Abstract
Chromium (Cr) is a common environmental contaminant due to industrial processes and anthropogenic activities such as mining of chrome ore, electroplating, timber treatment, leather tanning, fertilizer and pesticide, etc. Cr exists mainly in both hexavalent [Cr(VI)] and trivalent [Cr(III)] form, being Cr(VI) with non-degradability and potential to be hidden, thereby affecting surrounding environment and being toxic to human health. Therefore, researches on remediation of Cr pollution in the environment have received much attention. Biochar is a low-cost adsorbent, which has been identified as a suitable material for Cr(VI) immobilization and removal from soil and wastewater. This review incorporates existing literature to provide a detailed examination into the (1) Cr chemistry, the source and current status of Cr pollution, and Cr toxicity and health; (2) feedstock and characterization of biochar; (3) processes and mechanisms of immobilization and removal of Cr by biochar, including oxidation–reduction, electrostatic interactions, complexation, ion exchange, and precipitation; (4) applications of biochar for Cr(VI) remediation and the modification of biochar to improve its performance; (5) factors affecting removal efficiency of Cr(VI) with respect to its physico-chemical conditions, including pH, temperature, initial concentration, reaction time, biochar characteristics, and coexisting contaminants. Finally, we identify current issues, challenges, and put forward recommendations as well as proposed directions for future research. This review provides a thorough understanding of using biochar as an emerging biomaterial adsorbent in Cr(VI)-contaminated soils and wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is the seventh most abundant element on earth (Jaishankar et al. 2014), which is classified as the first carcinogenic element (Zhitkovich 2011) and is also considered as the fifth of potentially toxic elements (PTEs) (Ma et al. 2007). Cr exists in six oxidation states in the various ranges of Eh and pH in soil, among which trivalent [Cr(III)] and hexavalent [Cr(VI)] are the two stable states in the natural environment. The Cr(III) is deemed as an indispensable trace element for human health. Cr(III) is easily hydrolyzed in aqueous solution, and its compounds [i.e., Cr(OH)3, Cr2O3] are adsorbed by soil colloid to form less soluble and relatively inert complexes (Mishra and Bharagava 2016). While Cr(VI) is soluble, mobile, and poisonous in wide pH range of aqueous media, it mainly exists in the form of HCrO4−, Cr2O72−, and CrO42− (Reale et al. 2016; Ashraf et al. 2017). Cr(VI) is very reactive in soils and wastewater due to its solubility, non-degradability, mobility, and bioaccumulation (Jaishankar et al. 2014). This stage in soil is toxic, leads to adversely affect on the ecological sustainability, and potentially harmful to human health transmitted by a series of food chain (Mishra and Bharagava 2016; Antoniadis et al. 2018).
Recently, the environmental pollution of Cr has gained public attention due to its high concentrations in surrounding environment. In China, 20 hm2 of cultivated land is polluted by potentially toxic elements, which accounted for approximately 20% of the total arable land. The area of Cr-contaminated land constitutes 5.1% of the total PTEs-contaminated land, of which Cr pollution in China exceeds the risk value (GB 15618-2018) in the national soil sampling points by 1.1% (MEEPRC 2015). Some typical Cr(VI) concentrations of water or wastewater in contaminated sites vary in the range of 30–200 mg L−1 (e.g., Lu et al. 2006; Ai et al. 2008). However, the discharge of Cr(VI) concentration must be controlled under the limit of 0.05 mg L−1 in surface water (Zhou et al. 2016; Zelmanov and Semiat 2011) and 0.5 mg L−1 in wastewater in most countries (Costa and Klein 2006). Therefore, it is pertinent that the remediation methods should be aimed at decreasing the Cr(VI) concentration of effluents within permissible level.
Many techniques have been implemented to treat Cr(VI) pollution in soils and waters, including chemical reduction, electrochemical method, nanofiltration, membrane process, and biological remediation (Malaviya and Singh 2011; Dhal et al. 2013; Khalid et al. 2017; Jin et al. 2016). The adsorption method has emerged to be the most effective technique for Cr(VI) treatment because of its desirable characteristics such as no/minimum disturbance to the soil, economic efficiency, absence of secondary toxic slurries, in situ remediation, simple operation, and high selectivity (Velez et al. 2016; Jobby et al. 2018). In this regard, it is necessary to find practical materials that can adsorb and decrease Cr(VI) concentration or reduce Cr(VI) to Cr(III) to lower its toxicity.
Biochar is a stabilized, porous, fine-grained, and recalcitrant organic carbon compound (Panwar et al. 2019). It is produced under low (preferably zero) oxygen condition at temperatures usually varying from 300 to 1000 °C (Cha et al. 2016). Many researches have demonstrated that biochar could enhance carbon sequestration in soil environment (Nanda et al. 2016), increase crop yield (Crane-Droesch et al. 2013), control greenhouse gases emissions (Wu et al. 2018), improve soil quality (Agegnehu et al. 2017), decrease nutrient leaching (Yuan et al. 2016), and reduce irrigation and fertilizer requirements of agricultural soils (Ramlow et al. 2019). Due to its highly developed porosity, high degree of surface reactivity, and excellent adsorption capacity, biochar has been increasingly recognized as the great potential to remove organic contaminants (He et al. 2018; Huang et al. 2018; Luo et al. 2018) and PTEs (Shaheen et al. 2019; Sun et al. 2019), especially for Cr(VI) with highly redox activity (Fan et al. 2019; Zhou et al. 2019; Zhang et al. 2019) in soil and aqueous media.
Although there are many current studies on the effects of feedstock-specific biochar on Cr(VI) remediation available in the literature, few thorough reviews have thus far been published about Cr(VI) remediation by biochar. Indeed, the complete mechanisms and immobilization processes by biochar are still largely unrecognized, particularly for redox-sensitive PTEs including Cr(VI). The potential of Cr remediation by biochar under different reaction conditions also remain not reviewed, and the underlying essences of these influencing factors on removal efficiency even remain unresolved. Therefore, it is essential to underpin the modification mechanisms of biochar to improve its Cr sorption capability. Understanding these processes and mechanisms is also a key factor in evaluating reality and feasibility of applying biochar to remediate Cr(VI)-contaminated soils and wastewater.
Hence, this review focuses on the different dimensions of Cr remediation trials using biochar. Here, we slightly summarized Cr chemistry, the source and use of Cr, and toxic as well as health effects of Cr. We introduced the feedstock-dependent production of biochar and its application potential on Cr(VI) remediation. We also critically elucidated the processes and mechanisms of immobilization and removal of Cr by biochar, and latest research progress on biochar modification to improve Cr adsorption capacity, as well as the factors affecting removal efficiency of biochar for Cr(VI) in response to external conditions. Lastly, we identified current issues, challenges, and propose recommendations as well as future research directions in this field.
Chromium in the environment
Chromium species and characteristics
Understanding the chemical species of Cr is necessary to evaluate the ecological hazards and risk assessment concerned with Cr-contaminated soils and wastewater. The total Cr contents in soils usually do not explain its biogeochemical behavior, which is governed by different chemical species. Cr is in the form of different valence states, varying from 0 to VI, in which Cr(III) and Cr(VI) are the predominant and stable species in natural conditions (Fig. 1) (Choppala et al. 2013; Wang et al. 2015; Arian et al. 2018). Cr(III) mainly exists in the form of Cr(OH)2−5 , Cr(OH)−4 , Cr(OH)3, Cr(OH)+2 , Cr(OH)2+, and Cr3+ and usually combines easily with oxygen, sulfate, hydroxide, and organic matter (OM) to form insoluble chelates or is adsorbed by soil colloids to constitute Cr(III) precipitate, which has the characteristics of insolubility, low migration, and low bioavailability in the soil. Hence, Cr(III) toxicity to organisms is comparatively lower than Cr(VI) (Rakhunde et al. 2012; Saha et al. 2011). However, its disposal as soluble compounds into soils and waters may cause a serious health risk because Cr(III) will be oxidized to Cr(VI) in the natural environment (Fendorf et al. 2000). The Cr(VI) usually occurs as oxyanions with strong oxidation performance, namely Cr2O72−, HCrO4− (pH < 6.0), and CrO42− (pH > 6.0). These hexavalent compounds are highly soluble and mobile and easily migrate in soil and its pore water and hence are difficult to get adsorbed by soil colloid, thus causing seriously toxicity to living organisms (Dotaniya et al. 2014). The other transitional species including Cr(V), Cr(IV), and Cr(II) are normally unstable when occurred to oxidize and reduce between Cr(III) and Cr(VI) (Shahid et al. 2017).
Chromium sources and uses
High concentrations of Cr(VI) in the environment usually come from anthropogenic activities (Fig. 2), especially widespread usage in industries because of its corrosion resistance, strong crystalline structure, and yellow color. Cr is one of the basic materials in tanning, electroplating, printing, dyeing, papermaking, wood preservation, alloy making processes, etc. (Dhal et al. 2013; da Costa Cunha et al. 2016). In China alone, metal finishing is the major industrial source of Cr(VI) for Cr-containing discharge wastewater. Besides, the tannery is one of the largest industries; approximately 40 million tons per year of Cr-containing waste is discharged by tanneries around the world (Papp 2004). Correspondingly, the number of published articles regarding “Hexavalent chromium” is inevitably increasing year by year from 2000 to 2018 (Fig. 3). Mohan and Pittman (2006) have reported that the discharge of Cr is at 142 and 896 metric tons/year into water and soil at the global scale, respectively. Deng and Chen (2012) analyzed Cr content in industrial and solid waste, blast furnace slag, paint coating sludge, and solid waste (waste III) and reported that they have reached up to 2600, 1500, and 1400 mg Cr kg−1, respectively. Furthermore, these industrial wastes containing Cr are usually used as landfill materials to reclaim marshlands and tank dikes at some locations. The seeping and leaching of Cr(VI) from polluted soil into groundwater would increase Cr(VI) concentration (Jobby et al. 2018). Hence, the discharge of Cr-containing waste by industries onto land and into water bodies is the foremost source of Cr in our living environment.

(Modified from Xia et al. 2019)
The source of chromium contamination and Cr remediation technologies by biochar

(Data from Cao and Jin 2015) and the published articles regarding “Hexavalent chromium” (Data from Google Scholar)
The discharge of chromium from different industries in the year of 2010 in China
Ross (1994) has grouped the anthropogenic sources of Cr pollution into five categories: (1) Cr-containing emissions into the atmosphere, mainly coming from chemical manufacturing and metallurgical industries, and the burning of fossil fuels (coal, oil, and gas) (Saha et al. 2011); (2) operation of solid wastes, consisting of fly ash, coal, and rubbish (Dhal et al. 2013); (3) roadside soils contamination coming from Cr-containing asbestos brake linings in vehicles and atmospheric aerosols (Saha et al. 2011); (4) improper use of fertilizer and pesticide (Vogel et al. 2015), e.g., the Cr levels in some phosphate fertilizers are ranging from 30 to 3000 mg kg−1 (NRCC 1976); (5) discharge of industrial waste such as sewage, sewage sludge, and solid wastes in the process of industrial production, especially in the Cr residue stacking site, where Cr pollution is more serious (Vimercati et al. 2017).
