percent districts of the state of Rajasthan (CGWB, 2018). The state of Rajasthan is particularly vulnerable and is host to almost ten percent of the world's fluoride-affected habitations (Singh et al., 2011). The fluoride concentrations in Jalore, Jaipur, Ajmer, Nagur, Pali, Jodhpur, and Sirohi Districts with average 2 mg/L, are the highly affected (Hussain et al., 2012; Munoth et al., 2015; Singh et al., 2011). On both sides of the Aravalli mountain range, in the Tonk-Alwar-Bhilwara region in eastern Rajasthan, there is a high fluoride concentration that makes the water unfit for human consumption. The district of Bharatpur was reported to have the highest fluoride level of 8.70 mg/L in groundwater (Munoth et al., 2015).
66 million Indians are suffering from skeletal and dental fluorosis due to F− intrusion in groundwater (Kashyap et al., 2020). According to the USEPA, it is a frequent and harmful substance found in groundwater (Sohrabi et al., 2021). Its presence in drinking water can result in dental caries (at levels of less than 0.50 mg/L), fluorosis (at levels of 1.50–5 mg/L), and bone fluorosis (at levels of 5–40 mg/L) (Kimambo et al., 2019). Neurological impairments, cancer, arthritis, thyroid, infertility, and hypertension are all potential risks of F− contaminated water (Kimambo et al., 2019).
NO3− is another well-known contaminant that may be found in groundwater as a result of both natural and anthropogenic causes. Nitrogenous fertilizer use, poor waste disposal, sewage mismanagement, fertilizer runoff, and dumpsite leachates are all major contributor to NO3− pollution of groundwater resources. Long-term exposure to NO3− in drinking water can result in methemoglobinemia (also known as “blue baby syndrome”) in infants and stomach cancer in adults (WHO, 2017). Newborns, children and pregnant women are prone to have higher negative effects due to the high quantity of NO3− in groundwater (Eggers et al., 2018). Thyroid abnormalities, teratogenesis, abortions, cancer-causing, mutagenesis, and other ailments are linked to chronic intake of NO3− contaminated water (Wu et al., 2018).
NO3− pollution in the groundwater is a severe concern and all the states have contamination greater than 45 mg/l, (BIS permissible limit) (CGWB, 2018). The groundwater of Rajasthan state has a high nitrate concentration with levels ranging from 40 to 1000 mg/L (Ayyasamy et al., 2009). Various studies have been conducted in different districts of Rajasthan state to evaluate the groundwater quality, especially in reference to fluoride and nitrate. (Ahada & Suthar, 2017; Chaudhary & Satheeshkumar, 2018; Jandu et al., 2021; Joshi & Seth, 2011; Suthar et al., 2008, 2009). The majority of the region around Bhilwara, Rajsamand, Udaipur, and Dungarpur is classified as having a high nitrate content. Additionally, pockets of high nitrate concentration can be found all around in different regions (Munoth et al., 2015). The Jhalawar-Bundi-Baran belt, the area around Bikaner, south of Jaisalmer, and a few other spots throughout the rest of eastern Rajasthan have low to moderate concentrations of nitrate. The district of Chittaurgarh in Rajasthan recorded the highest level of nitrate at 1392 mg/L (Munoth et al., 2015).
In context to the scenario of groundwater contamination in Rajasthan, the present study was undertaken to investigate the potability and the significant risk associated with long—term consumption of F− and NO3− contaminated groundwater in the Churu District of Rajasthan. The study aims to appraise the water quality by comparing recommended limits by BIS and WHO and estimation health risk due ingestion of F− and NO3− through groundwater and take appropriate measure for safe and pure drinking water. The water quality index was used to assess the overall suitability of the water for drinking purposes. The correlation analysis calculated how anions and cations would affect the solubility and behavior of these contaminants in groundwater. Principle component analysis is a multivariate statistical approach for condensing a large number of variables into a few key components and used to reveal its source generation that impact groundwater quality.
Study region
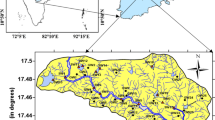
Churu District lies in north-eastern part of Rajasthan stretching between north latitudes 27°24 and 29°00 and east longitudes 73°40 and 75°38. It is stretched in an area of 16,830 km2 constituting approximately 4.92 percent of the total area of the state. Hanumangarh District borders it on the north, Bikaner District on the west, Nagaur District on the south, and Sikar and Jhunjhunu District and Hisar District on the southwest. The district's average annual rainfall is 269.60 mm (2006–2016). The southwest monsoon, which arrives in the district in the final week of June and leaves in the middle of September, accounts for over 95 percent of the total annual rainfall. Except for the monsoon season, the climate is mainly dry.
The aquifer system of Churu District may be separated into distinct hydrogeological units based on the nature of rock type and porosity. Unconsolidated alluvium, semi-consolidated sandstones, and consolidated schistose rocks are among the water-bearing formations in the Churu District. Aeolian sand and Quaternary alluvium forms an unconsolidated aquifer. Older and younger alluvium make up the aquifers, which cover 71% of the district's surface and are mostly made of sand from windblown and river sources. The alluvium is made up of fine to coarse-grained sand, gravel, and pebbles, as well as silt and clay with Kankar. Groundwater is present in an unconfined condition, and its saturation thickness ranges from a few meters to 60 m. Wells with specific capacities ranging from 1.85 to 19 lpm/m and medium to good transmittivity are available (CGWB, 2017). Sandstones (17.5%), schist (6.8%), and limestone (4.7%) aquifers are the district's next most notable aquifers. The saturation thickness, transmittivity levels of the aquifer ranges from 12 to 121 m; 8 to 1024 m2/day, respectively (CGWB, 2017). The southern and western portions of the district include significant amounts of hardrock aquifers. The groundwater in the faulted zone of limestone is fresh, and the aquifer produces a plentiful discharge. The majority of the district is covered with aeolian sand and quaternary alluvium, which is the region's primary source of groundwater (CGWB, 2017). The detailed stratigraphic data showing super-groups, groups, formation and lithology of Churu District taken from Geological Survey of India is given in Table 1.
