Abstract
Mitigating industrial air pollution is a big challenge, in such scenario screening of plants as a bio monitor is extremely significant. It requires proper selection and screening of sensitive and tolerant plant species which are bio indicator and sink for air pollution. The present study was designed to evaluate the Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API) of the common flora. Fifteen common plant species from among trees, herb and shrubs i.e. Chenopodium album (Chenopodiaceae), Parthenium hysterophorus (Asteraceae), Amaranthus viridis (Amaranthaceae), Lantana camara (Verbenaceaea), Ziziphus nummulari (Rhamnaceae), Silibum merianum (Asteraceae), Cannabis sativa (Cannabinaceae), Calatropis procera (Asclepediaceae), Ricinus communis (Euphorbiaceae), Melia azadirachta (Meliaceae), Psidium guajava (Myrtaceae), Eucalyptus globules (Myrtaceae), Broussonetia papyrifera (Moraceae), Withania somnifera (Solanaceae) and Sapium sabiferum (Euphorbiaceae) were selected growing frequently in vicinity of Marble industries in Potwar region. APTI and API of selected plant species were analyzed by determining important biochemical parameter i.e. total chlorophyll, ascorbic acid, relative water content and pH etc. Furthermore the selected vegetation was studied for physiological, economic, morphological and biological characteristics. The soil of studied sites was analyzed. It was found that most the selected plant species are sensitive to air pollution. However B. papyrifera, E. globulus and R. communis shows the highest API and therefore recommended for plantation in marble dust pollution stress area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nature has blessed Pakistan with more than 297 billion tons of marble reserves (Pakistan Stone Development Company, PASDEC), and this sale of raw marble to foreign buyers is a major source of foreign revenue. However, the marble production industry is a major waste-generating industry and a chief contributor of both liquid and solid waste to the environment. Fine particles with an aerodynamic diameter of <2 mm are produced during the cutting of marble, with around 25 % of these dust particles produced cutting marble slabs of 2 cm thickness from 1-m3 marble blocks (Yavuz Çelik and Sabah 2008). As marble passes through the processing stages, i.e., cutting, grinding, loading, polishing, buffing, not only is particulate matter (PM) released into the environment in the form of dust, but also liquid (water) in the form of slurry (Saini et al. 2011; Ashraf et al. 2014). The PM emitted from marble and other stone crushing industries make a significant contribution to the emission of fine suspended particles in the atmosphere (Belardi et al. 2013) which are the major pollutants contaminating the air. Since there is no technique to detect the impact of emitted pollutants at the point source, plants provide a means of filtering them out of air, somewhat like mopping the floor to remove dust. The response(s) of these plants to the pollutants can then be used to study the impacts of the pollutants on the plants’ physiology and morphology. However, the susceptibility and reaction of plants to air pollutants are variable. As a general rule, plant species which show the less resistance to the specific pollutant being studied are used as biological indicators as there is a synergistic action between plants and the air pollutant (Lakshmi et al. 2009). Many authors have worked on the impacts of pollutants on plants (Agbaire and Esiefarienrhe 2009; Agrawal and Tiwari 1997; Babu et al. 2013; Bakiyaraj and Ayyappan 2014; Belardi et al. 2013; Deepalakshmi et al. 2013; Grover et al. 2001; Joshi et al. 2011; Jyothi and Jaya 2010; Kabas et al. 2012; Klumpp et al. 2000; Kuddus et al. 2011; Kumar 2013; Lakshmi et al. 2009; Lima et al. 2000; Liu and Ding 2008; Loganathan and Ilyas 2012; Mahecha et al. 2013; Martos et al. 2000; Ninave et al. 2001; Nwadinigwe 2014; Overstreet et al. 2011; Pandey and Agrawal 1994; Prajapati 2012; Prajapati and Tripathi 2008; Radhapriya et al. 2012; Rai and Panda 2014; Raina et al. 2008; Randhi and Reddy 2012; Rawat and Banerjee 1996; Raza and Murthy 1988; Saini et al. 2011; Salami et al. 2004; Saxena and Ghosh 2013; Singh 2005; Singh and Rao 1983; Sudhalakhsmi et al. 2007; Thakar and Mishra 2010; Tripathi et al. 2009; Tripathi and Gautam 2007; Tsega and Prasad 2014; Yavuz Çelik and Sabah 2008).
One means to assess the response of plants to these pollutants is the Air Pollution Tolerance Index (APTI). The APTI is a unique value that is based on the concurrent analysis of four parameters, namely, total chlorophyll (TC) content in leaf extracts, ascorbic acid content, pH, and relative water content (RWC), rather than on each factor separately. It provides an insight into the resistance and tolerance of the test species towards air pollution by categorizing the plants in terms of their specific response to air pollution and then ranking them in order of sensitivity or tolerance to particulate air pollution (Kuddus et al. 2011; Rai and Panda 2014).
The objective of our study was to determine the sensitivity and air pollution tolerance of common plant species growing around industrial marble processing plants in the Potwar region. Using the APTI, biological and economic characteristics, and morphological features, we developed an Anticipated Performance Index (API) for selected flora. We found that the APTI and API are ideal indicator tools for recommending plant species for landscape plantation in the vicinity of marble industries.