Maximum allowable levels of Cr in soils and water
Based on the critical values in soils formulated by Kabata-Pendias (2010), the trigger action value (TAV) and the maximum allowable concentration (MAC) are 50–450 and 50–200 mg Cr kg−1, respectively. Certainly, the environmental quality standards of Cr for agricultural soil are the most concerned issue for human health, and regulations are vary from one country to another (Antoniadis et al. 2019). In China, government department, in order to safeguard the agricultural production and protect human health, have regulated the risk value of Cr limits of the environmental quality standards (EQS) under different pH ranges of pH < 6.5, 6.5 ≤ pH ≤ 7.5, pH > 7.5. For the dry lands, the limitations are ≤ 150, ≤ 200, ≤ 250 mg kg−1, and for paddy fields the corresponding values are ≤ 250, ≤ 300, ≤ 350 mg kg−1, respectively (GB 15618-2018). The maximum allowable concentration of total Cr for agricultural soils in Serbia, Austria, Poland, and Czech Republic are 100, 100, 150, and 100–200 mg Cr kg−1, respectively (Ding et al. 2014), while the acceptable concentration for Canada has been assessed at 64 mg kg−1 concerning the protection of environmental quality and human health (CCME 2015). The health investigation levels (HIL) of Cr is also in view of the land use practices in the contaminated sites. For Australia, HIL for Cr(VI) in residential areas, recreational, and commercial/industrial areas is 100–500, 300, and 3600 mg kg–1, respectively (NEPM 2013). Correspondingly, the critical value of Cr is regulated with 30 mg kg−1 for Cr(VI) and 1000 mg kg−1 for total Cr in the commercial land in China, respectively (Lyu et al. 2018).
The maximum recommended concentration of Cr(VI) in drinking water for many countries is limited to 0.05 mg L−1 (Lilli et al. 2015), while the maximum total Cr level in drinking water set by USEPA (1995) is 0.1 mg L−1. Zayed and Terry (2003) defined that the critical limits for Cr(III) levels in sea water, fresh water, and irrigation water are 50, 8, and 5 μg L−1, while these for Cr(VI) are 1, 1, and 8 μg L−1, respectively. The surface and wastewater discharge, to some extent, affect groundwater quality, especially for drinking water. In China, the discharge limit of Cr(VI) is regulated with 0.05 mg L−1 in surface water (Zhou et al. 2016; Zelmanov and Semiat 2011) and 0.5 mg L−1 in wastewater (PRC 1996). Nevertheless, in some areas developed by ultramafic rocks, the recorded values in groundwater resources remarkably exceed the limits of 0.05 mg L−1 by the World Health Organization (WHO) for drinking water, e.g., the value of the province of La Spezia, Italy, the Mojave Desert, USA, and New Caledonia have reached 0.005–0.073, 0.06, and 0.7 mg L−1, respectively (Fantoni et al. 2002; Ball and Izbicki 2004).
Cr toxicity and health
Chromium is a common dietary supplement (Table 1). The Cr(III), an necessary trace element for human health which exerts an indispensable function in the metabolism of sugar, lipid and proteins. In addition, Cr(III) is also found in different organs of the human beings, and hair has the most accumulation of Cr with 0.234–3.8 mg kg−1. However, high concentrations Cr(III) in the human body can cause negative effects on cellular structures. Headlam and Lay (2016) have evidenced that Cr(III) in dietary supplements can be partly oxidized to Cr(IV), Cr(V), and Cr(VI) in vivo through intracellular oxidation in the process of metabolism. Compared with Cr(III), Cr(VI) is about 100 times more toxic to living organisms. Most importantly, Cr is a non-degradable metal element (Jobby et al. 2018), which persists in nature environment and enriches in a series of food chain. Therefore, it can reach ecological risk for environment and detrimental levels for human beings over time. This warns us that people should eat less Cr-containing foods.
The health problem in human results from the high concentrations of Cr(VI) in surrounding environments which exceeds the global allowable levels in soils and groundwater (Gil-Cardeza et al. 2014; Shahid et al. 2017; Ballesteros et al. 2017). The toxic sites have been reported that there examined over 300 Cr-contaminated sites around the world, and it was estimated that about 16 million people are at risk due to Cr exposure (Pure Earth 2018; Jobby and Desai 2017). For example, the Cr(VI) concentration in the drinking water has increased the village-level human carcinogenic incidences in some areas of Liaoning Province, China (Beaumont et al. 2008).
Cr(VI) is considered highly poisonous because it causes serious harm to human beings such as lung cancer, kidney dysfunction, diarrhea, ulcers, kidney, liver, and gastric damage, as well as eye and skin irritations (Fig. 2) (Cefalu and Hu 2004). It not only causes high mutagenicity, carcinogenicity, and teratogenicity but also leads to birth defects and decreases reproductive health (Saha et al. 2011; Mishra and Bharagava 2016). CrO42− can substitute the SO42− and will transport into human cells through the sulfate transport system (Zhitkovich 2011). The Cr(VI) toxicity is mainly attributed to its easy diffusion penetrating the cell membrane and accompanied with the Cr(VI) reduction to Cr(III) in cells creating free radicals such as peroxo ions (O2H−), hydroxyl ions (OH−), superoxide ions (O2−), and nascent oxygen (O), which may directly lead to DNA alterations and some related toxic effects (Jaishankar et al. 2014). Cellular metabolism of Cr(VI) bring about both non-oxidative and oxidative processes of DNA damage, of which Cr-DNA binding is one of the most common and specific types. It has been reported that this reaction has been observed in reduction reactions of different cultured cells in vitro, thus leading to mutations and chromosomal breaks in cells (Zhitkovich 2011). Moreover, the electrostatic interaction would occur between the negatively charged phosphate groups on DNA and the positively charged Cr(III) species, thereby forming Cr(III)-DNA complexes with mutagenicity and toxicity. These complexes will further affect the normal DNA replication, transcription and then further cause mutagenesis.
Some researchers have reported that the occurrence of DNA single-strand breaks was related to Cr(VI) metabolism (e.g., Messer et al. 2006), which can seriously impact the function of cells and further cause cancers in the kidney, liver, and lungs. In addition, different passivation would take place when the skin directly contacted with Cr(VI), and this would cause dermatitis, dermal corrosion, and dermal necrosis (Zhang et al. 2008). We found that exposures to Cr(VI) via dust inhalation for occupational people obviously increase the hazard of cancers in the respiratory system (Costa and Klein 2006).
Feedstock, production of biochar, and its applications for Cr remediation in soils and wastewater
In recent years, many researchers have been developing economical, environmentally friendly and practical remediation technologies for Cr(VI) contamination, in which biomaterial remediation is considered as an alternative and promising technique (Yahya et al. 2015). Biochar, with high sorption capacity owing to favorable porosity, large specific surface area, and surface activity, is being widely applied for the remediation of PTEs-contaminated soil and water resources (Xie et al. 2015; Shaheen et al. 2019).
Biochar is a carbon-rich material pyrolyzed by thermal decomposition of biomass under oxygen-limited conditions at temperature usually < 900 °C (Ahmed et al. 2016; Xin et al. 2017). The feedstock source and calcination temperature are the pivotal factors influencing the sorption efficiency of biochar for PTEs-contaminated soils and water. It is obtained from various biomass feedstocks through different thermal conditions (constant temperature, heating rate and residence time), in which the slow pyrolysis is one of the most widely adopted method due to its moderate treatment conditions and optimization of biochar production yields (Fig. 4) (Tripathi et al. 2016). Biochar has attracted more attention because of low cost, a wide range of raw materials, the convenience of raw material, relatively high sorption performance, and no secondary pollution (Wu et al. 2012; Sud et al. 2008; Keng et al. 2014).
Schematic diagram of various production processes of different feedstock-derived biochar. (Referred from Shaheen et al. 2019b). Note: In the left section, A is the wheat straw, representing agricultural and forest residues; B is cattle manure, representing animal wastes; C is sewage sludge, representing industrial by-products; D is waste tires, representing the non-conventional material. In the right of the chart, a is columnar biochar; b is granular biochar; c is powder biochar
Biochars are made from various feedstocks, which mainly consists of four categories that include: (1) agricultural and forestry residual biomass; (2) animal wastes; (3) industrial wastes; and (4) non-conventional wastes. Agricultural and forestry biomass are considered as the major contributor to biochar feedstock (Jindo et al. 2014; Inyang et al. 2016). Annual production of agricultural residues, on a global scale, can be used for biochar making that has been predicted about 500 million tons (Duku et al. 2011). Most residues are by-products and direct waste from processing and harvesting of crops, including corn stalks (Dong et al. 2017), soybean, peanut shells (Yuan et al. 2011), sugarcane bagasse (Nie et al. 2018), wheat straw (Lyu et al. 2017), bamboo, rice straw (Lu et al. 2017), coconut fiber (Wu et al. 2017), cassava residue (Huang et al. 2018), and forestry wood waste (Sun et al. 2019). Biochar feedstock derived from animal waste include dead animal including poultry and pigs (Yang et al. 2017; He et al. 2018; Qin et al. 2018), pig manure (Song et al. 2018), poultry litter (bedding materials, feathers, and spilled feed) (Sehrish et al. 2019), and dairy manure (Xu et al. 2013; Choppala et al. 2016; Mandal et al. 2017). More and more researchers are interested in the biochar feedstock of municipal and industrial wastes and their by-products. Some studies have indicated the biochar feedstocks generated from bioenergy facilities (e.g., digested residues) (Li et al. 2018) and wastewater treatment plants (e.g., sewage sludge) (Melo et al. 2019; Zhang and Tsang 2019). Alternative feedstock sources of some non-conventional materials for biochar production, including plastics (Gil et al. 2010), food wastes (Rhee and Park 2010), bones (Park et al. 2015), waste tires (Karakoyun et al. 2011), periwinkle shells (Bello and Ahmad 2011), diatoms and algae (Grierson et al. 2011; Yu et al. 2017), and bioenergy residues (Yao et al. 2015) are currently being explored.
There are a number of studies available in literature on Cr(VI) remediation in soils and waters by biochar (Table 2). A typical example is that the biochar derived from sugar beet tailing (SBT) has the maximum adsorption capacity of 123 mg g−1 under pH 2, and therefore, it has an excellent removal efficiency of Cr(VI) from aqueous media (Dong et al. 2011). Desorption and X-ray photoelectron spectroscopy experiment demonstrated that Cr(III) was the foremost form binding to SBT biochar. It was deduced that the Cr(VI) was first adsorbed by biochar via electrostatic attractions; then, it was reduced to Cr(III) by the OM contained in the biochar, and finally, complexation occur between Cr(III) and SBT biochar’s functional groups (Dong et al. 2011). The physicochemical properties of biochar suggests that it can act as an effective sorbent to absorb and remove Cr(VI) from wastewater or groundwater and can also be used as a reaction barrier for remedy efforts, which highlights the remediation potential of biochar as the filter medium for Cr(VI)-contaminated water.
Biochar also has a strong fixation capacity for PTEs in contaminated soils (Yang et al. 2016; Lu et al. 2017; Nie et al. 2018) including Cr (Yuan et al. 2017; Lyu et al. 2018). More importantly, biochar can ameliorate the soil structure and can be used as slow-release carriers of fertilizer and as an attachment sites for soil microbe (Li et al. 2019; Zhu et al. 2017), thereby it improves the potential of microbial remediation for Cr contamination (Wang et al. 2018). Hence, applications of biochar to fields have confirmed its effect on Cr(VI) sorption and reduction, and its potential on in situ remediation for PTEs-contaminated sites (Choppala et al. 2016; Mandal et al. 2017).
Processes and mechanisms of immobilization and removal of Cr in soils and wastewater
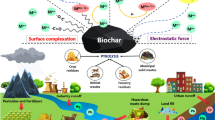
There are five mechanisms (Fig. 5) which govern the immobilization and removal of Cr from soils and wastewater that have been proposed in the literature (e.g., Inyang et al. 2016; Li et al. 2017), including metal complexation, electrostatic interaction, ion exchange, Cr(VI) reduction coupled subsequent sorption, and the formation of insoluble metal precipitates.