Materials and Methods
Sampling
The district was completely covered using systematic grid sampling (6 × 6 km2) as indicated in Fig. 1. A total of 515
water samples were collected in December, 2020 to February, 2021. Groundwater samples were collected using pre-treated polyethylene bottles (washed overnight in 10% nitric acid and rinsed many times with distilled water to eliminate contaminants sticking to the inside wall of the container). The samples were collected from different source including municipal supply, submersible, hand-pump, and tube-well which were operated for 2–3 min to evacuate any standing water in the pipe and obtain a consistent and fresh sample. To limit the possibility of errors, sample bottles were washed with source water before being collected to eliminate any residues of acid or distilled water.
Physiochemical analysis of water quality parameters
To assess the quality and potability of groundwater, a total of 16 water quality parameters were estimated as per APHA (2017). 7 in situ water quality parameters comprising pH, conductivity, TDS, salinity, DO, ORP, and temperature were measured in the field using a multi-parameter analyzer (Water Analyser, 371, Systronic). Total Hardness (TH) was tested by titrating with standard solutions of EDTA, Ca2+ hardness by titrating against standard EDTA and magnesium hardness by deducting Ca2+ hardness from TH. Total Alkalinity (TA) was calculated by titration with standard H2SO4 and relationships were used to calculate carbonate and bicarbonates in the water sample. The argentometric titration was used to determine the Cl−, photo-spectrometry (UV–Visible spectrophotometer, Labman) used to estimate F−, SO42−, phosphate, and NO3− using the SPADNS dye, turbidity, stannous chloride, and UV-spectrometric method, respectively.
Estimation of health risks due to F− and NO3 −
Groundwater pollution of F− and NO3− is often greater than that of surface water (Shirke et al., 2020). Ingestion and epidermal contact are two possible ways that people might be exposed to contaminated water. This approach was first utilized by the US Environmental Protection Agency (USEPA) to quantify the risk of exposure to harmful contaminants from polluted water (USEPA, 2006). In comparison to the oral route of exposure, the epidermal route of exposure is considered insignificant (Jandu et al., 2021). For both F− and NO3−, the general health risk was evaluated for three segments of the population: males, females, and children. F− and NO3− are taken to be non-carcinogenic in living beings. This non-carcinogenic human health risk assessment approach is extensively used to estimate the hazard of drinking contaminated water (Adimalla, 2019; Ali et al., 2019; Rao et al., 2020; Satyanarayana et al., 2017). The Chronic Daily Intake Dose (CDID) was determined using formula (1).
The values in the following equation are based on the most recent USEPA (2017) guideline, with some being changed and applied based on regional statistical data for residents of the researched area. Table 2 shows the specifics values of the various parameters considered for the human health risk assessment.
Hazard quotient
The hazard quotient was calculated as the ratio of Chronic Daily Intake Dose and reference dose (RD) of chronic oral exposure. The reference doses for F− and NO3− are 0.04 mg/kg/day and 1.6 mg/kg/day (USEPA, 2020). It was calculated by Eq. 2
Hazard Index
The hazard index was estimated by adding the hazard quotient due to the non-carcinogenic risk associated with the consumption of F− and NO3− in drinking water. It was computed using Eq. (3) as
HQf is the hazard quotient due to F− and HQn is due to NO3− present in water. The value of the hazard index is greater than one indicating significant non-carcinogenic health hazard due to consumption of contaminated water. Its value below one indicates that no significant health hazard is associated with the consumption of water (USEPA, 2014).
Water quality index (WQI)
The water quality index is the way to evaluate the overall quality of water by considering the effect of individual parameters. The quality of water was decided on the basic calculated WQI shown in Table 3. It was calculated by the weighted arithmetic index method using the following Eq. 4 (Brown et al., 1970)
where Wi is the unit weight of nth parameter considered in calculating the water quality index which is inversely proportional to the values of the recommended standards (Table 4), Qn is the quality rating of nth parameter taken to calculate the WQI. Unit weight (Wi) is calculated by Eq. 5:
where k is the constant proportionality and calculated as per Eq. 6, Sn is the permissible limit for a particular water quality parameter given by the standard agency
Quality rating (Qn) was calculated using Eq. 7:
where Pn is the actual value of the water parameter, Ci is the ideal concentration of parameter and is considered as 0 (Zero) except for pH and DO which are taken as 7 (Neutral) and 14.6 mg/L, respectively.
Quality control and assurance
All the chemicals were used of analytical grade and double distilled water was used throughout all analytical measurements. The analysis of water quality was ensured through procedural blank measurements, careful standardization, and spiked and triplicate measurements. The ion balance error percentage was estimated to ensure the accuracy of ions analysis. The ion balance error percent was estimated using the following Eq. 8:
The concentration of ions was converted from mg/L to meq/L by the following Eq. 9:
The percentage of ions balance error estimated for each sample was below 10% percent, which reflects the accuracy and reliability of analytical data (Rao et al., 2020). The reproducibility of analytical data was below 5%.
Statistical analysis
Descriptive statistical analysis of water quality data was performed using Microsoft excel professional plus 2019. The correlational analysis aids to determine the dependence and independence of water quality parameters. The significance of the Pearson correlation coefficient between the two parameters was determined with a 0.05 and 0.01 alpha level and a 95% and 99% confidence interval, respectively, and it was estimated using Origin 2019. Principal component analysis is a data reduction tool and which helps to infer major factors influencing the water quality. It is the common method for reducing data complexity. In this study, the sources of pollutants were revealed using principal component analysis (PCA). As a result, the different combinations of the ions in form of PCs can reveal details about the origins and sources of the geochemical processes as well as the poor quality of the groundwater. The Principal component analysis was performed using Origin 2019 software.