Study area
The study area was the Potwar region of Pakistan. Due to rapid housing development and urbanization, the demand for construction material has been increasing rapidly in recent years, resulting in the increased production of construction materials, such as marble, stones, crusher, among others. Environmental problems in the area have been increasing in parallel, especially air pollution. During 2013 we sampled plants under both sunny and dry weather conditions in the study area in order to estimate the air pollution tolerance and identify sensitive plants.
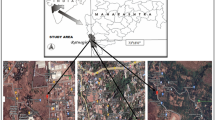
The selected area for study was in the vicinity of industrial marble processing plants where marble dust due to crushing and other processes was clearly visible on flora (Fig. 1). The area is also characterized by low rain fall and has been subjected to extensive deforestation, with vegetation becoming scarce.
Materials and methods
Fifteen plant species found in the vicinity of the marble processing plants, namely, Amaranthus viridus, Broussonetia papyrifera, Calatropis procera, Cannabis sativa, Chenopodium album, Eucalyptus globules, Lantana camara, Melia azadirachta, Parthenium hysterophorus, Psidium guajava, Ricinus communis, Sapium sabiferum, Silibum merianum, Withania somnifera and Ziziphus nimmularia, were selected for the study. Plant species were selected for inclusion in the study based on: the direction of air flow; plant abundance; presence/absence of visible morphological impacts of pollution on foliage; economic significance of the specific plant species. Fresh leaves were collected from the selected plants at a height of 2–4 m for trees, and the top leaves for herbs and shrubs; all collections were made during the peak crushing time, which was usually in the morning. The selected leaves were collected in ten replicates and immediately placed into a polythene bag and stored with dry ice in a container. Plants collected from experimental sites (i.e., sites close to marble processing plants) formed the experimental (polluted) group, and those collected from garden sites at a distance from the plants formed the control group.
Leaves were analyzed for RWC according to Liu and Ding (2008), and pH was determined according to Rai and Panda (2014). The collected leaves were further analyzed for TC content (Nwadinigwe 2014) and ascorbic acid (Keller and Schwager 1977). The values were then computed into the formulas given by Singh and Rao (1983) and APTI was determined using the following formula.
where A is ascorbic acid content (mg/g), T is for TC content (mg/g), P is the pH of leaf extracts, and R is the RWC of the leaf (%).
Ascorbic acid concentration was calculated using the formula:
where W is the weight of the fresh leaf, V 1 is the volume of the supernatant, and V is the total volume of the mixture.
The selected plants were also analyzed for total amount of dust deposited on leaf surface with respect to leaf area following the method of Prusty et al. (2005), with leaf area calculated according to Saini et al. (2011). The amount of dust deposited was calculated by first determining the difference in weight between freshly collected leaves in the field and the same washed leaves (dried leaves); the difference was divided by the area of the respective leaf. The number of stomata, their distribution, and the number of blocked and unblocked stomata were calculated per square centimeter.
Replicate soil samples were also taken from the study area, both where the plants were collected and close to the crushing units. These samples were analyzed for soil moisture and pH; the results were calculated as the mean ± standard deviation.
Results
The values for TC content, ascorbic acid content, RWC, and pH of the leaves sampled from the selected plants were determined for calculating the APTI value of each plant species (Table 1). Plants collected from control and polluted sites were surveyed prior to the study. Control plants were collected from residential gardens assessed to be situated outside the area affected by the marble processing plants, and experimental (polluted) plants were collected in the close vicinity of the marble processing plants. The APTI was used to establish a hierarchy of species tolerance to air pollution. The APTI calculated for the selected species ranged between a maximum of 17 (Parthenium hysterophorus) and a minimum of 11 (Silybum marianum) at the control site, and from a maximum of 16 (P. hysterophorus) to a minimum of 10 (S. marianum) at the polluted site. Based on the APTI value, the plant species were characterized as shown in Table 1 and Fig. 2, where an APTI value of <1 indicates a very sensitive response, between 1 and 16, a sensitive response, between 17 and 29, an intermediate response, and between 30 and 100, a tolerant response (Randhi and Reddy 2012).
The RWC of the plants at the control sites were found to range from a maximum of 100 % (Lantana camara) to a minimum of 29 % (P. hysterophorus), while the RWC of plants at the polluted site ranged from a maximum of 81 % (L. camara) to a minimum of 21.3 % (P. hysterophorus) (Table 1). The mean concentration of ascorbic acid in plants from the polluted and control sites is shown in Table 1, ranging from a maximum of 10 (P. hysterophorus) to a minimum of 3.8 (Cannabis sativa) at the control site and from a maximum of 9.5 (P. hysterophorus) to a minimum of 2 (S. merianum) at the polluted site. The TC content of plants ranged from a maximum of 6.6 (P. hysterophorus) to a minimum of 2.5 (S. merianum) at the control site and from a maximum of 5.6 (P. hysterophorus) to a minimum of 2 (Withania somnifera and S. marianum) at the polluted site (Table 1). The pH of the leaf extracts ranged from a maximum of 9.25 (Chenopodim album) to a minimum of 7.1 (Eucalyptus globules) at the control sites and from a maximum of 9 (P. hysterophorus) to a minimum of 7.1 (Ricinius communis) in the vicinity of the marble processing plant (polluted site). Comparison of the APTI values for the selected plant species from both sites revealed that the APTI value was higher at the control sites than at the polluted site for each plant species. For plants at the control site the APTI values ranged from a maximum of 17 (P. hysterophorus) to a minimum of 10.4 (C. sativa), whereas at polluted site the range of APTI was a maximum of 16 (P. hysterophorus) and a minimum of 10.4 (S. merianum) (Table 1). The selected plant species were evaluated to determine the API index on basis of parameters and scale given in Tables 2, 3, 4.