(Modified from Li et al. 2017)
Conceptual illustrations of Cr sorption mechanisms by biochar
Complexation
Both outer- and inner-sphere complexation processes of Cr with biochar control the immobilization and removal process. While the former is physical sorption where the metal ions can be directly adsorbed on the surface of biochar, the latter is involved in the interactions between metals and specific ligand forming the multi-atom structures (i.e., complexes). Specially, the oxygen-containing functional groups (e.g., phenolic, carboxyl, and lactonic) on biochars were evidenced to effectively bind Cr(III), following the first step involving the reduction of Cr(VI) to Cr(III) in the binding process (Li et al. 2017; Rosales et al. 2017). Oxygen content in biochar has also been demonstrated to increase with pyrolysis time or aging time, and this may be owing to the oxidation of biochar surface and then the formation of carboxyl groups (Harvey et al. 2011), and therefore metal complexation may enhance over time.
Electrostatic interaction
Electrostatic interaction between PTEs and charged biochar surfaces is another immobilization mechanism. The electrostatic process depends on pH in the media solution and point of zero charge (PZC) of biochar (Mukherjee et al. 2011; Dong et al. 2011). Biochar has been shown to adsorb both positively and negatively charged metal(loid) species, including CrOn−x (Solaiman and Anawar 2015). Chromium, not like other PTEs, shows a different behavior with biochar in soils and aqueous environments. The Cr species control its adsorption or immobilization mechanisms by biochar (Mandal et al. 2017; Li et al. 2017). When solution pH is more than pHPZC, the negatively charged biochar combines with metal cations such as Cr3+; when solution pH is less than pHPZC, the positively charged biochar combines with metal anions such as Cr2O72−, CrO42−, and HCrO4− (Shaheen et al. 2019). It was reported that pHPZC would improve with the increase in pyrolysis temperature in the process of biochar production, because the quantity of negatively charged functional groups (i.e., –COH, –COO−, and –OH) on biochars decreased, thus resulting in less negatively surface charges and increased pHPZC (Yuan et al. 2011). It has been reported that electrostatic attractions are more likely to occur between positively charged biochar surfaces and negatively charged Cr(VI) at pH 2, which will reach the maximum sorption capacity (Dong et al. 2011). In addition, another study demonstrated that carbonization high temperatures (> 400 °C) could facilitate the formation of graphene structures in biochars, which contribute to the occurrence of electrostatic attractions (Keiluweit and Kleber 2009).
Ion exchange
Sorption of PTEs through the exchange of ionizable protons/cations on biochar surfaces is another primary mechanism. The cation exchange capacities (CEC) of most biochars are comparatively high, partly because of their negatively charged surface which promotes affinity for PTEs such as Cr(III). CEC of different types of biochar significantly varies ranging from 4.5 to 40 cmol kg−1 (Bird et al. 2011; Rondon et al. 2007; Uzoma et al. 2011). Mineral components in biochar, such as potassium (K), magnesium (Mg), calcium (Ca), and phosphorus (P), are in charge of metal sorption from Cr-contaminated sites, especially aqueous environments. They can generate ion exchange or precipitate with PTEs and decrease their bioavailability (Li et al. 2017). During sorption of Cr cations onto biochar, base cations like Na+, K+, Ca2+, and Mg2+ were released into the solution media from biochar through cation exchange process (Uchimiya et al. 2010).
Sorption-coupled reduction
The sorption of Cr(VI) may be related to the redox reactions because of the high redox potential value (+ 1.3 V) of Cr(VI) (Yuan et al. 2017; Choppala et al. 2016). Biochar usually contains carbon reaching up to 30–70%, a source of protons which are essential for Cr(VI) reduction and serves as an immobilizer for Cr(III) (Rajapaksha et al. 2013). Biochar derived from agricultural and forestry residual biomass may yield abundant functional groups (e.g., C–O, C–OH, C–O–R), as the feedstocks have complicated heteropolysaccharides associated with galactose, arabinose, galacturonic acid, and some pectin substances (Aksu and Isoglu 2005). These oxygen functional groups can act as electron donors (π electrons) that promote the reduction of Cr(VI) to Cr(III) to facilitate surface sorption (Dong et al. 2014; Choppala et al. 2016; Shaheen et al. 2019).
Moreover, this reduction was also fulfilled by π-electrons offered by the disordered polycyclic aromatic hydrocarbons sheets (Wang et al. 2010). Specifically, biochar, pyrolyzed under higher temperatures, has more well-ordered graphene sheets, which is estimated to be a better donator of π-electrons (Xu et al. 2019). Wood-based biochars have a large amount of oxygen (8–12%) as wood feedstock contain more lignin, celluloses, and hemicelluloses. These oxygen-containing compounds, such as catechol, diols, unsaturated anhydrosugars, and substituted catechol, are normally conducive to reducing Cr(VI) to Cr(III) (Mohan et al. 2011; Ahmad et al. 2014).
The “sorption-reduction theory” is a more persuasive explanation of the removal and immobilization of Cr(VI) by biochar in aqueous environment. “Sorption-reduction” mechanism is grouped into (a) direct reduction mechanism, which Cr(VI) is reduced to Cr(III) by the electrons on biochar surfaces, and Cr(III) is complexed with the functional group on biochar surfaces or stranded in water (Dong et al. 2014); and (b) indirect reduction mechanism, mainly involving three steps: (1) Cr(VI) binds to the positively charged functional groups on the surface of biochar; (2) Cr(VI) is reduced to Cr(III) by the electrons on the surface of biochar; and (3) Cr(III) is released into water through positively charged functional groups due to electron repulsion or complexed with other adjacent functional groups on the surface of biochar (Park et al. 2005; Zhou et al. 2016). Hence, the reduction of Cr(VI) to Cr(III) accompanied with Cr(III) complexation is a foremost mechanism for Cr(VI) remediation by biochar (Mandal et al. 2017; Xia et al. 2019).
Application of biochar to Cr-contaminated soils and sediments increase the content of soil organic matter (SOM) and thus can promote adsorption of Cr(VI) (Antoniadis et al. 2017, 2018). The involved mechanisms are as follows: On the one hand, SOM promotes microbe growth, thereby motivating biotic reduction of Cr(VI) (Palansooriya et al. 2019); on the other hand, it creates a reduced condition and alters redox potential through proliferation of the microbes (Bolan et al. 2003b), facilitating the reduction of Cr(VI) to Cr(III) (Banks et al. 2006; Shaheen et al. 2014; Rinklebe et al. 2016); lastly, Dong et al. (2014) found that dissolved organic matter (DOM) derived from biochar can act as both electron acceptor and donor, and it was a better reductant than oxidant because it has more positive effect on Cr(VI) reduction (Kunhikrishnan et al. 2017).
Precipitation
Precipitation includes surface/micro-precipitation and co-precipitation, the former occurs between the negatively charged hydroxyl on the biochar surface and positively reduced Cr(III); the latter is that mineral components (e.g., CO32−, PO43−, and SO42−) in biochar which contributes to bind with cationic PTEs including Cr to form co-precipitation. A few studies have proposed inorganic mineral species in biochar exist crystalline mineral compound, including calcite (CaCO3), gonnardite [(Na, Ca)2(Si, Al)5O10·3H2O], quartz (SiO2), and garnet (Ca3Al2(SiO4)(OH)8) (Huggins et al. 2016; Tripathi et al. 2016; Abdel-Fattah et al. 2015).
Reduction of anionic Cr(VI) to cationic Cr(III) species increases the pH of variable charge surfaces like biochar (Bolan et al. 2003a). This Cr(VI) reduction-induced increase in pH creates additional negative charges on biochar, thereby facilitating the adsorption of positively charged Cr(III) species. The increase in pH also accelerates the precipitation of Cr(III) as Cr(OH)3, thereby enhancing its immobilization process.
Modification of biochar to improve Cr sorption
Although biochar has the ability to adsorb PTEs from contaminated sites, its capacity is usually lower than other sorbents such as granular activated carbon (GAC). Hence, approaches to manipulate biochar to improve PTE sorption have been described by many studies, such as physical/mechanical modification, chemical modification, biological modification, immobilization, combining with mineral sorbents, and magnetic modification (Fomina and Gadd 2014). These modification processes will increase surface area, porosity, pHPZC, functional groups, activation in surface area, which can alter Cr sorption processes and efficiency in biochar (Ahmed et al. 2016; Mandal et al. 2017) (Fig. 6). In recent years, the modification technology mainly focused on polyethylenimine modification (Ma et al. 2014), chitosan loading (Mandal et al. 2017), β-cyclodextrin/poly (l-glutamic acid) modification (Jiang et al. 2017), ultraviolet modified biochar (UV-BC) (Peng et al. 2018), diluted sulfuric acid-assisted MgO-coated biochar (Xiao et al. 2018), biochar-supported carboxymethyl cellulose (CMC)-stabilized nanoscale iron sulfide (FeS) composite (CMC-FeS-biochar) (Lyu et al. 2018), nanoscale zero-valent iron stabilization (Fan et al. 2019), acid (HCl)/base (NaOH)/ethanol (C2H5OH) treatment (Zhou et al. 2019), etc. Specifically, Gan et al. (2015) synthesized Zn-biochar nanocomposites derived from sugarcane bagasse, and it showed an increase in Cr(VI) removal efficiency by 1.2–2.0 times higher than that of pristine biochar. Specific surface areas and oxygen-containing functional groups of biochars obviously increased by UV irradiation, and thus, the Cr(VI) adsorption increased from 1.11 mg g−1 for BC to 20.04 mg g−1 for UV-BC (Peng et al. 2018). From X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) analysis, it has been inferred that the mechanisms of Cr(VI) adsorption by UV-BC were the surface complexation coupled Cr(VI) reduction. Similarly, γ-Fe2O3 was loaded onto the peanut hull-derived biochar and the amount of Cr(VI) adsorption enhanced 1–2 orders of magnitude that of the pristine biochar (Han et al. 2016). Low-cost walnut shell biochars modified with β-cyclodextrin-chitosan (β-CCWB) were synthesized, and the removal efficiency of Cr(VI) was found to be much higher for β-CCWB (93%) than for pristine biochar (27%). From the FTIR spectral data and XPS analysis, the Cr(VI) removal mechanisms mainly include the electrostatic attraction between negatively charged Cr(VI) and the positively charged biochar surface, and the complexation between Cr(III) ions and functional groups of β-CCWB, and the reduction of Cr(VI) to Cr(III) (Huang et al. 2016).
Investigation of recent literature has indicated that nanoscale zero-valent iron (nZVI) is the most wide application modification material. These modified biochars contain nanoparticles of mineral phases of Fe with redox activity, which can serve as catalysts for redox reactions such as the reduction of Cr(VI) to Cr(III); the magnetized biochars also contribute to their easy recovery from wastewater after the adsorption and removal of Cr(VI) (Ahmed et al. 2016).
Factors affecting remediation capability of Cr in soils and wastewater
The removal efficiency of biochar for Cr depends on the biosorption system, and it is influenced by different factors, including pH in solution media, temperature, reaction time and speed, initial concentration of Cr, the amount of biochar dosage, physical and chemical properties of biochar, as well as the other competing pollutants in reaction systems.
pH
Compared with other PTEs, the pH-dependent sorption of Cr(VI) results in differences in speciation (Fig. 1), and it also affects the dissociation of the active functional groups (–OH, –COOH, and –NH2) on biochar surface (Dong et al. 2011). Most studies showed low pH is conducive to the Cr(VI) sorption, and the maximum sorption point is usually at pH 2 (e.g., Zhang et al. 2017; Choppala et al. 2018; Xu et al. 2019). Cr mainly exists in the forms of HCrO4− and H2CrO4 under low pH, and it will gradually convert into CrO42− and Cr2O72− when the solution pH increases (Shahid et al. 2017). This behavior might be interpreted by the strong protonation on the surface of biochar at low pH, which favors the occurrence of electrostatic interactions between the positively charged functional groups on biochar surface and the negatively charged chromate ions (Abdel-Fattah et al. 2015; Shaheen et al. 2019). No significant adsorption of Cr(VI) occurs under the condition of pH values higher than 6.0 owing to the competition between the Cr anions (e.g., HCrO4−, Cr2O72−) and OH− anions for adsorption sites (Miretzky and Cirelli 2010).