Results and discussion
Groundwater quality analysis
To evaluate the quality of the groundwater in Rajasthan's Churu District, systematic comprehensive sampling was implemented and 515 groundwater samples were taken. The samples were clear, odorless, and taken from potable water sources. Due to the region's dry and semiarid climate, there aren’t many surface water resources in the area, and locals mostly rely on groundwater for drinking needs. A summary of all the water quality parameters is shown in Table 5. The pH ranged varied from slightly acidic to alkaline range (6.96–9.50) with a mean and median value of 7.87 and 7.85, respectively. 92.43% of the samples fell within the BIS and WHO-recommended limit. According to BIS and WHO, the pH range for drinking water should be between 6.5 and 8.5. (BIS, 2012, WHO, 2017). The pH of 39 (7.57%) samples was found to be in the alkaline range, exceeding the BIS and WHO-recommended limit. TDS was found to range between 100.48 mg/L and 19,622.40 mg/L. TDS value was above the desired limit of 500 mg/L according to BIS in 427 (82.91%) of the samples. Furthermore, it was found that TDS surpassed the permitted limit of 2000 mg/L set by BIS in 180 (34.95%) of the samples. TDS exceeded the WHO-recommended limit in 336 (65.24%) of the samples. According to Freeze and Cherry, (1979) classification (Table 6), 179 samples (34.76%) fell into the fresh water group, whereas 325 samples (63.11%) belonged to the brackish type. Even more than 10,000 mg/L of TDS was present in 11 (2.14%) samples. These groundwater samples were placed into the saline category. With an average value of 3274.21 µS/cm, the conductivity of groundwater samples was found to vary from 157.00 µS/cm to 30,660.00 µS/cm. In 339 (65.83%) of the samples, the EC value was higher than the BIS-recommended limit of 1500 µS/cm, whereas the remaining samples were within the permissible range. EC value less than 1500 µS/cm indicates low enrichment of salts, classified as type I; between 1500 µS/cm and 3000 µS/cm indicates the moderate enrichment of salts, classified as type II and greater than 3000 µS/cm indicates the high enrichment of salts, classified as type III (Rao et al., 2012). EC value in 66% of samples was higher than the recommended limit of 1500 µS/cm as per BIS while others were within the standard limit. 29% of samples had moderate enrichment of salts while 37% of samples had high enrichment of salts from source rocks. This is because moderate and high salts enriched water samples belong to topographic lows (northern region), and follow the underground stream flow (Rao et al., 2012). The EC is also determined by the dissolved salts and results from the leaching and dissolution of host rocks aquifer material (Hem, 1991). An important measure to understand the nature and quality of water is ORP (oxidation–reduction potential) that ranged between 5 and 356 mV. It shows that water is primarily oxic (oxidizing), which is considered to be beneficial. The observed salinity value ranged from 73.79 mg/L to 14,410.20 mg/L, with an average value of 1534.18 mg/L. In 324 (62.91%) of the samples, the F− level was below the BIS-desirable limit. The F− concentration in 76 samples above the BIS and WHO permissible level. According to BIS and WHO, the permissible limit of F− in groundwater is 1.5 mg/L whereas the desired limit is 1.0 mg/L as per BIS. (BIS, 2012; WHO, 2017). Water containing 1 mg/L F− is needed for microbiological protection and to prevent tooth decay, while water containing more than 1.5 mg/L F− can lead to skeletal and dental fluorosis. As a result, dental fluorosis and skeletal fluorosis are likely to be caused by 14.76% of samples. The high concentration of F− in groundwater increases the incidence of issues including dental fluorosis, yellowing of tooth, aching in the shoulders and legs, twisting of the bones, and deformities (Kothari et al., 2021). F− pollution in groundwater had a significant impact on the north and north-eastern, as well as some central portions of the Churu District, as illustrated in Fig. 2.
The dissolution of F−in groundwater is influenced by the geological composition. In the area, there are granite gneisses, schist, quaternary alluvium, and aeolian sand rocks that are primarily responsible for significant F− contents in the groundwater (Selvakumar et al., 2017). Both natural (geogenic) and anthropogenic sources contribute to fluoride in groundwater. The minerals such apatite, biotite, hornblende, and clay are the natural contributor while agricultural fertilizers especially phosphatic are the anthropogenic contributor of fluoride in groundwater having pH in alkaline range (Subba Rao, 2018). Previous investigation in northern Rajasthan conducted by Suthar et al. (2008) overserved that 90% of the samples were having the fluoride content higher than recommended limit of BIS.
The Cl− content in 237(46.02%) samples was exceeding the desirable limit of BIS. However, 39 (7.57%) of samples, the Cl− was greater than the permissible limit of 1000 mg/L as per BIS. The recommended limit of Cl− for drinking water is 200 mg/L as per the guidelines of WHO (WHO, 2017). 55.73% samples were observed have Cl− content higher than the recommended limit of WHO. Along with geological composition, Cl− concentration in groundwater is influenced by anthropogenic activities consisting of improper management of septic tanks, sewage disposal, and animal waste, which causes the mixing of sewage in the groundwater table. However, it also depends upon soil porosity and rock permeability of the area (Kothari et al., 2021). The Cl− level of 72% of the samples was below the WHO limit. Groundwater samples from the Churu District were found to contain NO3− in concentrations ranging from 0.80 mg/L to 498.70 mg/L, with a mean value of 44.57 mg/L.; 45 mg/L is the desired/acceptable limit of NO3− in drinking water (BIS, 2012); 50 mg/L as per WHO (WHO, 2017) 147 (28.54%) had NO3− level above the BIS permissible limits. However, 128 (24.85%) of the samples even went beyond the WHO-recommended limit. Nitrate has non-lithological source and under natural circumstances it doesn't exceed above 10 mg/L (Hem, 1991; Rao et al., 2021b). Therefore, the increasing NO3− content above this threshold is a sign of human-caused pollution (Wu & Sun, 2016). The possible source of nitrate contamination includes sewage waste, septic tank leaks, agricultural fertilizer, and animal waste on the aquifer system resulting in NO3− contamination of groundwater (Ullah et al., 2022). The NO3− contamination of groundwater largely affects the north, south, and south eastern regions as shown in Fig. 3.
The findings were consistent with those of earlier reports in the nearby district (Jandu et al., 2021). With a mean and median value of 86.93 mg/L and 67.90 mg/L, the SO42− was found to vary from 1.00 mg/L to 863.41 mg/L. It exceeded the BIS-desirable limit of 200 mg/L in 30 (5.83%) samples and the BIS permissible limit of 400 mg/L in 12 (2.33%) samples. The water may taste bad due to the unusually high sulfate level. In addition to anthropogenic inputs, the source of SO42− is minerals in the aquifer that come into contact with water and release SO42−, which gets dissolved in groundwater. The amount of phosphate in drinking water has no upper limit. Phosphate levels in groundwater were observed to be between BDL and 0.27 mg/L. The mean value of the TH was measured to be 918.08 mg/L, ranging from 24.31 mg/L to 10,649.50 mg/L. 407 samples of groundwater, or 79.03 percent, were found to fall into the very hard category, and 41 samples (7.96%) were found to fall into the soft category (Table 7).