The dust deposition capacity of 15 roadside plants at both sites is presented in Fig. 3, which shows that the dust deposition capacity was greater among plants exposed to air pollution, with a maximum of 0.65 mg/cm2 for S. merianum and a minimum of 0.09 mg/cm2 for A. viridus. Figure 4 shows the mean leaf surface area of plants at both sites. In terms of leaf deposition, the trend for trees was Broussonetia papyrifera > Psidium gujava > Melia azadirachta > E. globules > Sapium sabiferum, for shrubs, Silibum marianum > R. communis > Ziziphus nummularia > Calatropis procera > C. sativa > Lantana camara > P. hysterophorus, and for herbs, W. somnifera > C. album > Amaranthus viridis. The difference among plants was significant (p < 0.05).
The pH of the soils at both the control and polluted sites were basic, with a mean pH of 8.31 ± 0.47 at the polluted site compared to a mean pH of 7.76 ± 0.47 at the control sites. Soil EC values were higher in polluted soils than in soils samples at the control sites (5.95 ± 2.42 vs. 2.63 ± 1.82, respectively) (Fig. 5). Soil moisture content at sites moisture content (MC) and moisture factor (MF) from the polluted site was 2.14 ± 1.48 and 1.29 ± 0.39, respectively; at control sites MC and MF, the respective soil moisture contrent was 1.83 ± 0.85 and 1.42 ± 0.488 (Fig. 5).
Discussion
The degradation of air quality is of major environmental concern as it affects living organisms not only at many urban and industrial sites, both flora and fauna, but also in the surrounding regions (Kuddus et al. 2011). The major constituents of PM emitted from marble processing plants are calcium (Ca) and magnesium (Mg) compounds. A considerable amount of these compounds accumulates on the soil, water, and vegetation surrounding the industrial sites (Ademoroti 1996). In such cases, flora in the vicinity of the pollutant-emitting sources absorb, accumulate, and assimilate the contaminants during normal gaseous exchange and nutrient uptake via the soil, causing degradation of their leaf structure and physiology, such as that reported in our study, where the plants growing in the vicinity of the marble processing plant showed significant signs of visible injuries and chlorosis of leaves. Alkaline airborne particles, such as limestone, damage plant surfaces (Jeffrey Brandt and Rhoades 1973) and harden the leaves, resulting in a loss of flexibility in leaf texture (Prajapati 2012; Joshi and Swami 2007). Plants growing in such areas show stunted growth, hard leaves, and reduced leaf area, as reported by Salami et al. (2004). The results of our study indicate that plants subjected to pollution from the marble processing plant had a significant reduction in leaf size, necrosis and chlorosis—changes which affect photosynthetic activities. Liu and Ding (2008) suggest that leaves provide the surface area on which the pollutants are deposited, subsequently accumulating and ultimately absorbed by the plant. Consequently, the plant removes pollutants from the air by absorption, deposition, and aerosols over leaf surfaces and through the fallout of PM on the vegetation (Rawat and Banerjee 1996).
We observed that plants in the polluted site had a lower average leaf area than those at the control sites (Fig. 4). The leaves of R. communis, C. procera, C. sativa, E. globulus, and B. papyrifera showed a considerable reduction of leaf area at the polluted site while the same species at control site exhibited a relatively larger leaf surface area. These results are in agreement with the results obtained by Seyyednejad and Koochak (2011) who observed a reduction in leaf area in E. cammaldulensis growing at a polluted site exposed to air pollutants. In our study, the leaf area was very similar in L. camara, W. somnifera, and P. guajava growing at the polluted and control sites.
The dust that is deposited on leaves ultimately forms a thick coating (Raina et al. 2008), thereby preventing sunlight penetration. We noted, as expected, greater dust deposition on plants in the vicinity of the marble processing plant compared to the control plants. Leaf position, area, geometry, phyllotaxy, shape, height of plant, and type of canopy all determine the dust-intercepting capacity of the leaf (Nowak 1994), as well as the presence or absence of hair and cuticle and the length of the petiole (Prajapati and Tripathi 2008). We noted that the greatest deposition of dust was on S. merianum, R. communis, W. somnifera, B. papyrifera, L. camara, and C. procera, likely due to the relative wide lamina, straight orientation, and hairy or rough surface of the leaves of these plants, thereby providing a large surface area for dust retention. Plants with a wide lamina surface have the greatest dust deposition, and a rough surface of the lamina also leads to a high dust content. The leaves of these plants are also close to the ground and heavily covered with dust. Thakar and Mishra (2010) also found this potential in L. camara. This dust-retaining capacity results impacts the sensitive plants which are more susceptible to pollution as it affects its biochemical and physiological features (Singh 2005). Less dust was deposited on E. globules due to its aspect, height, and thin lamina of its leaves.