Temperature
Due to the endothermic sorption of Cr(VI) by most biochars (e.g., Jiang et al. 2017; Lyu et al. 2017), the adsorption capability of PTEs by biochar generally increases with the increasing temperature. This indicates that an increase in temperature provides sufficient energy to metal ions to conquer a diffuse double-layer and sequester into the internal structure of biochar (Liu and Zhang 2009). Thus, the diffusion rate of adsorbents and the probability of contact with the sorption sites also increased due to the increases in the kinetic energy and surface activity of biochar, which facilitates an increase in sorption effect with the increasing temperature (Mohan et al. 2011). However, higher temperatures possibly damage the physical microstructure of the adsorbent (Park et al. 2010), thereby impacting the sorption capacity for PTE including Cr.
Reaction time and speed
The basic reaction conditions include contact time, agitation time, and speed. Biochar feedstocks have different lignin and cellulose contents; thus, there exist differences in the functional groups and adsorption sites. The sorption rate will decrease when the sorption sites are gradually occupied, whereby the sorption process reaches the equilibrium state (Deveci and Kar 2013; Jain et al. 2013; Ullah et al. 2013). Besides, the agitation time and speed also influence the sorption rate of Cr by minimizing the mass transfer resistance; however, it may cause damages to the physical structure of adsorbent (Park et al. 2010).
Initial concentration and biochar dosage
The quantity of adsorbed Cr(VI) per unit weight of biosorbent can be increased by the increasing initial concentration of Cr(VI), but decrease the percentage of removal efficiency (Barakat 2011; Fomina and Gadd 2014). Generally, the higher exchangeable capacity of PTEs evidently increase in response to the addition of biochar; however, the immobilization effects will decrease at higher initial concentrations of PTEs (Jiang et al. 2012). It is demonstrated that the biochar application dosage contributes to a greater percentage of PTEs removal (Chen et al. 2011). For example, Dong et al. (2011) reported that the percent of Cr(VI) sorption improved from 19.8 to 88.5% when the dosage of biochar increased from 0.2 to 8.0 g L−1. However, the amount of biochar application at higher rate decreased the sorption efficiency of PTEs, which are possibly because of the formation of aggregates between the biochar particles, thus reducing the available surface area for PTEs sorption (Chen et al. 2011). Dong et al. (2017) found that the lower mass of BC which loaded with nZVI could not prevent the aggregation of nZVI particles on the surface of BC; however, excessive BC would obstruct the active sites of nZVI, thereby leading to the decrease of reduction capacity of nZVI. More importantly, the soil pH usually increased, and the soil CEC and zeta potential decreased in response to biochar addition, which would affect the complexation and electrostatic attraction processes.
Biochar characteristics
The sorption performance of biochar is a function of its characteristics such as specific surface area, pHPZC, pore size, and functional groups on biochar surface, which in turn mainly depends on biochar feedstock and pyrolysis temperature (Li et al. 2017). In general, biomass rich in cellulose (e.g., husks) mainly develops microporous-structured biochar, while biomass rich in lignin (e.g., coconut shell and bamboo) predominantly yields macroporous-structured biochar (Joseph et al. 2007). Grassy and non-woody biochars, containing high oxygen contents and acidic surface sites, usually have higher CECs than woody-derived biochars with low oxygen-containing functional groups (Harvey et al. 2011). Bird et al. (2011) reported that the CEC of algae-derived biochar is 29–41 cmol kg−1 and extractable K, Ca, and Mg vary from 27 to 485 cmol kg−1 compared to biochars derived from higher plants. Hence, the feedstock of biochar largely determines the adsorption capability.
Elevated pyrolysis temperature usually results in larger pore size and surface area of biochar. However, in some cases, porous structure of biochar may be blocked or even destroyed by tar, thus leading to decrease in surface area and lower porosity under high temperature (Jin et al. 2016). Besides, when pyrolytic temperature increases, the carbon content will increase accompanied by a decrease in oxygen and hydrogen contents, indicating an increase in the carbonization of the chars (Chun et al. 2004). Shen et al. (2012) demonstrated that Cr(VI) sorption by biochar decreased drastically from 31.1 to 4.10 mg g−1 with increasing pyrolysis temperature from 250 to 600 °C; at the same time, the acidic functional groups (carboxyl, lactonic, and phenolic) decreased sharply from 1.78 to 0.12 mmol g−1 because of the decrease in the O/C molar ratio. Consistently, some studies have also reported that Cr(VI) sorption capacity by biochars was relatively higher under pyrolysis low temperature (e.g., Han et al. 2016; Zhou et al. 2016).
Moreover, many studies have proved that biochar pH increases with increasing pyrolysis temperature (e.g., Zhang et al. 2015a, b; Jin et al. 2016; Subedi et al. 2016). This is mainly related with three factors: (1) higher temperature leads to more ash component that positively correlated with biochar pH (Jin et al. 2016); (2) the increase in base cations and carbonates contributes to increased pH (Yuan et al. 2011); (3) acidic functional groups (e.g., –COOH) decrease under high temperature (Al-Wabel et al. 2013). In addition, Cr(VI) sorption capacity would lower under longer pyrolysis residence time. With residence time of biochar pyrolysis increasing from 1 to 2 h, Cr(VI) sorption by municipal sludge-derived biochars decreased from 69.0–118 mg g−1 to 19.6–45.2 mg g−1 at temperature 400–600 °C, which is mainly because of loss of functional groups (Zhang et al. 2013).
In conclusion, biochar possesses high pH, CEC, and surface areas produced at higher temperatures, while it has more stable C–O complexes and active sites produced at lower temperature (Kumar et al. 2011). The C–O-containing functional groups are important for various sorption/chemical reaction potentials (Xie et al. 2015). Hence, the production processes are very important to attain maximum sorption capability.
Coexisting contaminants
In general, more than one PTE coexist in contaminated soils or wastewater. Therefore, the sorption of PTEs by biochars was affected by the concentration and presence of these coexisting contaminants in media. The effects of coexisting ions on the targeted metal sorption capability depend on specific metal speciation, coexisting ions concentration and charge, and the nature of biochars. The research results are inconsistent in different reaction environments. The inhibitory effects for the biosorption of Cr(III) and Cr(VI) ions are: SO42− > Cl− ≈ NO3− and NO3− > Cl− > SO42−, respectively (Michalak et al. 2013). However, Zhu et al. (2018) reported that Cr(VI) removal efficiency is weakly inhibited by the presence of coexisting anions (SO42−, PO43−, and NO3−). In contrast, Shang et al. (2017) reported that the presence of SO42− and humic acid promoted Cr(VI) removal at both low and high concentrations, while the HCO3− inhibited the reaction rate. The amount of Cr(VI) sorption by β-cyclodextrin·poly(l-glutamic acid)-modified biochar (CGA-biochar) gradually decreased with increasing ionic strength in solution (Na+, K+, Ca2+, and Mg2+) varying from 0 to 1 mmol L−1 (Jiang et al. 2017). This phenomenon might be explained by the hindrance of electrostatic interactions between Cr(VI) ions and the charges on the CGA-biochar surface (Gan et al. 2015). Relative to monovalent cations (Na+ and K+), divalent cations (Ca2+ and Mg2+) resulted in a lower removal efficiency of Cr(VI) (Jiang et al. 2017). This indicated that the electrostatic attraction of bivalent cations was more intense than that of monovalent cations, which would bring about stronger hindrance of electrostatic interaction between Cr(VI) ions and the charges on CGA-biochar surface (Hu et al. 2014). For coexisting organic contaminants, Wang et al. (2016) found that pyrene inhibit the adsorption efficiency of Cr(VI) when pyrene and Cr(VI) coexist in the media, as the inner complex exists between the hydroxyl groups on pineapple peel-derived biochar (PABC) and pyrene flushbonading the H bond with Cr(VI).
Concluding remarks, recommendations, and future challenges
Chromium is a highly potential toxic element, which is considered as a priority pollutant impacting ecosystems and human health. Biochar, having the advantages of a wide range of feedstock materials, no secondary pollution, low cost, high sorption capacity, and relatively simple operation, provides greater practical value and significance for Cr contamination (Xie et al. 2015; Zama et al. 2018). However, there are still some issues in the practical process of Cr(VI) remediation. Therefore, in order to fully exploit field application and efficient sorption for Cr pollution, we should comprehend the detailed processes and mechanisms of Cr(VI) remediation by biochar and its multiple influencing factors as well as the intrinsic nature of these affecting factors. Here, we put forward recommendations and challenges for biochar practice, thus providing a theoretical basis for further study of the mechanisms of Cr(VI) remediation.
The research areas that require further study are as follows:
-
1.
The necessity to modify biochar through environmentally friendly green methods to introduce more active functional groups, increase surface area, improve the mechanical strength and chemical stability, such as chitosan, diatomite carrier, acid/base modification, nanoscale zero-valent iron (nZVI), etc., in order to get the best remediation effect of Cr(VI). However, nZVI, as the most widely used modifier in recent studies, has some undesirable effects. nZVI tends to agglomerate rapidly due to its nanosize effect and magnetic interaction, which then reduces its reactivity and mobility and weakens its remediation efficiency, especially in contaminated soils (Xu and Zhao 2007; He and Zhao 2005). Moreover, nanoparticles, to some extent, may impact soil properties and decrease soil fertility because of their stronger activity, which negatively affects soil re-use and plant regeneration (McBride and MartÍnez 2000; Kumpiene et al. 2008).
-
2.
Generally, both PTEs and organic pollutants often coexist in contaminated sites. It is necessary to bolster the study of sorption of Cr by biochar in the presence of multiple contaminants in contaminated soil and wastewater, especially in the presence of inorganic metals. Because some functional groups for the adsorption of PTEs are chemically similar, competition for binding sites of PTEs will occur, thus influencing immobilization effect for individual metal in metal-mixed contaminated sites. The immobilization effects of biochar on PTEs and other contaminants in field polluted soils need to be assessed in the future.
-
3.
The Cr recycling and regeneration of biochar are two important factors for the prospective application of biochar. At present, the inherent problem is that although Cr(VI) is reduced to Cr(III) with less bioavailability, the total contaminant concentrations in soils remains to be unchanged. It may become bioavailable with time via advanced decomposition of SOM and natural weathering process. Thus, it is necessary to clarify the probability and efficiency of suitable methods to dispose the adsorbed biochar safely after the completed Cr sorption. However, there are few studies on the methods for recycling that are concerned with economical and environmental impacts.
-
4.
It has been well demonstrated that the sorption-coupled reduction is the primary mechanism of Cr(VI) remediation by biochar. It is essential to explore and differentiate the mechanisms of electron transfer regarding biochars, which comes from their electrical conductivity (transferring electrons between two bio/chemical entities) and redox functional groups (accepting or donating electrons) on the surface. The sorption-reduction mechanism of pollutant removal by biochar needs to be further studied. A combination of tools which contributes to understanding how Cr(VI) is bound to biochar, including X-ray diffraction (XRD), micro-X-ray fluorescence (μ-XRF), scanning electron microscope (SEM), energy-dispersive spectroscopy (EDS), extended bulk-EXAFS spectroscopy, Fourier transform-infrared spectroscopy (FTIR), and Brunauer–Emmett–Teller (BET) techniques.
-
5.
Some studies indicated that biochar itself may contain organic pollutants or other PTEs that produce toxic substances in the process of Cr(VI) remediation. Hence, it is necessary to study the advance environmental toxicological effect of biochar counteracting the soil and water resources.
-
6.