There are 200 mg/L and 600 mg/L acceptable and permissible limits for hardness in samples of drinking water, respectively (BIS, 2012). The hardness value was higher than the desired limit in 397 (77.09%) groundwater samples and greater than the permissible limit in 214 (41.55%) samples. Only 71 samples (13.79%) had TH values below the allowable level, while the remaining 444 samples (86.21%) had TH values over the WHO guideline limit of 100 mg/L. Because of the local geology, significant cations like Ca2+ and magnesium dissolve in groundwater, contributing to its hardness. With an average of 172.80 mg/L, the Ca2+ level was found to range from 4.40 mg/L to 2571.40 mg/L. Ca2+ has an acceptable and permissible limit of 75 mg/L and 200 mg/L, respectively (BIS, 2012). Only 98 samples (19.303%) had Ca2+ levels that were over the permissible limit, whereas 312 samples (60.58%) exceeded the BIS and WHO Ca2+ desired limit. In 60.58% of the samples, the magnesium level was higher than the BIS's desired limit, but in 27.18% of the samples, it was higher than the permitted limit. The magnesium content had, however, exceeded the WHO-recommended limit in 44.85 percent of the groundwater samples. With the exception of 212 (41.16%) samples, TA in the remaining samples was below the 600 mg/L permitted level, but in 388 (75.33%) of the samples, it was above the BIS-acceptable range. With a mean value of 567.96 mg/L, the bicarbonate value was observed to range from 75.00 mg/L to 2250.00 mg/L. As phenolphthalein alkalinity was absent, the total alkalinity was only due to bicarbonate the high content of bicarbonate is because of soil atmosphere having higher partial pressure of CO2 which is the consequence of decomposing organic materials and root respiration that can enter the groundwater system. The soil CO2 then reacts with water to create HCO3−. Higher HCO3− in water indicates that mineral dissolution is more prevalent (Stumm and Morgan, 1996).
Health risk analysis due to F− and NO3 −
As F− and NO3− levels in the study area are greater than the BIS and WHO permitted limits. As a result, the health risk assessment had been conducted, and Table provides information on the estimated chronic Daily Intake Dose (CDID) for males, females, and children (Table 1s, supplementary information). According to certain specifications set forth for various gender and age criteria of residents of the studied area, the CDID calculates the F− or NO3− taken by men, women, and children. The Hazard Quotients (HQ) are used to calculate the non-carcinogenic risk of drinking water with F− and NO3−. When HQ is larger than 1, it means that drinking either F− or NO3− contaminated water poses a serious health risk. By combining the HQ values from F− and NO3−, the Hazard Index (HI) was determined. According to the USEPA (2014), HI values more than one suggest considerable risks from consuming F− and NO3− contaminated water. The estimated HI values for males, female, and children were 0.06–13.68, 0.07–14.93, and 0.07–14.07, respectively. ArcGIS 10.3 software was used to display the HI value for a groundwater sample taken from a specific location (Fig. 4). The HI value of 393 (76.31 percent) groundwater samples for men, 403 (78.25 percent) groundwater samples for women, and 397 (77.08 percent) groundwater samples for children was greater than 1, indicating considerable health risks associated with groundwater drinking. Because females had the highest average lifetime and exposure duration among the three, HI value was most critical for them. Females, children, and men were listed in decreasing order of the Hazard Index.
Water quality index (WQI)
The computation of the water quality index accounts for the contribution of each water quality parameter to the overall quality and potability of the water. The range of the water quality index was found to be 4.46 to 220.37. Figure 5 displays the various WQI categories, the quantity of samples, and their cumulative percentage. The results showed that 81 (15.73%) and 156 (30.29%) of the samples fell into the excellent (represented by the green circle in Fig. 6) and good (represented by the light green tringle in Fig. 6) categories, respectively, and could be utilized for industrial, agricultural, and drinking purposes.
144 (27.96%) groundwater samples were found to be in the poor category of WQI and are not appropriate for drinking but are suitable for industrial and irrigation purposes. 12.23% was found to have a very low WQI rating and could be utilized only for irrigation. 71 (13.78%) of the samples from the Churu District were found to have WQI values higher than 100, the lowest possible quality and unusable for any purpose. The groundwater from these sites has to undergo some sort of treatment before use. The north-northeast, central, and southern parts of the district account for the majority of samples with WQI values more than 100. Maximum F− and NO3− contamination in the groundwater was discovered in this area of the district. The high levels of F− and NO3− in the groundwater are the most likely reason for the elevated WQI in these samples. This is well depicted by comparing interpolation maps of F−, NO3− and WQI as shown in Figs. 2, 3 and 6, respectively.
Descriptive statistical analysis
Descriptive statistical analysis was performed for all samples and water quality parameters. Statistical parameters like mean, median, standard error, standard deviation, skewness, kurtosis, range and quartile was estimated. It was observed that data of all variables not symmetric (as the value of skewness is not equal to zero). Skewness in data could be positive or negative. If skewness coefficient is less than 0.5 and −0.5 is considered somewhat symmetric but not perfect. If skewness coefficient is greater than 0.5 and less than 1 or less than -0.5 but greater than −1, indicates the moderately skewed data. If it is greater than 1 and less than −1, indicates high degree of skewness in data. Except ORP, all the water quality parameters are positively skewed as shown in Table 6, i.e., median value is less than mean value. This indicated that water quality parameters values in 50% of samples were relatively higher than median which shifted the mean value at higher side. Similarly, NO3− dataset was highly positively skewed (3.56). The kurtosis infers about the peakedness and flatness in the dataset. The peaked data (Leptokurtic) infers that most value in the data lie around mean value and in more flat distribution (platykurtic), the values in data are away from the mean value of dataset. The kurtosis value 3 follow the normal distribution (Mesokurtic) of dataset while less than 3 or greater than 3 indicates platykurtic or leptokurtic data, respectively. In this study, debarring pH, ORP, DO, F−, TA and bicarbonates, the kurtosis in all water quality parameters is greater than 3 indicates Leptokurtic data, i.e., distribution has thin peak and thicker tale. Leptokurtic data as depicted from Table 8. TA and bicarbonate had 2.19 kurtosis resulting distribution of data somewhat similar to Mesokurtic while rest were platykurtic. Range indicates the extent of dataset distribution, calculated subtracting maximum from minimum. The third quartile of F− (1.09) was greater than desirable limit of 1 mg/L indicates that 25% samples had crossed the desirable limit of BIS but second quartile of F− (as median value is 0.74) of samples are less than desirable limit indicating 50% samples were less than the desirable limit as per the BIS. In case of NO3−, 3rd quartile was 49.65, indicates that approximately 75% samples had the NO3− content less than the standard limit of 50 mg/L as per WHO. The detailed description of all statistic of parameters is given in Table 8.