Stomata regulate the efficiency of photosynthesis and exchange of gases both inside and outside the plant. In this study total number of clogged and unclogged stomata in both abaxial and adaxial stomata in 1 mm2 of leaf area were counted. There were a higher number of clogged stomata in leaves from the polluted site than in those from the control site (Table 5). Extensive stomatal clogging was also observed in the leaves of roadside herbs exposed to vehicular exhaust, with the a higher total number of stomata on the abaxial surface than on the adaxial surface. Sharma et al. (1980) reported stomatal abnormalities in plants exposed to pollution. We found a lower number of clogged stomata B. papyrifera, likely due to the presence of protective trichomes on the abaxial epidermis which do not allow dust to come into direct contact with leaves. This protection from trichomes enables this plant to survive in stress conditions. Similar results for B. papyrifera have been reported. Extensive stomatal clogging was observed in C. sativa, C. procera, Psidium gujava, Ricinius communis, W. somnifera, and A. viridis at the polluted site. Dust deposits on the leaves, when emitted from a point source, cause stomata clogging (Prajapati and Tripathi 2008). In terms of total numbers of stomata, on both the abaxial and adaxial epidermis, there were more in plants at the control site than in those at the polluted site. It is also reported that the number of stomata in C. sativa decreased in plants growing in the vicinity of the pollution source, with C. sativa plants in the control area having an increased total number of stomata on both the abaxial and adaxial surface. In contrast to C. sativa, we found that M. azadirachta had a higher total number of stomata on its abaxial side in plants growing at the polluted site in comparison to the control site. The total number of stomata in Z. nummularia was observed to be higher in plants growing at the polluted site than in those growing at the control site. However, one the adaxial surface, the total number of clogged stomata was less (72) in plants at the polluted site than at the control site (90). The opposite trend was seen for the abaxial surface, where the clogging of stomata was higher at the polluted site. It is reported that stomata are present on both sides of Chenopodium album, but are more abundant on the abaxial surface than on the adaxial surface. Similar results were observed in this study for C. album. Parthenium hysterophorus showed a totally different behavior to all other selected plants, with the total number of stomata present on the abaxial and adaxial surfaces at the polluted site being 64 and 41, respectively, and at the control site, 70 (abaxial) and 45 (adaxial). In R. communis, there were 32 and 11 clogged stomata on the abaxial surface at the polluted site and control site, respectively, and in S. sabiferum, the total number of stomata was higher on the abaxial surface of plants growing at the polluted site. Of all plants growing at the polluted site, Silibum marianum had the highest total number of stomata, on both the abaxial and adaxial surfaces. This plant was highly sensitive to the pollution emitted by the marble process plant. Beckett et al. (2000) reported that trees with a fine and complex leaf structure effectively capture pollutant particles, with is in line with general observation that the amount of dust captured by the leaf largely depends on the external structure of the leaf and leaf geometry. Iqbal and Shafig (2001) reported on the impact of dust deposition on the stomata. These authors found that alkaline dust causes foliar injuries to leaves which could be easily observed in particular on leaves of short plants, such as those in our study (W. somnifera, C procera, A. viridus), clogging the stomata present on the surface of leaf. Plants located in close vicinity to the factory showed an increased amount of Ca encrusted on the surface of the leaves, resulting in the erosion of the epicuticular wax; in addition, a large number of stomata were plugged with cuticular wax or hairs.
Leaf membrane permeability is affected by air pollutants, and an indicator of leaf relative membrane permeability is its EC. An increase in membrane permeability has been observed in plants exposed to air pollutants such as sulfur dioxide and PM. Air pollutants cause solutes to leak out, leading to an increase in EC, but other environmental stresses, such as a decrease in soil moisture content or frost injury, may lead to the same changes. Marble dust is alkaline and reacts with the cell membrane to cause foliar injury (Prajapati and Tripathi 2008). In our study, the membrane permeability of the plants was higher at the polluted site than at the control sites due to extensive dust deposition on the leaves. There was also a decrease in soil moisture content. Water stress causes tissue desiccation and electrolyte leakage. The presence of acidic gases in the atmosphere may also account for the increased membrane permeability in plants at the polluted site.
Table 1 illustrates the chlorophyll contents observed in plants growing in polluted and control sites. Almost all of the studied plants showed a marked reduction in TC content when the control and polluted sites were compared. Similar results were reported by Tripathi and Gautam (2007). We found the maximum and minimum TC content at the control site to be 7 mg/g (C. procera) and 2.5 mg/g (S. marianum), respectively; at the polluted site these values were 5.6 mg/g (P. hysterophorus) and 2 mg/g (W. somnifera and S. marianum), respectively. TC content is an indicator of the photosynthesis activity, growth, and developmental progress of a plant and varies from one plant to other.