Biochar, due to its designable surface chemistry and better sorption performance, offers great potential in various engineering applications. However, there are rarely few published literatures about cost–benefit studies of biochars through different production processes, e.g., biomass availability, production scale, raw material collection and transportation, calcination temperature, residence time, and biochar modification. The absence of these data about all parameters during the production process complexes the estimation of the economic cost of biochar. Hence, large-scale application in different contaminated sites is not realistic, and it is still in the laboratory stage or only suitable for small-scale, intensive application at severe sites.
-
7.
In order to achieve effective, large-scale production of biochar and its implications for application to contaminated sites as an engineered material, the predicted models among feedstock composition, production conditions and biochar properties must be established.
Still, there still exists a huge gap between successful implementation and laboratory findings of biochar for the remediation of Cr(VI)-contaminated sites under field conditions. Most studies are a batch mode of remediation for small quantities of effluents. Therefore, it is necessary to develop a suitable operational strategy, for the continuous removal of Cr(VI) from the industrial effluents to handle this global risk. Application of biochar in redox-sensitive environments such as aquatic media (e.g., lakes and wetlands) is an effective method to detoxify and reduce PTEs, including Cr(VI) (Yuan et al. 2017). Overall, using biochar for the remediation of Cr-contaminated soil and wastewater is a promising technique aiming to substitute the expensive, traditional, less-efficient, and non-applicable methods (e.g., physical and chemical remediation technologies) to the cost-effective, high performance, easily applicable, and emerging biomaterials.
References
Abdel-Fattah, T. M., Mahmoud, M. E., Ahmed, S. B., Huff, M. D., Lee, J. W., & Kumar, S. (2015). Biochar from woody biomass for removing metal contaminants and carbon sequestration. Journal of Industrial and Engineering Chemistry,22, 103–109. https://doi.org/10.1016/j.jiec.2014.06.030.
Agegnehu, G., Srivastava, A. K., & Bird, M. I. (2017). The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Applied Soil Ecology,119, 156–170. https://doi.org/10.1016/j.apsoil.2017.06.008.
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., et al. (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere,99, 19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071.
Ahmed, M. B., Zhou, J. L., Ngo, H. H., Guo, W., & Chen, M. (2016). Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresource Technology,214, 836–851. https://doi.org/10.1016/j.biortech.2016.05.057.
Ai, Z., Cheng, Y., Zhang, L., & Qiu, J. (2008). Efficient removal of Cr(VI) from aqueous solution with Fe@Fe2O3 core–shell nanowires. Environmental Science and Technology,42(18), 6955–6960. https://doi.org/10.1021/es800962m.
Aksu, Z., & İşoğlu, İ. A. (2005). Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochemistry,40(9), 3031–3044. https://doi.org/10.1016/j.procbio.2005.02.004.
Al-Wabel, M. I., Al-Omran, A., El-Naggar, A. H., Nadeem, M., & Usman, A. R. (2013). Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresource Technology,131, 374–379. https://doi.org/10.1016/j.biortech.2012.12.165.
Antoniadis, V., Polyzois, T., Golia, E. E., & Petropoulos, S. A. (2017). Hexavalent chromium availability and phytoremediation potential of Cichorium spinosum as affect by manure, zeolite and soil ageing. Chemosphere,171, 729–734. https://doi.org/10.1016/j.chemosphere.2016.11.146.
Antoniadis, V., Shaheen, S., Levizou, E., Shahid, M., Niazi, N. K., Vithanage, M., et al. (2019). A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: Are they protective concerning health risk assessment?—A review. Environmental International. https://doi.org/10.1016/j.envint.2019.03.039.
Antoniadis, V., Zanni, A. A., Levizou, E., Shaheen, S. M., Dimirkou, A., Bolan, N., et al. (2018). Modulation of hexavalent chromium toxicity on Οriganum vulgare in an acidic soil amended with peat, lime, and zeolite. Chemosphere,195, 291–300. https://doi.org/10.1016/j.chemosphere.2017.12.069.
Arian, M. B., Ali, I., Yilmaz, E., & Soylak, M. (2018). Nanomaterial’s based chromium speciation in environmental samples: A review. TrAC Trends in Analytical Chemistry. https://doi.org/10.1016/j.trac.2018.03.014.
Ashraf, A., Bibi, I., Niazi, N. K., Ok, Y. S., Murtaza, G., Shahid, M., et al. (2017). Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. International Journal of Phytoremediation,19(7), 605–613. https://doi.org/10.1080/15226514.2016.1256372.
Ball, J. W., & Izbicki, J. A. (2004). Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Applied Geochemistry,19(7), 1123–1135. https://doi.org/10.1016/j.apgeochem.2004.01.011.
Ballesteros, S., Rincón, J. M., Rincón-Mora, B., & Jordán, M. M. (2017). Vitrification of urban soil contamination by hexavalent chromium. Journal of Geochemical Exploration,174, 132–139. https://doi.org/10.1016/j.gexplo.2016.07.011.
Banks, M. K., Schwab, A. P., & Henderson, C. (2006). Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere,62(2), 255–264. https://doi.org/10.1016/j.chemosphere.2005.05.020.
Barakat, M. A. (2011). New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry,4(4), 361–377. https://doi.org/10.1016/j.arabjc.2010.07.019.
Beaumont, J. J., Sedman, R. M., Reynolds, S. D., Sherman, C. D., Li, L. H., Howd, R. A., et al. (2008). Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology. https://doi.org/10.1097/EDE.0b013e31815cea4c.
Bello, O. S., & Ahmad, M. A. (2011). Response surface modeling and optimization of remazol brilliant blue reactive dye removal using periwinkle shell-based activated carbon. Separation Science and Technology,46(15), 2367–2379. https://doi.org/10.1080/01496395.2011.595756.
Bielicka, A., Bojanowska, I., & Wisniewski, A. (2005). Two faces of chromium-pollutant and bioelement. Polish Journal of Environmental Studies,14(1), 5–10.
Bird, M. I., Wurster, C. M., de Paula Silva, P. H., Bass, A. M., & De Nys, R. (2011). Algal biochar–production and properties. Bioresource Technology,102(2), 1886–1891. https://doi.org/10.1016/j.biortech.2010.07.106.
Bolan, N. S., Adriano, D. C., & Curtin, D. (2003a). Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Advances in Agronomy,78(21), 5–272. https://doi.org/10.1016/S0065-2113(02)78006-1.
Bolan, N. S., Adriano, D. C., Natesan, R., & Koo, B. J. (2003b). Effects of organic amendments on the reduction and phytoavailability of chromate in mineral soil. Journal of Environmental Quality,32(1), 120–128. https://doi.org/10.2134/jeq2003.1200.
Cao, H., & Jin, W. (2015). Industrial Cr(VI) pollution analysis and control strategies in China. In The 5th international symposium on metallomics, Guangxi, China.
CCME. (2015). Canadian soil quality guidelines for the protection of environmental and human health. Canada Council of Ministers of the Environment Winnipeg.
Cefalu, W. T., & Hu, F. B. (2004). Role of chromium in human health and in diabetes. Diabetes Care,27(11), 2741–2751. https://doi.org/10.2337/diacare.27.11.2741.
Cha, J. S., Park, S. H., Jung, S. C., Ryu, C., Jeon, J. K., Shin, M. C., et al. (2016). Production and utilization of biochar: A review. Journal of Industrial and Engineering Chemistry,40, 1–15. https://doi.org/10.1016/j.jiec.2016.06.002.
Chen, X., Chen, G., Chen, L., Chen, Y., Lehmann, J., McBride, M. B., et al. (2011). Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresource Technology,102(19), 8877–8884. https://doi.org/10.1016/j.biortech.2011.06.078.
Choppala, G., Bolan, N., Kunhikrishnan, A., & Bush, R. (2016). Differential effect of biochar upon reduction-induced mobility and bioavailability of arsenate and chromate. Chemosphere,144, 374–381. https://doi.org/10.1016/j.chemosphere.2015.08.043.
Choppala, G., Bolan, N., Lamb, D., & Kunhikrishnan, A. (2013). Comparative sorption and mobility of Cr(III) and Cr(VI) species in a range of soils: Implications to bioavailability. Water, Air, and Soil pollution,224(12), 1699. https://doi.org/10.1007/s11270-013-1699-6.
Choppala, G., Kunhikrishnan, A., Seshadri, B., Park, J. H., Bush, R., & Bolan, N. (2018). Comparative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils. Journal of Geochemical Exploration,184, 255–260. https://doi.org/10.1016/j.gexplo.2016.07.012.
Choudhary, B., & Paul, D. (2018). Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. Journal of Environmental Chemical Engineering,6(2), 2335–2343. https://doi.org/10.1016/j.jece.2018.03.028.
Choudhary, B., Paul, D., Singh, A., & Gupta, T. (2017). Removal of hexavalent chromium upon interaction with biochar under acidic conditions: Mechanistic insights and application. Environmental Science and Pollution Research,24(20), 16786–16797. https://doi.org/10.1007/s11356-017-9322-9.
Chun, Y., Sheng, G., Chiou, C. T., & Xing, B. (2004). Compositions and sorptive properties of crop residue-derived chars. Environmental Science and Technology,38(17), 4649–4655. https://doi.org/10.1021/es035034w.
Costa, M., & Klein, C. B. (2006). Toxicity and carcinogenicity of chromium compounds in humans. Critical Reviews in Toxicology, 36(2), 155–163.
Crane-Droesch, A., Abiven, S., Jeffery, S., & Torn, M. S. (2013). Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environmental Research Letters,8(4), 044049. https://doi.org/10.1088/1748-9326/8/4/044049.
da Costa Cunha, G., Peixoto, J. A., de Souza, D. R., Romão, L. P. C., & Macedo, Z. S. (2016). Recycling of chromium wastes from the tanning industry to produce ceramic nanopigments. Green Chemistry,18(19), 5342–5356. https://doi.org/10.1039/C6GC01562J.
Deng, H. Y., & Chen, G. C. (2012). Progress in research on microbial remediation technologies of chromium-contaminated soil. Earth and Environment,40, 466–472. https://doi.org/10.14050/j.cnki.1672-9250.2012.03.018.
Deveci, H., & Kar, Y. (2013). Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. Journal of Industrial and Engineering Chemistry,19(1), 190–196. https://doi.org/10.1016/j.jiec.2012.08.001.
Dhal, B., Thatoi, H. N., Das, N. N., & Pandey, B. D. (2013). Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. Journal of Hazardous Materials,250, 272–291. https://doi.org/10.1016/j.jhazmat.2013.01.048.
Ding, C., Li, X., Zhang, T., Ma, Y., & Wang, X. (2014). Phytotoxicity and accumulation of chromium in carrot plants and the derivation of soil thresholds for Chinese soils. Ecotoxicology and Environmental Safety,108, 179–186. https://doi.org/10.1016/j.ecoenv.2014.07.006.
Dong, H., Deng, J., Xie, Y., Zhang, C., Jiang, Z., Cheng, Y., et al. (2017). Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. Journal of Hazardous Materials,332, 79–86. https://doi.org/10.1016/j.jhazmat.2017.03.002.
Dong, X., Ma, L. Q., Gress, J., Harris, W., & Li, Y. (2014). Enhanced Cr(VI) reduction and As (III) oxidation in ice phase: Important role of dissolved organic matter from biochar. Journal of Hazardous Materials,267, 62–70. https://doi.org/10.1016/j.jhazmat.2013.12.027.
Dong, X., Ma, L. Q., & Li, Y. (2011). Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. Journal of Hazardous Materials,190(1–3), 909–915. https://doi.org/10.1016/j.jhazmat.2011.04.008.
Dotaniya, M. L., Thakur, J. K., Meena, V. D., Jajoria, D. K., & Rathor, G. (2014). Chromium pollution: A threat to environment—A review. Agricultural Reviews,35(2), 153–157. https://doi.org/10.5958/0976-0741.2014.00094.4.