Correlation analysis
A correlation study was conducted to see whether the water quality parameters shown in Table 9 might be correlated. The Pearson correlation coefficient determines if there is a positive or negative connection between the parameters. The correlation coefficient was determined by taking into account 12 variables, including pH, TDS, EC, ORP, salinity, DO, F−, Cl−, NO3−, SO42−, TH, and TA. The correlation between the two parameters, however, also depends on the significance level of the analysis as determined by the t test, not merely the value of the Pearson correlation coefficient. The two-tailed t-test was used, and the p value was computed. The p value less than 0.01 (99 percent level of confidence) for correlation coefficients considered highly significant marked by double asterisks as shown in Table 9, and the coefficient bearing a p value less than 0.05 (95 percent level of confidence) was considered significant and was denoted by a single asterisk. Because the estimation of the parameters is reliant on one another, EC, TDS, and salinity have a linear relationship, and p values indicate that the correlation coefficient is significant. The correlation between TDS and Cl− (r = 0.797**), NO3− (r = 0.668**), TH (r = 0.939**), and TA (r = 0.395**) was found to be quite strong. The long-term flow of water in the groundwater zone is responsible for the leaching of anions and cations from rocks into groundwater, as shown by the very significant correlation of TDS with these parameters (indicated with double asterisks denoting p value is less than 0.01). F− has been found to significantly weakly correlate with salinity, TDS, EC, and DO. TDS (r = 0.395**), F− (r = 0.158**), Cl− (r = 0.303**), NO3− (r = 0.343**), and TH (r = 0.315**) were all shown to have a positive, statistically significant correlation with TA. The dissolution of calcium and magnesium carbonate rocks in the groundwater was indicated by the positive correlation of TA and TH. Most other measures have a strong negative correlation with dissolved oxygen.
Principle component analysis
The multivariate statistical analytical technique known as principle component analysis (PCA) condenses a large number of variables into a small number of principle components (PCs) that account for the majority of the variance in the data (Panghal et al., 2021). A huge dataset's variation and compositional patterns can be explained using this method, which also yields conclusions that are reasonable, impartial, trustworthy, and scientific. As a result, a multivariate dataset is summarized while reducing initial information loss, streamlining the data structure, and creating new, uncorrelated variables known as principle components from the original variables (Ahada & Suthar, 2017). The varimax rotation was employed on principal components (PCs) and Kaiser’s criterion was considered to select PCs having eigenvalues greater than one (Rao et al., 2020). Table 10 displays the PCs with the eigenvalue, factor loadings, percent variance, eigenvalue percent, and cumulative percent variance. Each PC's individual eigenvalue percent is shown in the scree plot (Fig. 7). The higher the parameter’s loading indicates the greater influence toward groundwater chemistry. Water quality parameter with loadings greater than 0.75, 0.75 to 0.50, and 0.50 to 0.30 are categorized as strong, moderate, and weak, respectively, based on the explained variance. PC 1 has eigenvalue of 6.90 which accounted for a variance of 43.11 percent. TDS, EC, salinity, Cl−, TH, and magnesium were contributed to the PC 1, which had numerous positive factor loadings larger than 0.3 (bold in Table 10 and represented in Fig. 8a). PC 1 revealed that both geogenic and anthropogenic sources influenced the groundwater quality in the study area (Ullah et al., 2021). TDS, EC, salinity, TH, and magnesium may be high due to erosion, dissolution, and leaching as a result of water–rock interaction, inferencing PC 1 contributors had geogenic origin (Rao et al., 2020; Ullah et al., 2021). While chloride sourced from domestic waste and agricultural additives imply anthropogenic influence (Gugulothu et al., 2022; Rashid et al., 2022). Ahada and Suthar (2017) in their study of groundwater analysis in North Rajasthan concluded that both geogenic and anthropogenic factors influenced the groundwater quality. PC 2 had eigenvalue of 1.83 which accounted for a variance of 11.43 percent. TA and bicarbonate are responsible for the factor loadings in PC 2 that are more than 0.3 as shown in Table 10 and Fig. 8b. As it was previously mentioned that the pH is controlled by the presence of soil CO2 combining with rainfall to generate HCO3−. The greater HCO3− concentration in the groundwater (the mean is 567.96 mg/L; Table 3) suggested that mineral dissolution predominates (Stumm and Morgan, 1996). PC 2 had shown that the natural factor is observed to influence nature of water PC 3 had eigenvalue of 1.23 which accounted for a variance of 7.68 percent. Only for two parameters, i.e., F− and SO42− of PC 3 displayed factor loadings greater than 0.3 as shown in Fig. 8c. F− generally dissolves from host rocks containing fluoride, indicated geogenic origination while sulfate in PC 3 indicated the dissolution of minerals like gypsum applied for soil amendment (Gugulothu et al., 2022). Eigenvalue of PC 4 was 1.10 which accounted for a variance of 6.89 percent. As shown in Fig. 8d, PC 4 also had two parameters, F− and phosphate which had factor loading larger than 0.3. The primary source of F− in groundwater is the dissolving of F− bearing minerals (hornblende, biotite, and apatite) found in rocks of the region and its dissolution more prevalent in an alkaline (HCO3−) environment indicated its geogenic origin (Reddy et al., 2016; Subba Rao et al., 2017). An enrichment of F− in groundwater is also due to the use of phosphate fertilizers, which infer the anthropogenic intervention. (Ayoob & Gupta, 2006).