TC content is related to the amount of dust deposited on the leaf surface (Fig. 2), with plants showing a marked reduction in TC content having a high deposition capacity (e.g., A. viridis, L. camara, S. marianum). This deposition interferes with the penetration of sunlight into the leaf and hinders the process of pigment formation. The alkaline nature of marble dust (Raina et al. 2008) causes foliar injuries when deposited on the leaf surface, as was clearly visible on S. marianum leaves. The decrease in the pigment content causes yellowing of leaves which, in combination with leaf injuries, causes premature leaf fall and low plant productivity (Prajapati 2012). We found that the TC content of leaves varied from species to species (Table 1), but the age of the leaf and the environmental stresses are also known to be risk factors. The decrease in TC content of plants found on the polluted site in our study confirms that the primary target site is the chloroplast (Grover et al. 2001). This is the most damaging effect of particle deposition since TC is an index of plant productivity (Bakiyaraj and Ayyappan 2014), with chloroplast degradation used as an environmental indicator (Ninave et al. 2001). The present study reveals that TC content varied with the pollution status of the area.
The mean ascorbic acid content in the leaves of the sampled plants at the polluted and control sites is given in Table 1. At the control site, maximum ascorbic acid content was found in P. hysterophorus (10 mg/g) and the minimum in P. guajava, whereas at the polluted site it ranged from 9.5 mg/g (P. hysterophorus) to 3.5 mg/g (E. globules). The reduced ascorbic acid content in the leaves of these and other plant species at the polluted site supports the sensitivity of these plants towards air pollutants (Jyothi and Jaya 2010). Leaf ascorbic acid content was found to be decreased with soil contamination and air pollution in Tibouchina pulchra saplings (Klumpp et al. 2000). Since ascorbic acid is an antioxidant that is present in actively growing plant parts, its content influences the plant’s resistance to air pollutants. The level of pollution resistance increases with increasing level of ascorbic acid (Lima et al. 2000), whereas a decrease in ascorbic acid content is an indicator of deterioration in the plant defense systems, as ascorbic acid is consumed during removal of the cytotoxic radicals generated in response to pollutants that penetrate the leaves (Pandey and Agrawal 1994).
All plants showed a general trend towards a basic pH (Table 1), ranging from 7.1 to 9.25 at the control site, and from 7.6 to 9 at the polluted site. The pH of the leaf extract of plants at the polluted site tended to be basic due to the effects of alkaline marble dust deposition (CaCO3). Radhapriya et al. (2012) reported similar findings in plants in the vicinity of cement industries. L. camara, W. somnifera, C. procera, B. papyrifera, and A. viridis had a slightly basic pH at the control site. A low pH is an indicator of a sensitivity to air pollution. A neutral pH was seen for P. gujava, E. globules, and R. communis at the control site. Alkalinity in the leaf extract pH may be accounted for the alkaline soil in the polluted area. Joshi et al. (2011) believe that the alkaline and basic nature of pollutants is responsible for chlorophyll degradation through stomatal blocking and phaephytin production.
The RWC (Table 1) of the plants ranged from 85.21 % (P. guajava) to 21.3 % (P. hysterophorus) at the polluted site and from 100 % (L. camara) to 21.3 % (B. papyrifera and P. hysterophorus) at the control site. The RWC of a leaf is an indicator of the water content present relative to its full turgidity, and this measurement is associated with the protoplasmic permeability of cells, with permeable cells resulting in the loss of water and dissolved nutrients and, ultimately, early leaf senescence (Agrawal and Tiwari 1997). Hence, plants with a high RWC may be tolerant to pollutants in a polluted environment (Jyothi and Jaya 2010). Air pollutants cause a decrease in the RWC of leaves, indicating a disturbed physiological status of the plant (Deepalakshmi et al. 2013). Lakshmi et al. (2009) proposed that the RWC of intermediately tolerant plant species should be in the range 58–84 % and that of sensitive plant species should range from 51.3 to 84 %. Bakiyaraj and Ayyappan (2014) reported the range of RWC in plants at a control site to be 94.5–36.07, while at the experimental site it ranged from 95.9 to 36.9. The RWC in most of the plant species examined in our study fell within the intermediate range (29–59 %) and sensitive range (23–100 %), demonstrating a variation in the RWC in different areas and plants.
L. camara had an RWC of 100 % at the control site and 81 % at the polluted site, thereby demonstration changes in physiological balance under stress conditions. This change also serves as an indicator of drought resistance in plants since water is crucial factor in plant life. Under stress (pollution) conditions, a high water content maintains a good physiological balance in plants as transpiration rates are higher than normal under stress conditions. A high level of RWC favors drought resistance. Air pollutants therefore cause a reduced transpiration rate and damage to the leaf pull engine that takes up water rom the soil via roots, with the result that plants neither absorb minerals nor cool down their leaves. Swami et al. (2004) reported a marked reduction in the RWC of P. hysterophorus due to the impact of pollutants on the transpiration rate in leaves.
The APTI has been used to rank species in terms of their tolerance to air pollution (Raza and Murthy 1988; Singh and Rao 1983). In our study, P. hysterophorus and C. procera exhibited the greatest tolerance at the control site, and C. procera exhibited the greatest tolerance at the polluted site. The lowest tolerance at both the control site and polluted site was expressed by S. marianum. The tolerance levels of all the species under study ranged from 11 to 17 at control sites and from 10 to 15.25 at the polluted site (Table 1). The tolerance of the species under study in decreasing order at the polluted site is as follows: for shrubs, P. hysterophorus > C. procera > R. communis > S. sabiferum > L. camara; for trees, P. gujava > M. azadirachta > B. papyrifera > C. album > Z. nummularia > E. globules; for herbs, A. viridis > W. somnifera > C. sativa > S. marianum. Overall, plants growing in the control environment had a higher APTI than those growing at the polluted site. P. hysterophorus, S. sabiferum, C. sativa, S. marianum and C. procera showed consistent performance at both sites. However, among the trees, P guajava, Shrub P. hysterophorus and herb C album are more tolerant.