Duku, M. H., Gu, S., & Hagan, E. B. (2011). Biochar production potential in Ghana—A review. Renewable and Sustainable Energy Reviews,15(8), 3539–3551. https://doi.org/10.1016/j.rser.2011.05.010.
Fan, Z., Zhang, Q., Gao, B., Li, M., Liu, C., & Qiu, Y. (2019). Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere,217, 85–94. https://doi.org/10.1016/j.chemosphere.2018.11.009.
Fantoni, D., Brozzo, G., Canepa, M., Cipolli, F., Marini, L., Ottonello, G., et al. (2002). Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environmental Geology,42(8), 871–882. https://doi.org/10.1007/s00254-002-0605-0.
Fendorf, S., Wielinga, B. W., & Hansel, C. M. (2000). Chromium transformations in natural environments: The role of biological and abiological processes in chromium (VI) reduction. International Geology Review,42(8), 691–701. https://doi.org/10.1080/00206810009465107.
Fomina, M., & Gadd, G. M. (2014). Biosorption: Current perspectives on concept, definition and application. Bioresource Technology,160, 3–14. https://doi.org/10.1016/j.biortech.2013.12.102.
Gan, C., Liu, Y., Tan, X., Wang, S., Zeng, G., Zheng, B., et al. (2015). Effect of porous zinc–biochar nanocomposites on Cr(VI) adsorption from aqueous solution. RSC Advances,5(44), 35107–35115. https://doi.org/10.1039/C5RA04416B.
GB 15618-2018.
Gheju, M. (2011). Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water, Air, and Soil pollution,222(1–4), 103–148. https://doi.org/10.1007/s11270-011-0812-y.
Ghorbani-Khosrowshahi, S., & Behnajady, M. A. (2016). Chromium (VI) adsorption from aqueous solution by prepared biochar from Onopordom Heteracanthom. International Journal of Environmental Science and Technology,13(7), 1803–1814. https://doi.org/10.1007/s13762-016-0978-3.
Gil, M. V., Fermoso, J., Pevida, C., Pis, J. J., & Rubiera, F. (2010). Intrinsic char reactivity of plastic waste (PET) during CO2 gasification. Fuel Processing Technology,91(11), 1776–1781. https://doi.org/10.1016/j.fuproc.2010.07.019.
Gil-Cardeza, M. L., Ferri, A., Cornejo, P., & Gomez, E. (2014). Distribution of chromium species in a Cr-polluted soil: Presence of Cr(III) in glomalin related protein fraction. Science of the Total Environment,493, 828–833. https://doi.org/10.1016/j.scitotenv.2014.06.080.
Grierson, S., Strezov, V., & Shah, P. (2011). Properties of oil and char derived from slow pyrolysis of Tetraselmis chui. Bioresource Technology,102(17), 8232–8240. https://doi.org/10.1016/j.biortech.2011.06.010.
Han, Y., Cao, X., Ouyang, X., Sohi, S. P., & Chen, J. (2016). Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr(VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere,145, 336–341. https://doi.org/10.1016/j.chemosphere.2015.11.050.
Harvey, O. R., Herbert, B. E., Rhue, R. D., & Kuo, L. J. (2011). Metal interactions at the biochar–water interface: energetics and structure–sorption relationships elucidated by flow adsorption microcalorimetry. Environmental Science anf Technology,45(13), 5550–5556. https://doi.org/10.1021/es104401h.
He, F., & Zhao, D. (2005). Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environmental Science and Technology,39(9), 3314–3320. https://doi.org/10.1021/es048743y.
He, L., Fan, S., Müller, K., Wang, H., Che, L., Xu, S., et al. (2018). Comparative analysis biochar and compost-induced degradation of di-(2-ethylhexyl) phthalate in soils. Science of the Total Environment,625, 987–993. https://doi.org/10.1016/j.scitotenv.2018.01.002.
Headlam, H. A., & Lay, P. A. (2016). Spectroscopic characterization of genotoxic chromium(v) peptide complexes: Oxidation of chromium(iii) triglycine, tetraglycine and pentaglycine complexes. Journal of Inorganic Biochemistry,162, 227–237. https://doi.org/10.1016/j.jinorgbio.2016.06.015.
Hu, X. J., Liu, Y. G., Zeng, G. M., You, S. H., Wang, H., Hu, X., et al. (2014). Effects of background electrolytes and ionic strength on enrichment of Cd (II) ions with magnetic graphene oxide–supported sulfanilic acid. Journal of Colloid and Interface Science,435, 138–144. https://doi.org/10.1016/j.jcis.2014.08.054.
Huang, P., Ge, C., Feng, D., Yu, H., Luo, J., Li, J., et al. (2018). Effects of metal ions and pH on ofloxacin sorption to cassava residue-derived biochar. Science of the Total Environment,616, 1384–1391. https://doi.org/10.1016/j.scitotenv.2017.10.177.
Huang, X., Liu, Y., Liu, S., Tan, X., Ding, Y., Zeng, G., et al. (2016). Effective removal of Cr(VI) using β-cyclodextrin–chitosan modified biochars with adsorption/reduction bifuctional roles. RSC Advances,6(1), 94–104. https://doi.org/10.1039/C5RA22886G.
Huggins, T. M., Haeger, A., Biffinger, J. C., & Ren, Z. J. (2016). Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Research,94, 225–232. https://doi.org/10.1016/j.watres.2016.02.059.
Inyang, M. I., Gao, B., Yao, Y., Xue, Y., Zimmerman, A., Mosa, A., et al. (2016). A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Critical Reviews in Environmental Science and Technology,46(4), 406–433. https://doi.org/10.1080/10643389.2015.1096880.
Jain, M., Garg, V. K., & Kadirvelu, K. (2013). Chromium removal from aqueous system and industrial wastewater by agricultural wastes. Bioremediation Journal,17(1), 30–39. https://doi.org/10.1080/10889868.2012.731450.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology,7(2), 60–72. https://doi.org/10.2478/intox-2014-0009.
Jiang, L., Liu, S., Liu, Y., Zeng, G., Guo, Y., Yin, Y., et al. (2017). Enhanced adsorption of hexavalent chromium by a biochar derived from ramie biomass (Boehmeria nivea (L.) Gaud.) modified with β-cyclodextrin/poly (l-glutamic acid). Environmental Science and Pollution Research,24(30), 23528–23537. https://doi.org/10.1007/s11356-017-9833-4.
Jiang, T. Y., Jiang, J., Xu, R. K., & Li, Z. (2012). Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere,89(3), 249–256. https://doi.org/10.1016/j.chemosphere.2012.04.028.
Jin, W., Du, H., Zheng, S., & Zhang, Y. (2016). Electrochemical processes for the environmental remediation of toxic Cr(VI): A review. Electrochimica Acta,191, 1044–1055. https://doi.org/10.1016/j.electacta.2016.01.130.
Jindo, K., Mizumoto, H., Sawada, Y., Sanchez-Monedero, M. A., & Sonoki, T. (2014). Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences,11(23), 6613–6621. https://doi.org/10.5194/bg-11-6613-2014.
Jobby, R., & Desai, N. (2017). Bioremediation of heavy metals. In P. Kumar, B. R. Gurjar, & J. N. Govil (Eds.), Biodegradation and bioremediation. Environmental science and engineering (pp. 201–220). New Delhi: Studium Press.
Jobby, R., Jha, P., Yadav, A. K., & Desai, N. (2018). Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere,207, 255–266. https://doi.org/10.1016/j.chemosphere.2018.05.050.
Joint FAO/WHO food standards programme, Codex alimentarius commission, Twenty-fourth session, Geneva, Switzerland, 2–7 July 2001.
Joseph, S. D., Downie, A., Munroe, P., Crosky, A., & Lehmann, J. (2007). Biochar for carbon sequestration, reduction of greenhouse gas emissions and enhancement of soil fertility; A review of the materials science. In Proceedings of the Australian combustion symposium (pp. 130–133).
Kabata-Pendias, A. (2010). Trace elements in soils and plants. CRC press.
Karakoyun, N., Kubilay, S., Aktas, N., Turhan, O., Kasimoglu, M., Yilmaz, S., et al. (2011). Hydrogel-biochar composites for effective organic contaminant removal from aqueous media. Desalination,280(1–3), 319–325. https://doi.org/10.1016/j.desal.2011.07.014.
Keiluweit, M., & Kleber, M. (2009). Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environmental Science and Technology,43(10), 3421–3429. https://doi.org/10.1021/es8033044.
Keng, P. S., Lee, S. L., Ha, S. T., Hung, Y. T., & Ong, S. T. (2014). Removal of hazardous heavy metals from aqueous environment by low-cost adsorption materials. Environmental Chemistry Letters,12(1), 15–25. https://doi.org/10.1007/s10311-013-0427-1.
Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration,182, 247–268. https://doi.org/10.1016/j.gexplo.2016.11.021.
Kumar, S., Loganathan, V. A., Gupta, R. B., & Barnett, M. O. (2011). An assessment of U (VI) removal from groundwater using biochar produced from hydrothermal carbonization. Journal of Environmental Management,92(10), 2504–2512. https://doi.org/10.1016/j.jenvman.2011.05.013.
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Management,28(1), 215–225. https://doi.org/10.1016/j.wasman.2006.12.012.
Kunhikrishnan, A., Choppala, G., Seshadri, B., Wijesekara, H., Bolan, N. S., Mbene, K., et al. (2017). Impact of wastewater derived dissolved organic carbon on reduction, mobility, and bioavailability of As (V) and Cr(VI) in contaminated soils. Journal of Environmental Management,186, 183–191. https://doi.org/10.1016/j.jenvman.2016.08.020.
Li, H., Dong, X., da Silva, E. B., de Oliveira, L. M., Chen, Y., & Ma, L. Q. (2017). Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere,178, 466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072.
Li, J., Shen, F., Yang, G., Zhang, Y., Deng, S., Zhang, J., et al. (2018). Valorizing rice straw and its anaerobically digested residues for biochar to remove Pb(II) from aqueous solution. International Journal of Polymer Science. https://doi.org/10.1155/2018/2684962.
Li, Z., Song, Z., Singh, B. P., & Wang, H. (2019). The impact of crop residue biochars on silicon and nutrient cycles in croplands. Science of the Total Environment, 659, 673–680.
Lilli, M. A., Moraetis, D., Nikolaidis, N. P., Karatzas, G. P., & Kalogerakis, N. (2015). Characterization and mobility of geogenic chromium in soils and river bed sediments of Asopos basin. Journal of Hazardous Materials,281, 12–19. https://doi.org/10.1016/j.jhazmat.2014.07.037.
Liu, Z., & Zhang, F. S. (2009). Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. Journal of Hazardous Materials,167(1–3), 933–939. https://doi.org/10.1016/j.jhazmat.2009.01.085.
Lu, A., Zhong, S., Chen, J., Shi, J., Tang, J., & Lu, X. (2006). Removal of Cr(VI) and Cr(III) from aqueous solutions and industrial wastewaters by natural clino-pyrrhotite. Environmental Science and Technology,40(9), 3064–3069. https://doi.org/10.1021/es052057x.
Lu, K., Yang, X., Gielen, G., Bolan, N., Ok, Y. S., Niazi, N. K., et al. (2017). Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. Journal of Environmental Management,186, 285–292. https://doi.org/10.1016/j.jenvman.2016.05.068.
Luo, J., Li, X., Ge, C., Müller, K., Yu, H., Huang, P., et al. (2018). Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresource Technology,263, 385–392. https://doi.org/10.1016/j.biortech.2018.05.022.
Lyu, H., Tang, J., Huang, Y., Gai, L., Zeng, E. Y., Liber, K., et al. (2017). Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chemical Engineering Journal,322, 516–524. https://doi.org/10.1016/j.cej.2017.04.058.
Lyu, H., Zhao, H., Tang, J., Gong, Y., Huang, Y., Wu, Q., et al. (2018). Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere,194, 360–369. https://doi.org/10.1016/j.chemosphere.2017.11.182.