Conclusion
F− and NO3− contamination in groundwater of Rajasthan is most concerning issue. To determine the groundwater potability and health risk due to consumption of F− and NO3− contaminated groundwater an extensive and systematic research of the Churu District was conducted, and the potability of the water was assessed using the water quality index. Correlation was estimated to comprehend the possible interaction between cations and anions present in groundwater and PCA was used to discover potential factors influencing groundwater chemistry and variables sharing a common source of origin in groundwater. In the study area, 515 groundwater samples were taken from different locations in the Churu District and analyzed for 16 water quality parameters using the standard methods. The results showed that the values of all quality parameters, including pH, EC, TDS, F−, chloride, NO3−, sulfate, total hardness, calcium, magnesium, and total alkalinity, were higher than the recommended limit of BIS, 2012, and WHO, 2017. F− and NO3− surpassed the allowed limit according to BIS (2012) in 14.76 percent of samples, and NO3− in 28.54 percent of samples. The drinking of F− and NO3− contaminated water is associated with considerable non-carcinogenic health risk, according to the results of the risk assessment for males, females, and children. Indications of severe health risks from drinking groundwater were found in 397 groundwater samples for children, 403 groundwater samples for women, and 393 groundwater samples for men. Order of frequency of health risk was female > children > male. According to the water quality index, just 46.02 percent of groundwater samples could be utilized for drinking, with the remaining samples requiring treatment to make them potable. Without undergoing an appropriate treatment, 13.78 percent of samples cannot be used for any purpose. At a degree of confidence of 99 percent, a correlation study found that numerous factors were significantly associated. At the 0.01 level of significance, it was found that TDS was correlated positively with EC, salinity, chloride, NO3−, sulfate, total hardness, and total alkalinity. Total alkalinity (TA) was reported to have a substantial positive correlation with total hardness, NO3−, and chloride. Data on water quality are typically positively skewed and leptokurtic, according to descriptive statistics. According to PCA and Kaiser’s criterion was considered to select PCs having eigenvalues greater than one found that the four PCs are responsible for 69.11% of the data variance. It decreased the 16 parameters into the four main factors influencing the chemistry of groundwater's 16 important water quality metrics. PCA indicated that first component that significantly influenced the water quality of district have geogenic and anthropogenic origin. Appropriate corrective actions such as water treatment, supplies that are safe to drink, defluoridation filters, calcium and phosphorus rich foods, rainwater harvesting and catchment methods, proper domestic waste disposal, proper landfill and septic tank construction, limited use of chemical fertilizers, and public awareness of the health hazards associated with fluoride and nitrate can help to control the water pollution and manage the health risk due to due to consumption of water of the area.
References
Adimalla, N. (2019). Human and ecological risk assessment : An international spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India assessment from nitrate in drinking water from semi-arid. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2018.1508329
Adimalla, N., Marsetty, S. K., & Xu, P. (2020). Assessing groundwater quality and health risks of fluoride pollution in the Shasler Vagu (SV) watershed of Nalgonda India. Human and Ecological Risk Assessment, 26(6), 1569–1588. https://doi.org/10.1080/10807039.2019.1594154
Ahada, C. P., & Suthar, S. (2017). Hydrochemistry of groundwater in North Rajasthan, India: Chemical and multivariate analysis. Environmental Earth Sciences, 76(5), 1–16.
Ali, S., Fakhri, Y., Golbini, M., Thakur, S. K., Alinejad, A., Parseh, I., Shekhar, S., & Bhattacharya, P. (2019). Concentration of fluoride in groundwater of India: A systematic review, meta- analysis and risk assessment. Groundwater for Sustainable Development. https://doi.org/10.1016/j.gsd.2019.100224
APHA. (2017). Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association/American Water Works Association/ Water Environment Federation.
Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking water: A review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 36(6), 433–487.
Ayyasamy, P. M., Rajakumar, S., Sathishkumar, M., Swaminathan, K., Shanthi, K., Lakshmanaperumalsamy, P., & Lee, S. (2009). Nitrate removal from synthetic medium and groundwater with aquatic macrophytes. Desalination, 242(1–3), 286–296.
CGWB. (2017). Groundwater brochure, Churu district, Western region Jaipur, Central Ground Water Board, Ministry of Water Resources, Government of India.
Chaudhary, V., & Satheeshkumar, S. (2018). Assessment of groundwater quality for drinking and irrigation purposes in arid areas of Rajasthan. India. Applied Water Science, 8(8), 1–17.
CWC. (2019). Water and related statistics. Central Water Commission, Department of Water Resources, RD and GR, Ministry of Jal Shakti, Government of India, New Delhi, India.
Duvva, L. K., Panga, K. K., Dhakate, R., & Himabindu, V. (2022). Health risk assessment of nitrate and fluoride toxicity in groundwater contamination in the semi-arid area of Medchal. South India. Applied Water Science, 12(1), 1–21. https://doi.org/10.1007/s13201-021-01557-4
Eggers, M., Doyle, J., Lefthand, M., Young, S., Moore-Nall, A., Kindness, L., Medicine, R. O., Ford, T., Dietrich, E., Parker, A., Hoover, J., & Camper, A. (2018). Community engaged cumulative risk assessment of exposure to inorganic well water contaminants, crow reservation, Montana. International Journal of Environmental Research and Public Health, 15(1), 76. https://doi.org/10.3390/ijerph15010076
Freeze, R. A., & Cherry, J. A. (1979). Groundwater. Prentice-Hall.
Gugulothu, S., Subba Rao, N., Das, R., Duvva, L. K., & Dhakate, R. (2022). Judging the sources of inferior groundwater quality and health risk problems through intake of groundwater nitrate and fluoride from a rural part of Telangana India. Environmental Science and Pollution Research, 29(32), 49070–49091. https://doi.org/10.1007/s11356-022-18967-9
Hem, J. D. (1991). Study and interpretation of the chemical characteristics of natural water: USGS professional paper book 2254. Scientific Publishers.
Hussain, I., Arif, M., & Hussain, J. (2012). Fluoride contamination in drinking water in rural habitations of Central Rajasthan. India. Environmental Monitoring and Assessment, 184(8), 5151–5158.
Jandu, A., Malik, A., & Dhull, S. B. (2021). Fluoride and nitrate in groundwater of rural habitations of semiarid region of northern Rajasthan, India: A hydrogeochemical, multivariate statistical, and human health risk assessment perspective. Environmental Geochemistry and Health, 43(10), 3997–4026. https://doi.org/10.1007/s10653-021-00882-6
Joshi, A., & Seth, G. (2011). Hydrochemical profile for assessing the groundwater quality of Sambhar lake City and its Adjoining area. Environmental Monitoring and Assessment, 174(1), 547–554.