Different floras have a considerable variability in their susceptibility to air pollutants. The plants with high and low APT could serve as tolerant and sensitive indicator species, respectively. Sensitivity levels among plants to air pollutants vary among herbs, shrubs and trees. With the same values, a tree may be sensitive to a given pollutant while a shrub or herb may be tolerant. Therefore, the indices for different plant types should be considered separately (Singh and Rao 1983).
Figures 6, 7, 8, 9, and 10 show the linear regression plots of RWC, pH, TC content, and ascorbic acid content against the APTI of selected plants. The highest correlation was between TC content and ascorbic acid content and APTI, and the lowest correlation was between pH and RWC and APTI. These results indicate the significance of TC content and ascorbic acid content on tolerance index of plants.
The API of the plants were evaluated using following characteristics morphology, socioeconomic significance, and biochemical features, such as APTI value, plant habit, canopy structure, type of plant, laminar structure, among others. The results presented in Tables 2, 3, and 4 suggest that it would be advantageous from an environmental point of view to promote the growth of E. globules, B papyrifera, R. communis, S. sabiferum, and C. sativa around marble processing plants—in particular E. globules and B. papyrifera.
Pollution tolerance is greater among plants capable of maintaining a high RWC in a polluted environment (Jyothi and Jaya 2010). Plants are very important factors affecting the ecological balance by contributing to the cycling of nutrients and gases such as CO2 and O2. Plants can be adversely affects by air pollution either directly via the leaves or indirectly by soil acidification (Kumar 2013). Various ways can be used to remove environmental pollution, but the planting of trees in urban areas which are highly tolerant to the effects of airborne PM can have a beneficial effect on air quality. Integrated studies of tree effects on air pollution reveal that management of urban tree canopy cover could be a viable strategy to improve air quality and help meet clean air standards (Nowak et al. 2006).
The soils in the polluted site in our study had basic pH values (Fig. 8), indicating that the PM from the marble processing plants not only have an impact on the surrounding vegetation but also on the soil parameters [pH: 8.316 ± 0.4792 (polluted site) vs. 7.76 ± 0.436 (control site); EC: 2.63 ± 1.82 (polluted site) vs. 5.95 ± 2.42 (control site)]. The more basic pH at the polluted site is due to the deposition of marble dust which is chiefly composed of Ca and Mg carbonates. The soils at the controls site had a high moisture content due to the presence of heavy foliage and plant growth at this control site. Soil EC values were higher at the polluted site (Fig. 4) than at the control site showing the presence of clay. Soil EC varies with particle size and soil texture, with sandy soils having a lower EC and clay soils having a high EC (Overstreet et al. 2011). High values of EC at the polluted site were due to a high clay content in the soil. The EC of soil is closely related to the pH and other physical properties of the soil on which crop yield depends. Marble dust in soil leads to an increase in soil basicity (Sudhalakhsmi et al. 2007). Marble waste reduces the accumulation of metals in plant shoots due to the formation of metal carbonates that hinder the absorption of metals by plants (Kabas et al. 2012), thus affecting the growth of plants.
Conclusion
The results of this study reveal that the emissions from marble processing plants have a considerable impact on vegetation. Our comparison of plants from a polluted and control site revealed that the former suffered from multiple physiological and physiochemical damages. Tolerance of plant towards air pollutants is specific to a site and depends on the type and level of pollution. Plants constantly exposed to environment pollution absorb, accumulate, and assimilate pollutants, which impacts their leaf structure depending on their sensitivity level. The APTI and API of the plants studied here suggests that the cultivation of E. globules, B. papyrifera, R. communis, S. sabiferum, and C. sativa around marble processing plants should be promoted. In particular, E. globules and B. papyrifera are highly recommended at these sites as they could serve as a sink for air pollutants, while sensitive plants like W. somnifera and S. marianum, among others, could be effectively used as bioindicators of pollution and thereby facilitate decision-making on the most appropriate species for future plantation.
References
Ademoroti, C. (1996). Environmental chemistry and toxicology. Ibadan: Foludex Press Ltd.
Agbaire, P., & Esiefarienrhe, E. (2009). Air Pollution tolerance indices (apti) of some plants around Otorogun Gas Plant in Delta State, Nigeria. Journal of Applied Sciences and Environmental Management, 13, 11–14.
Agrawal, S., & Tiwari, S. (1997). Susceptibility level of few plants on basis of air pollution tolerance index. Indian Forester, 123, 319–322.
Ashraf, M. A., Khan, A. M., Ahmad, M., Aqib, S., Balkhair, K. S., & Bakar, N. K. A. (2014). Release, deposition and elimination of radiocesium (137Cs) in the terrestrial environment. Environmental Geochemistry and Health. doi:10.1007/s10653-014-9620-9
Babu, G. B., Parveen, S. N., Kumar, K. N., & Reddy, M. S. (2013). Evaluation of Air Pollution Tolerance Indices of Plant Species Growing in the Vicinity of Cement Industry and Yogi Vemana University Campus. Indian Journal of Advances in Chemical Science, 2, 16–20.