Ma, H. W., Hung, M. L., & Chen, P. C. (2007). A systemic health risk assessment for the chromium cycle in Taiwan. Environment International,33(2), 206–218. https://doi.org/10.1016/j.envint.2006.09.011.
Ma, Y., Liu, W. J., Zhang, N., Li, Y. S., Jiang, H., & Sheng, G. P. (2014). Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresource Technology,169, 403–408. https://doi.org/10.1016/j.biortech.2014.07.014.
Malaviya, P., & Singh, A. (2011). Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Critical Reviews in Environmental Science and Technology,41(12), 1111–1172. https://doi.org/10.1080/10643380903392817.
Mandal, S., Sarkar, B., Bolan, N., Ok, Y. S., & Naidu, R. (2017). Enhancement of chromate reduction in soils by surface modified biochar. Journal of Environmental Management,186, 277–284. https://doi.org/10.1016/j.jenvman.2016.05.034.
McBride, M. B., & MartÍnez, C. E. (2000). Copper phytotoxicity in a contaminated soil: Remediation tests with adsorptive materials. Environmental Science and Technology,34(20), 4386–4391. https://doi.org/10.1021/es0009931.
McNeill, L. S., McLean, J. E., Parks, J. L., & Edwards, M. A. (2012). Hexavalent chromium review, part 2: Chemistry, occurrence, and treatment. Journal-American Water Works Association,104(7), E395–E405. https://doi.org/10.5942/jawwa.2012.104.0092.
Melo, T. M., Bottlinger, M., Schulz, E., Leandro, W. M., de Oliveira, S. B., de Aguiar Filho, A. M., et al. (2019). Management of biosolids-derived hydrochar (Sewchar): Effect on plant germination, and farmers’ acceptance. Journal of Environmental Management,237, 200–214. https://doi.org/10.1016/j.jenvman.2019.02.042.
Messer, J., Reynolds, M., Stoddard, L., & Zhitkovich, A. (2006). Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radical Biology and Medicine,40(11), 1981–1992. https://doi.org/10.1016/j.freeradbiomed.2006.01.028.
Michalak, I., Chojnacka, K., & Witek-Krowiak, A. (2013). State of the art for the biosorption process—A review. Applied Biochemistry and Biotechnology,170(6), 1389–1416. https://doi.org/10.1007/s12010-013-0269-0.
Ministry of Ecology and Environment of the People’s Republic of China. (2015). http://www.zhb.gov.cn/hjzl/zghjzkgb/lnzghjzkgb/201605/P020160526564730573906.
Miretzky, P., & Cirelli, A. F. (2010). Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. Journal of Hazardous Materials,180(1–3), 1–19. https://doi.org/10.1016/j.jhazmat.2010.04.060.
Mishra, S., & Bharagava, R. N. (2016). Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. Journal of Environmental Science and Health, Part C,34, 1–32. https://doi.org/10.1080/10590501.2015.1096883.
Mohan, D., & Pittman, C. U., Jr. (2006). Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. Journal of Hazardous Materials,137(2), 762–811. https://doi.org/10.1016/j.jhazmat.2006.06.060.
Mohan, D., Rajput, S., Singh, V. K., Steele, P. H., & Pittman, C. U., Jr. (2011). Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. Journal of Hazardous Materials,188(1–3), 319–333. https://doi.org/10.1016/j.jhazmat.2011.01.127.
Mukherjee, A., Zimmerman, A. R., & Harris, W. (2011). Surface chemistry variations among a series of laboratory-produced biochars. Geoderma,163(3–4), 247–255. https://doi.org/10.1016/j.geoderma.2011.04.021.
Nanda, S., Dalai, A. K., Berruti, F., & Kozinski, J. A. (2016). Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste and Biomass Valorization,7(2), 201–235. https://doi.org/10.1007/s12649-015-9459-z.
NEPM (National Environmental Protection Council). (2013). National environmental protection measure (NEPM). Schedule B1: Guideline on investigation levels for soil and groundwater. https://www.comlaw.gov.au/Details/F2013C00288/Html/Volume_2.
Nie, C., Yang, X., Niazi, N. K., Xu, X., Wen, Y., Rinklebe, J., et al. (2018). Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere,200, 274–282. https://doi.org/10.1016/j.chemosphere.2018.02.134.
Palansooriya, K. N., Wong, J. T. F., Hashimoto, Y., Huang, L., Rinklebe, J., Chang, S. X., et al. (2019). Response of microbial communities to biochar-amended soils: A critical review. Biochar,1, 3–22.
Panwar, N. L., Pawar, A., & Salvi, B. L. (2019). Comprehensive review on production and utilization of biochar. SN Applied Sciences,1, 168. https://doi.org/10.1007/s42452-019-0172-6.
Papp, J. F. (2004). Chromium use by market in the United States. In Proceedings: Tenth international ferroalloys congress (pp. 770–778).
Park, D., Yun, Y. S., & Park, J. M. (2005). Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere,60, 1356–1364. https://doi.org/10.1016/j.chemosphere.2005.02.020.
Park, D., Yun, Y. S., & Park, J. M. (2010). The past, present, and future trends of biosorption. Biotechnology and Bioprocess Engineering,15(1), 86–102. https://doi.org/10.1007/s12257-009-0199-4.
Park, J. H., Cho, J. S., Ok, Y. S., Kim, S. H., Kang, S. W., Choi, I. W., et al. (2015). Competitive adsorption and selectivity sequence of heavy metals by chicken bone-derived biochar: Batch and column experiment. Journal of Environmental Science and Health, Part A,50(11), 1194–1204. https://doi.org/10.1080/10934529.2015.1047680.
Peng, Z., Zhao, H., Lyu, H., Wang, L., Huang, H., Nan, Q., et al. (2018). UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environmental Science and Pollution Research,25(11), 10808–10819. https://doi.org/10.1007/s11356-018-1353-3.
PRC. (1996). Integrated wastewater discharge standard. EPSPRC GB 8978, PRC, China.
Pure Earth. (2018). Fact sheet: Chromium (2015)—Pure earth [online] Available at: http://www.pureearth.org/fact-sheet-chromium-2015/. Accessed 11 February 2018.
Qin, P., Wang, H., Yang, X., He, L., Müller, K., Shaheen, S. M., et al. (2018). Bamboo-and pig-derived biochars reduce leaching losses of dibutyl phthalate, cadmium, and lead from co-contaminated soils. Chemosphere,198, 450–459. https://doi.org/10.1016/j.chemosphere.2018.01.162.
Rajapaksha, A. U., Alam, M. S., Chen, N., Alessi, D. S., Igalavithana, A. D., Tsang, D. C., et al. (2018). Removal of hexavalent chromium in aqueous solutions using biochar: Chemical and spectroscopic investigations. Science of the Total Environment,625, 1567–1573. https://doi.org/10.1016/j.scitotenv.2017.12.195.
Rajapaksha, A. U., Vithanage, M., Ok, Y. S., & Oze, C. (2013). Cr (VI) formation related to Cr(III)-muscovite and birnessite interactions in ultramafic environments. Environmental Science and Technology,47(17), 9722–9729. https://doi.org/10.1021/es4015025.
Rakhunde, R., Deshpande, L., & Juneja, H. D. (2012). Chemical speciation of chromium in water: A review. Critical Reviews in Environmental Science and Technology,42(7), 776–810. https://doi.org/10.1080/10643389.2010.534029.
Ramlow, M., Foster, E. J., Del Grosso, S. J., & Cotrufo, M. F. (2019). Broadcast woody biochar provides limited benefits to deficit irrigation maize in Colorado. Agriculture, Ecosystems & Environment,269, 71–81. https://doi.org/10.1016/j.agee.2018.09.017.
Reale, L., Ferranti, F., Mantilacci, S., Corboli, M., Aversa, S., Landucci, F., et al. (2016). Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere,145, 98–105. https://doi.org/10.1016/j.chemosphere.2015.11.047.
Rhee, S. W., & Park, H. S. (2010). Effect of mixing ratio of woody waste and food waste on the characteristics of carbonization residue. Journal of Material Cycles and Waste Management,12(3), 220–226. https://doi.org/10.1007/s10163-010-0291-z.
Rinklebe, J., Shaheen, S. M., Schröter, F., & Rennert, T. (2016). Exploiting biogeochemical and spectroscopic techniques to assess the geochemical distribution and release dynamics of chromium and lead in a contaminated floodplain soil. Chemosphere,150, 390–397. https://doi.org/10.1016/j.chemosphere.2016.02.021.
Rondon, M. A., Lehmann, J., Ramírez, J., & Hurtado, M. (2007). Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biology and Fertility of Soils,43(6), 699–708. https://doi.org/10.1007/s00374-006-0152-z.
Rosales, E., Meijide, J., Pazos, M., & Sanromán, M. A. (2017). Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresource Technology,246, 176–192. https://doi.org/10.1016/j.biortech.2017.06.084.
Ross, S. M. (1994). Sources and forms of potentially toxic metals in soil-plant systems. Toxic Metals in Soil-Plant Systems, 3–25.
Saha, R., Nandi, R., & Saha, B. (2011). Sources and toxicity of hexavalent chromium. Journal of Coordination Chemistry,64(10), 1782–1806. https://doi.org/10.1080/00958972.2011.583646.
Sehrish, A. K., Aziz, R., Hussain, M. M., Rafiq, M. T., Rizwan, M., Muhammad, N., et al. (2019). Effect of poultry litter biochar on chromium (Cr) bioavailability and accumulation in spinach (Spinacia oleracea) grown in Cr-polluted soil. Arabian Journal of Geosciences,12(2), 57. https://doi.org/10.1007/s12517-018-4213-z.
Shaheen, S. M., Niazi, N. K., Hassan, N. E., Bibi, I., Wang, H., Tsang, D. C., et al. (2019). Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. International Materials Reviews,64(4), 216–247. https://doi.org/10.1080/09506608.2018.1473096.
Shaheen, S. M., Rinklebe, J., Rupp, H., & Meissner, R. (2014). Lysimeter trials to assess the impact of different flood–dry-cycles on the dynamics of pore water concentrations of As, Cr, Mo and V in a contaminated floodplain soil. Geoderma,228, 5–13. https://doi.org/10.1016/j.geoderma.2013.12.030.
Shahid, M., Shamshad, S., Rafiq, M., Khalid, S., Bibi, I., Niazi, N. K., et al. (2017). Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil–plant system: A review. Chemosphere,178, 513–533. https://doi.org/10.1016/j.chemosphere.2017.03.074.
Shang, J., Zong, M., Yu, Y., Kong, X., Du, Q., & Liao, Q. (2017). Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. Journal of Environmental Management,197, 331–337. https://doi.org/10.1016/j.jenvman.2017.03.085.
Shen, Y. S., Wang, S. L., Tzou, Y. M., Yan, Y. Y., & Kuan, W. H. (2012). Removal of hexavalent Cr by coconut coir and derived chars—The effect of surface functionality. Bioresource Technology,104, 165–172. https://doi.org/10.1016/j.biortech.2011.10.096.
Solaiman, Z. M., & Anawar, H. M. (2015). Application of biochars for soil constraints: Challenges and solutions. Pedosphere,25(5), 631–638. https://doi.org/10.1016/S1002-0160(15)30044-8.
Song, C., Shan, S., Müller, K., Wu, S., Niazi, N. K., Xu, S., et al. (2018). Characterization of pig manure-derived hydrochars for their potential application as fertilizer. Environmental Science and Pollution Research,25(26), 25772–25779. https://doi.org/10.1007/s11356-017-0301-y.
Su, H., Fang, Z., Tsang, P. E., Zheng, L., Cheng, W., Fang, J., et al. (2016). Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. Journal of Hazardous Materials,318, 533–540. https://doi.org/10.1016/j.jhazmat.2016.07.039.