Kashyap, C. A., Ghosh, A., Singh, S., Ali, S., Singh, H. K., Chandrasekhar, T., & Chandrasekharam, D. (2020). Distribution, genesis and geochemical modeling of fluoride in the water of tribal area of Bijapur district, Chhattisgarh, central India. Groundwater for Sustainable Development, 11, 100403. https://doi.org/10.1016/j.gsd.2020.100403
Kaur, L., Rishi, M. S., Sharma, S., Sharma, B., Lata, R., & Singh, G. (2019). Hydrogeochemical characterization of groundwater in alluvial plains of river Yamuna in northern India: An insight of controlling processes. Journal of King Saud University-Science, 31(4), 1245–1253. https://doi.org/10.1016/j.jksus.2019.01.005
Kimambo, V., Bhattacharya, P., Mtalo, F., Mtamba, J., & Ahmad, A. (2019). Fluoride occurrence in groundwater systems at global scale and status of defluoridation–State of the art. Groundwater for Sustainable Development, 9, 100223. https://doi.org/10.1016/j.gsd.2019.100223
Kothari, V., Vij, S., Sharma, S. K., & Gupta, N. (2021). Correlation of various water quality parameters and water quality index of districts of Uttarakhand. Environmental and Sustainability Indicators, 9, 100093. https://doi.org/10.1016/j.indic.2020.100093
Li, P., & Qian, H. (2018). Water resources research to support a sustainable China. International Journal of Water Resources Development, 34(3), 327–336. https://doi.org/10.1080/07900627.2018.1452723
Munoth, P., Tiwari, K., & Goyal, R. (2015). Fluoride and nitrate groundwater contamination in Rajasthan, India: A review. In 20th Inter. Conference on Hydraulics, Water Resources, and River Engineering. IIT Roorkee, India. (Vol. 2, No. 2859.6241)
Nair, P. K. M., & Augustine, L. F. (2018). Country-specific nutrient requirements & recommended dietary allowances for Indians : Current status & future directions. The Indian Journal of Medical Research. https://doi.org/10.4103/ijmr.IJMR
NIN-ICMR. (2011). Dietary guidelines for Indians -A Manual. National Institute of Nutrition-Indian Council of Medical Research.
NITI Aayog. (2020). Government of India. Retrieved July 01, 2020 from, https://niti.gov.in/content/life-expectancy
Panghal, V., Singh, A., Kumar, R., Kumari, G., Kumar, P., & Kumar, S. (2021). Soil heavy metals contamination and ecological risk assessment in Rohtak urban area, Haryana (India). Environmental Earth Sciences, 80(21), 1–20. https://doi.org/10.1007/s12665-021-10028-7
Rahman, A., Mondal, N. C., & Tiwari, K. K. (2021). Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan. India. Scientific Reports, 11(1), 1–13. https://doi.org/10.1038/s41598-021-88600-1
Rao, N. S., Dinakar, A., & Kumari, B. K. (2021a). Appraisal of vulnerable zones of non-cancer-causing health risks associated with exposure of nitrate and fluoride in groundwater from a rural part of India. Environmental Research, 202, 111674. https://doi.org/10.1016/j.envres.2021a.111674
Rao, N. S., Dinakar, A., Sravanthi, M., & Kumari, B. K. (2021b). Geochemical characteristics and quality of groundwater evaluation for drinking, irrigation, and industrial purposes from a part of hard rock aquifer of South India. Environmental Science and Pollution Research, 28(24), 31941–31961. https://doi.org/10.1007/s11356-021-12404-z
Rao, N. S., Ravindra, B., & Wu, J. (2020). Human and ecological risk assessment : An international geochemical and health risk evaluation of fluoride rich groundwater in Sattenapalle Region, Guntur district, Andhra Pradesh, India. Human and Ecological Risk Assessment: an International Journal. https://doi.org/10.1080/10807039.2020.1741338
Rao, S. N., Surya Rao, P., Venktram Reddy, G., Nagamani, M., Vidyasagar, G., & Satyanarayana, N. L. V. V. (2012). Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh. India. Environmental Monitoring and Assessment, 184(8), 5189–5214. https://doi.org/10.1007/s10661-011-2333-y
Rashid, A., Ayub, M., Khan, S., Ullah, Z., Ali, L., Gao, X., Li, C., El-Serehy, H. A., Kaushik, P., & Rasool, A. (2022). Hydrogeochemical assessment of carcinogenic and non-carcinogenic health risks of potentially toxic elements in aquifers of the Hindukush ranges, Pakistan: insights from groundwater pollution indexing, GIS-based, and multivariate statistical approaches. Environmental Science and Pollution Research, 29(50), 75744–75768. https://doi.org/10.1007/s11356-022-21172-3
Reddy, A. G. S., Reddy, D. V., & Kumar, M. S. (2016). Hydrogeochemical processes of fluoride enrichment in Chimakurthy pluton, Prakasam district, Andhra Pradesh. India. Environmental Earth Sciences, 75(8), 1–17.
Satyanarayana, E., Dhakate, R., Kumar, D. L., Ravindara, P., & Muralidhara, M. (2017). Hydrochemical characteristics of groundwater quality with special reference to fluoride concentration in parts of Mulugu-Venkatapur Mandals, Warangal district, Telangana. Journal of Geological Society of India, 89, 247–258. https://doi.org/10.1007/s12594-017-0597-8
Shirke, K. D., Kadam, A., & Pawar, N. J. (2020). Human and Ecological Risk Assessment : An International Health risk assessment and prevalence of fluoride in groundwater around the geological diversity of Ambadongar South Gujarat, India. Human and Ecological Risk Assessment: an International Journal. https://doi.org/10.1080/10807039.2020.1858270
Singh, P., Rani, B., Singh, U., & Maheshwari, R. (2011). Fluoride contamination in ground water of Rajasthan and its mitigation strategies. Journal of Pharmaceutical and Bio-Medical Sciences, 6(6), 1–12.