Bakiyaraj, R., & Ayyappan, D. (2014). Air pollution tolerance index of some terrestrial plants around an industrial area. International Journal of Modern Research and Reviews, 2, 1–7.
Beckett, K. P., Freer Smith, P., & Taylor, G. (2000). Effective tree species for local air quality management. Journal of Arboriculture, 26, 12–19.
Belardi, G., Vignaroli, G., Plescia, P., & Passeri, L. (2013). The assessment of particulate matter emitted from stone-crushing industry by correlating rock textures with particles generated after comminution and dispersed in air environment. Environmental Science and Pollution Research, 20, 4711–4728.
Deepalakshmi, A., Ramakrishnaiah, H., Ramachandra, Y., & Radhika, R. (2013). Roadside plants as bio-indicators of urban air pollution. IOSR Journal of Environmental Science, Toxicology and Food Technology, 3, 10–14.
Grover, A., Kapoor, A., Satya Lakshmi, O., et al. (2001). Understanding molecular alphabets of the plant abiotic stress responses. Current Science, 80, 206–216.
Iqbal, M. Z., & Shafig, M. (2001). Periodical effect of cement dust pollution on the growth of some plant species. Turk. J. Bot, 25, 19–24.
Jeffrey Brandt, C., & Rhoades, R. W. (1973). Effects of limestone dust accumulation on lateral growth of forest trees. Environmental Pollution (1970), 4, 207–213.
Joshi, N., Bora, M., & Haridwar, U. (2011). Impact of air quality on physiological attributes of certain plants. Report and Opinion, 3, 42–47.
Joshi, P., & Swami, A. (2007). Physiological responses of some tree species under roadside automobile pollution stress around city of Haridwar, India. The Environmentalist, 27, 365–374.
Jyothi, S. J., & Jaya, D. (2010). Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. Journal of Environmental Biology, 31, 379–386.
Kabas, S., Faz, A., Acosta, J. A., et al. (2012). Effect of marble waste and pig slurry on the growth of native vegetation and heavy metal mobility in a mine tailing pond. Journal of Geochemical Exploration, 123, 69–76.
Keller, T., & Schwager, H. (1977). Air pollution and ascorbic acid. European Journal of Forest Pathology, 7, 338–350.
Klumpp, G., Furlan, C. M., Domingos, M., & Klumpp, A. (2000). Response of stress indicators and growth parameters of Tibouchina pulchra Cogn. exposed to air and soil pollution near the industrial complex of Cubatão, Brazil. Science of the Total Environment, 246, 79–91.
Kuddus, M., Kumari, R., & Ramteke, P. W. (2011). Studies on air pollution tolerance of selected plants in Allahabad city, India. Journal of Environmental Research and Management, 2, 042–046.
Kumar, M. (2013). Identification and evaluation of air pollution tolerance index of selected avenue tree species of urban Bangalore, India. International Journal of Emerging Technologies in Computational and Applied Sciences, 13, 388–390.
Lakshmi, P. S., Sravanti, K. L., & Srinivas, N. (2009). Air pollution tolerance index of various plant species growing in industrial areas. The Ecoscan, 2, 203–206.
Lima, J. S., Fernandes, E., & Fawcett, W. (2000). Mangifera indica and Phaseolus vulgaris in the bioindication of air pollution in Bahia, Brazil. Ecotoxicology and Environmental Safety, 46, 275–278.
Liu, Y.-J., & Ding, H. (2008). Variation in air pollution tolerance index of plants near a steel factory: Implication for landscape-plant species selection for industrial areas. WSEAS Transactions on Environment and development, 4, 24–32.
Loganathan, M., & Ilyas, M. M. (2012). Impact of cement dust pollution on morphology and histology in some medicinallly signifiicant plants. Pharmacie Globale (IJCP), 11, 23–28.
Mahecha, G., Bamniya, B., Nair, N., & Saini D. (2013). Air pollution tolerance index of certain plant species—A study of Madri Industrial Area, Udaipur (Raj.), India. International Journal of Innovative Research in Science, Engineering and Technology, 2, 7927–7929.
Martos, I., Ferreres, F., & Tomás-Barberán, F. A. (2000). Identification of flavonoid markers for the botanical origin of Eucalyptus honey. Journal of Agricultural and Food Chemistry, 48, 1498–1502.
Ninave, S., Chaudhari, P., Gajghate, D., & Tarar, J. (2001). Foliar biochemical features of plants as indicators of air pollution. Bulletin of Environmental Contamination and Toxicology, 67, 133–140.
Nowak, D. J. (1994). Air pollution removal by Chicago’s urban forest (pp. 63–81). Results of the Chicago urban forest climate project: Chicago’s urban forest ecosystem.
Nowak, D. J., Crane, D. E., & Stevens, J. C. (2006). Air pollution removal by urban trees and shrubs in the United States. Urban Forestry & Urban Greening, 4, 115–123.
Nwadinigwe, A. (2014). Air pollution tolerance indices of some plants around Ama industrial complex in Enugu State, Nigeria. African Journal of Biotechnology, 13, 1231–1236.