Subedi, R., Taupe, N., Pelissetti, S., Petruzzelli, L., Bertora, C., Leahy, J. J., et al. (2016). Greenhouse gas emissions and soil properties following amendment with manure-derived biochars: Influence of pyrolysis temperature and feedstock type. Journal of Environmental Management,166, 73–83. https://doi.org/10.1016/j.jenvman.2015.10.007.
Sud, D., Mahajan, G., & Kaur, M. P. (2008). Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresource Technology,99(14), 6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064.
Sun, Y., Iris, K. M., Tsang, D. C., Cao, X., Lin, D., Wang, L., et al. (2019). Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater. Environment International,124, 521–532. https://doi.org/10.1016/j.envint.2019.01.047.
Tripathi, M., Sahu, J. N., & Ganesan, P. (2016). Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renewable and Sustainable Energy Reviews,55, 467–481. https://doi.org/10.1016/j.rser.2015.10.122.
Uchimiya, M., Lima, I. M., Thomas Klasson, K., Chang, S., Wartelle, L. H., & Rodgers, J. E. (2010). Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. Journal of Agricultural and Food Chemistry,58(9), 5538–5544. https://doi.org/10.1021/jf9044217.
Ullah, I., Nadeem, R., Iqbal, M., & Manzoor, Q. (2013). Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecological Engineering,60, 99–107. https://doi.org/10.1016/j.ecoleng.2013.07.028.
USEPA. 1995. USEPA National primary drinking water regulations chromium. EPA No. B11-F-95-002d-T, USEPA, Washington, DC.
Uzoma, K. C., Inoue, M., Andry, H., Fujimaki, H., Zahoor, A., & Nishihara, E. (2011). Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use and Management,27(2), 205–212. https://doi.org/10.1111/j.1475-2743.2011.00340.x.
Velez, P. A., Talano, M. A., Paisio, C. E., Agostini, E., González, P. S. (2016) Synergistic effect of chickpea plants and as a natural system for chromium phytoremediation. Environmental Technology 38(17), 2164–2172
Vimercati, L., Gatti, M. F., Gagliardi, T., Cuccaro, F., De Maria, L., Caputi, A., et al. (2017). Environmental exposure to arsenic and chromium in an industrial area. Environmental Science and Pollution Research,24(12), 11528–11535. https://doi.org/10.1007/s11356-017-8827-6.
Vogel, C., Radtke, M., Reinholz, U., Schäfers, F., & Adam, C. (2015). Chemical state of chromium, sulfur, and iron in sewage sludge ash based phosphorus fertilizers. ACS Sustainable Chemistry and Engineering,3(10), 2376–2380. https://doi.org/10.1021/acssuschemeng.5b00678.
Wang, C., Gu, L., Ge, S., Liu, X., Zhang, X., & Chen, X. (2018). Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(VI) co-contaminated soil. Environmental Technology. https://doi.org/10.1080/09593330.2018.1441328.
Wang, C., Gu, L., Liu, X., Zhang, X., Cao, L., & Hu, X. (2016). Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. International Biodeterioration and Biodegradation,111, 78–84. https://doi.org/10.1016/j.ibiod.2016.04.029.
Wang, X. S., Chen, L. F., Li, F. Y., Chen, K. L., Wan, W. Y., & Tang, Y. J. (2010). Removal of Cr(VI) with wheat-residue derived black carbon: Reaction mechanism and adsorption performance. Journal of Hazardous Materials,175(1–3), 816–822. https://doi.org/10.1016/j.jhazmat.2009.10.082.
Wang, Y., Peng, B., Yang, Z., Chai, L., Liao, Q., Zhang, Z., et al. (2015). Bacterial community dynamics during bioremediation of Cr(VI)-contaminated soil. Applied Soil Ecology,85, 50–55. https://doi.org/10.1016/j.apsoil.2014.09.002.
Wu, D., Senbayram, M., Zang, H., Ugurlar, F., Aydemir, S., Brüggemann, N., et al. (2018). Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Applied Soil Ecology,129, 121–127. https://doi.org/10.1016/j.apsoil.2018.05.009.
Wu, W., Li, J., Lan, T., Müller, K., Niazi, N. K., Chen, X., et al. (2017). Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Science of the Total Environment,576, 766–774. https://doi.org/10.1016/j.scitotenv.2016.10.163.
Wu, W., Yang, M., Feng, Q., McGrouther, K., Wang, H., Lu, H., et al. (2012). Chemical characterization of rice straw-derived biochar for soil amendment. Biomass and Bioenergy,47, 268–276. https://doi.org/10.1016/j.biombioe.2012.09.034.
Xia, S., Song, Z., Jeyakumar, P., Shaheen, S. M., Rinklebe, J., Ok, Y. S., et al. (2019). A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Critical Reviews in Environmental Science and Technology. https://doi.org/10.1080/10643389.2018.1564526.
Xiao, R., Wang, J. J., Li, R., Park, J., Meng, Y., Zhou, B., et al. (2018). Enhanced sorption of hexavalent chromium [Cr(VI)] from aqueous solutions by diluted sulfuric acid-assisted MgO-coated biochar composite. Chemosphere,208, 408–416. https://doi.org/10.1016/j.chemosphere.2018.05.175.
Xie, T., Reddy, K. R., Wang, C., Yargicoglu, E., & Spokas, K. (2015). Characteristics and applications of biochar for environmental remediation: A review. Critical Reviews in Environmental Science and Technology,45(9), 939–969. https://doi.org/10.1080/10643389.2014.924180.
Xin, Y., Cao, H., Yuan, Q., & Wang, D. (2017). Two-step gasification of cattle manure for hydrogen-rich gas production: Effect of biochar preparation temperature and gasification temperature. Waste Management,68, 618–625. https://doi.org/10.1016/j.wasman.2017.06.007.
Xu, X., Cao, X., Zhao, L., Wang, H., Yu, H., & Gao, B. (2013). Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environmental Science and Pollution Research,20(1), 358–368. https://doi.org/10.1007/s11356-012-0873-5.
Xu, X., Huang, H., Zhang, Y., Xu, Z., & Cao, X. (2019). Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environmental Pollution,244, 423–430. https://doi.org/10.1016/j.envpol.2018.10.068.
Xu, Y., & Zhao, D. (2007). Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research,41(10), 2101–2108. https://doi.org/10.1016/j.watres.2007.02.037.
Yahya, M. A., Al-Qodah, Z., & Ngah, C. Z. (2015). Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renewable and Sustainable Energy Reviews,46, 218–235. https://doi.org/10.1016/j.rser.2015.02.051.
Yang, X., Liu, J., McGrouther, K., Huang, H., Lu, K., Guo, X., et al. (2016). Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environmental Science and Pollution Research,23(2), 974–984. https://doi.org/10.1007/s11356-015-4233-0.
Yang, X., Lu, K., McGrouther, K., Che, L., Hu, G., Wang, Q., et al. (2017). Bioavailability of Cd and Zn in soils treated with biochars derived from tobacco stalk and dead pigs. Journal of Soils and Sediments,17(3), 751–762. https://doi.org/10.1007/s11368-015-1326-9.
Yao, Y., Gao, B., Wu, F., Zhang, C., & Yang, L. (2015). Engineered biochar from biofuel residue: Characterization and its silver removal potential. ACS Applied Materials & Interfaces,7(19), 10634–10640. https://doi.org/10.1021/acsami.5b03131.
Yu, K. L., Lau, B. F., Show, P. L., Ong, H. C., Ling, T. C., Chen, W. H., et al. (2017). Recent developments on algal biochar production and characterization. Bioresource Technology,246, 2–11. https://doi.org/10.1016/j.biortech.2017.08.009.
Yuan, H., Lu, T., Wang, Y., Chen, Y., & Lei, T. (2016). Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma,267, 17–23. https://doi.org/10.1016/j.geoderma.2015.12.020.
Yuan, J. H., Xu, R. K., & Zhang, H. (2011). The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresource Technology,102(3), 3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018.
Yuan, Y., Bolan, N., Prévoteau, A., Vithanage, M., Biswas, J. K., Ok, Y. S., et al. (2017). Applications of biochar in redox-mediated reactions. Bioresource Technology,246, 271–281. https://doi.org/10.1016/j.biortech.2017.06.154.
Zama, E. F., Reid, B. J., Arp, H. P. H., Sun, G. X., Yuan, H. Y., & Zhu, Y. G. (2018). Advances in research on the use of biochar in soil for remediation: A review. Journal of Soils and Sediments,18, 2433–2450. https://doi.org/10.1007/s11368-018-2000-9.
Zayed, A. M., & Terry, N. (2003). Chromium in the environment: Factors affecting biological remediation. Plant and Soil,249(1), 139–156. https://doi.org/10.1023/a:1022504826342.
Zelmanov, G., & Semiat, R. (2011). Iron (Fe3+) oxide/hydroxide nanoparticles-based agglomerates suspension as adsorbent for chromium (Cr6+) removal from water and recovery. Separation and Purification Technology,80(2), 330–337. https://doi.org/10.1016/j.seppur.2011.05.016.
Zhang, J., Chen, S., Zhang, H., & Wang, X. (2017). Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars. Bioresource Technology,238, 484–491. https://doi.org/10.1016/j.biortech.2017.04.081.
Zhang, J., Liu, J., & Liu, R. (2015a). Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresource Technology,176, 288–291. https://doi.org/10.1016/j.biortech.2014.11.011.
Zhang, H., Voroney, R. P., & Price, G. W. (2015b). Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biology & Biochemistry,83, 19–28. https://doi.org/10.1016/j.soilbio.2015.01.006.
Zhang, M., Chen, Z., Chen, Q., Zou, H., Lou, J., & He, J. (2008). Investigating DNA damage in tannery workers occupationally exposed to trivalent chromium using comet assay. Mutation Research/Genetic Toxicology and Environmental Mutagenesis,654(1), 45–51. https://doi.org/10.1016/j.mrgentox.2008.04.011.
Zhang, S., Lyu, H., Tang, J., Song, B., Zhen, M., & Liu, X. (2019). A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere,217, 686–694. https://doi.org/10.1016/j.chemosphere.2018.11.040.
Zhang, W., Mao, S., Chen, H., Huang, L., & Qiu, R. (2013). Pb (II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresource Technology,147, 545–552. https://doi.org/10.1016/j.biortech.2013.08.082.
Zhang, W., & Tsang, D. C. (2019). Sludge-derived biochar and its application in soil fixation. Biochar from Biomass and Waste. https://doi.org/10.1016/B978-0-12-811729-3.00013-3.
Zhitkovich, A. (2011). Chromium in drinking water: Sources, metabolism, and cancer risks. Chemical Research in Toxicology,24(10), 1617–1629. https://doi.org/10.1021/tx200251t.
Zhou, J., Chen, H., Thring, R. W., & Arocena, J. M. (2019). Chemical pretreatment of rice straw biochar: Effect on biochar properties and hexavalent chromium adsorption. International Journal of Environmental Research,13(1), 91–105. https://doi.org/10.1007/s41742-018-0156-1.
Zhou, L., Liu, Y., Liu, S., Yin, Y., Zeng, G., Tan, X., et al. (2016). Investigation of the adsorption–reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresource Technology,218, 351–359. https://doi.org/10.1016/j.biortech.2016.06.102.
Zhu, S., Huang, X., Wang, D., Wang, L., & Ma, F. (2018). Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere,207, 50–59. https://doi.org/10.1016/j.chemosphere.2018.05.046.
Zhu, X., Chen, B., Zhu, L., & Xing, B. (2017). Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environmental Pollution, 227, 98–115.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xia, S., Song, Z., Jeyakumar, P. et al. Characteristics and applications of biochar for remediating Cr(VI)-contaminated soils and wastewater. Environ Geochem Health 42, 1543–1567 (2020). https://doi.org/10.1007/s10653-019-00445-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00445-w