Sohrabi, N., Kalantari, N., Amiri, V., Saha, N., Berndtsson, R., Bhattacharya, P., & Ahmad, A. (2021). A probabilistic-deterministic analysis of human health risk related to the exposure to potentially toxic elements in groundwater of Urmia coastal aquifer (NW of Iran) with a special focus on arsenic speciation and temporal variation. Stochastic Environmental Research and Risk Assessment, 35(7), 1509–1528. https://doi.org/10.1007/s00477-020-01934-6
Stumm, W., & Morgan, J. J. (1981). Aquatic chemistry: Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. A Wiley-Interscience-Publication
Subba Rao, N. (2011). High-fluoride groundwater. Environmental Monitoring and Assessment, 176(1–4), 637–645. https://doi.org/10.1007/s10661-010-1609-y
Subba Rao, N. (2018). Groundwater quality from a part of Prakasam District, Andhra Pradesh India. Applied Water Science, 8(1), 1–18. https://doi.org/10.1007/s13201-018-0665-2
Subba Rao, N. (2021). Spatial distribution of quality of groundwater and probabilistic non-carcinogenic risk from a rural dry climatic region of South India. Environmental Geochemistry and Health, 43(2), 971–993. https://doi.org/10.1007/s10653-020-00621-3
Subba Rao, N., Deepali Marghade, A., Dinakar, I., Chandana, B., Sunitha, B., & Ravindra, T. B. (2017). Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environmental Earth Sciences. https://doi.org/10.1007/s12665-017-7093-8
Subba Rao, N., Dinakar, A., Surya Rao, P., Rao, P. N., Madhnure, P., Prasad, K. M., & Sudarshan, G. (2016). Geochemical processes controlling fluoride-bearing groundwater in the granitic aquifer of a semi-arid region. Journal of the Geological Society of India, 88(3), 350–356. https://doi.org/10.1007/s12594-016-0497-3
Subba Rao, N., Ravindra, B., & Wu, J. (2020). Geochemical and health risk evaluation of fluoride rich groundwater in Sattenapalle Region, Guntur district, Andhra Pradesh. India. Human and Ecological Risk Assessment, 26(9), 2316–2348. https://doi.org/10.1080/10807039.2020.1741338
Subba Rao, N., Subrahmanyam, A., & Babu Rao, G. (2013). Fluoride-bearing groundwater in Gummanampadu Sub-basin, Guntur District, Andhra Pradesh. India. Environmental Earth Sciences, 70(2), 575–586. https://doi.org/10.1007/s12665-012-2142-9
Suthar, S., Bishnoi, P., Singh, S., Mutiyar, P. K., Nema, A. K., & Patil, N. S. (2009). Nitrate contamination in groundwater of some rural areas of Rajasthan. India. Journal of Hazardous Materials, 171(1–3), 189–199.
Suthar, S., Garg, V. K., Jangir, S., Kaur, S., Goswami, N., & Singh, S. (2008). Fluoride contamination in drinking water in rural habitations of Northern Rajasthan. India. Environmental Monitoring and Assessment, 145(1), 1–6.
Tanwer, N., Anand, P., Batra, N., Kant, K., Gautam, Y. P., & Sahoo, S. K. (2022a). Temporal Distribution of Uranium and its Correlation with Varied Physicochemical Parameters in the Groundwater Samples of Panipat, Haryana, India. Journal of the Geological Society of India. 98, 926–932. https://doi.org/10.1007/s12594-022-2096-9
Tanwer, N., Deswal, M., Khyalia, P., & Singh, J. (2023). Fluoride and nitrate in groundwater : a comprehensive analysis of health risk and potability of groundwater of Jhunjhunu district of Rajasthan, India. Environmental Monitoring and Assessment, 1–21. https://doi.org/10.1007/s10661-022-10886-z
Tanwer, N., Khyalia, P., Meena Deswal, J. S., & Laura, B. K. (2022b). Spatial distribution of uranium in groundwater and its health risk assessment in Haryana, India. Rasayan Journal of Chemistry, 15(01), 343–349. https://doi.org/10.31788/RJC.2022.1516608
Ullah, Z., Yifan, X., Zeng, X.-C., Rashid, A., Ali, A., Iqbal, J., Almutairi, M. H., Aleya, L., Abdel-Daim, M. M., & Shah, M. (2022). Non-carcinogenic health risk evaluation of elevated fluoride in groundwater and its suitability assessment for drinking purposes based on water quality index. International Journal of Environmental Research and Public Health, 19(15), 9071. https://doi.org/10.3390/ijerph19159071
USEPA. (2006). USEPA region III risk-based concentration table: technical background information. United States Environmental protection Agency
USEPA. (2014). Human Health Evaluation Manual, Supplemental Guidance: Update of Standard Default Exposure Factors-OSWER Directive 9200.1–120, p. 6). United States Environmental Protection Agency, Washington, DC, USA.
USEPA. (2017). Regional screening levels (RSLs)–generic tables; Retrieved May 10, 2022, from https://www.epa.gov/risk/regional-screening-levels-rsls-generictables.
USEPA. (2020). Regional screening levels (RSLs)–generic tables; Retrieved May 10, 2022, from, https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables
Wagh, V. M., Panaskar, D. B., Mukate, S. V., & Nashik, R. B. (2019). Human and Ecological Risk Assessment : An International Nitrate associated health risks from groundwater of Kadava River Basin Nashik, Maharashtra, India. Human and Ecological Risk Assessment: An International Journal, 0(0), 1–19. https://doi.org/10.1080/10807039.2018.1528861
WHO. (2017). Guidelines for drinking water quality: Fourth edition incorporating the first addendum. World Health Organization.
Wu, J., & Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Exposure and Health, 8(3), 311–329.
Wu, J., Yin, Y., & Wang, J. (2018). Hydrogen-based membrane biofilm reactors for nitrate removal from water and wastewater. International Journal of Hydrogen Energy, 43(1), 1–15. https://doi.org/10.1016/j.ijhydene.2017.10.178
Acknowledgements
The TSC-4, NRFCC, BRNS, HPD, HS&E Group, BARC, and NUP members who continuously supported the project's execution are thanked by the authors. Maharshi Dayanand University in Rohtak provided all the facilities needed to complete this project, which the authors are extremely grateful for. The authors thank Abhishek Kumar, a research scholar in the geography department at Maharshi Dayanand University, Rohtak, Haryana, for his technical of ArcGIS.
Funding
This study was financed by the Department of Atomic Energy's Board of Research in Nuclear Sciences under the National Uranium Project, with sanction number. 36(4)/14/18/2017-BRNS dated 04-12-2017.
Author information
Authors and Affiliations
Contributions
Naresh Tanwer has done experimental, analysed data and wrote initially draft of manuscript. Meena Deswal helped in experimental and analysis of data. Pradeep Khyalia helped in experimental and analysis of data. Jitender Singh Laura conceptualized the work and critical reviewing of manuscript. Babita Khosla conceptualised the work, drafting and critical reviewing of manuscript. All authors have read, understood, and have complied the work done in the paper
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with the publication
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanwer, N., Deswal, M., Khyalia, P. et al. Assessment of groundwater potability and health risk due to fluoride and nitrate in groundwater of Churu District of Rajasthan, India. Environ Geochem Health 45, 4219–4241 (2023). https://doi.org/10.1007/s10653-023-01485-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01485-z