Overstreet, C., Barbosa, R., Burns, D., et al. (2011). Using electrical conductivity to determine nematode management zones in alluvial soils of the mid-South. In: Proceedings of the Beltwide Cotton Conferences, pp. 252–258.
Pandey, J., & Agrawal, M. (1994). Evaluation of air pollution phytotoxicity in a seasonally dry tropical urban environment using three woody perennials. New Phytologist, 126, 53–61.
Prajapati, S. K. (2012). Ecological effect of airborne particulate matter on plants. Environmental Skeptics & Critics, 1, 12–22.
Prajapati, S. K., & Tripathi, B. (2008). Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. Journal of Environmental Quality, 37, 865–870.
Prusty, B., Mishra, P., & Azeez, P. (2005). Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicology and Environmental Safety, 60, 228–235.
Radhapriya, P., Gopalakrishnan, A. N., Malini, P., & Ramachandran, A. (2012). Assessment of air pollution tolerance levels of selected plants around cement industry, Coimbatore, India. Journal of Environmental Biology, 33, 635–641.
Rai, P. K., & Panda, L. L. S. (2014). Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Quality, Atmosphere and Health, 7, 93–101.
Raina, A., Rathore, V., & Sharma, A. (2008). Effect of stone crusher dust on leaves of Melia azedarach Linn. and Dalbergia sissoo Roxb. in Jammu (J&K). Nature, Environment and Pollution Technology, 7, 279.
Randhi, U. D., & Reddy M. A. (2012). Evaluation of Tolerant plant species in Urban Environment: A case study from Hyderabad, India. Universal Journal of Environmental Research & Technology, 2, 300–304.
Rawat, J., & Banerjee, S. (1996). Urban forestry for improvement of environment. Energy Environment Monitor, 12, 109–116.
Raza, S., & Murthy, M. (1988). Air pollution tolerance index of certain plants of Nacharam industrial area, Hyderabad. Indian Journal of Botony, 11, 91–95.
Saini, Y., Bhardwaj, N., & Gautam, R. (2011). Effect of marble dust on plants around Vishwakarma Industrial Area (VKIA) in Jaipur, India. Journal of Environmental Biology, 32, 340–344.
Salami, A., Farounbi, A., & Muoghalu, J. (2004). Effect of cement production on vegetation in a part of southwestern Nigeria. Tanzania Journal of Science, 28, 69–82.
Saxena, P., & Ghosh, C. (2013). Ornamental plants as sinks and bioindicators. Environmental Technology, 34, 3059–3067.
Seyyednejad, S. M., & Koochak, H. (2011) A study on air pollution affects on Eucalyptuscamaldulensis. In:Proceedings of the International Conference on Environmental, Biomedical and Biotechnology, pp. 98–101.
Sharma, G., Chandler, C., & Salemi, L. (1980). Environmental pollution and leaf cuticular variation in Kudzu (Pueraria lobata Willd.). Annals of Botany, 45, 77–80.
Singh, P. K. (2005). Plants as indicators of air pollution: an Indian experience. Indian Forester, 131, 71–80.
Singh, S., & Rao, D. (1983). Evaluation of plants for their tolerance to air pollution. In: Proceedings of the Symposium on Air Pollution Control, pp. 218–24.
Sudhalakhsmi, C., Velu, V., & Thiyagarajan, T. (2007). Redox potential in the rhizosphere soil of rice hybrid as mediated by crop management options. Research Journal of Agriculture and Biological Sciences, 3, 299–301.
Swami, A., Bhatt, D., & Joshi, P. (2004). Effects of automobile pollution on sal (Shorea robusta) and rohini (Mallotus phillipinensis) at Asarori, Dehradun. Himal J Environ Zool, 18, 57–61.
Thakar, B., & Mishra, P. (2010). Dust collection potential and air pollution tolerance index of tree vegetation around Vedanta Aluminium Limited, Jharsuguda. The Bioscan, 3, 603–612.
Tripathi, A. K., & Gautam, M. (2007). Biochemical parameters of plants as indicators of air pollution. Journal of Environmental Biology, 28, 127–132.
Tripathi, A., Tiwari, P. B., & Mahima, Singh D. (2009). Assessment of air pollution tolerance index of some trees in Moradabad city, India. Journal of Environmental Biology, 30, 545–550.
Tsega, Y. C., & Prasad, A. D. (2014). Variation in air pollution tolerance index and anticipated performance index of roadside plants in Mysore, India. Journal of Environmental Biology, 35, 185–190.
Yavuz Çelik, M., & Sabah, E. (2008). Geological and technical characterisation of Iscehisar (Afyon–Turkey) marble deposits and the impact of marble waste on environmental pollution. Journal of Environmental Management, 87, 106–116.
Acknowledgments
This research is supported by High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04 from the Ministry of Education Malaysia. Thanks also for the support by UMRG (RG257-13AFR) and FRGS (FP038-2013B).
Conflict of interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted by the Editor-in-Chief. After a thorough investigation carried out according to the Committee on Publication Ethics guidelines, we have strong reason to believe that the peer review process was compromised.
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10653-016-9812-6.
About this article
Cite this article
Noor, M.J., Sultana, S., Fatima, S. et al. RETRACTED ARTICLE: Estimation of Anticipated Performance Index and Air Pollution Tolerance Index and of vegetation around the marble industrial areas of Potwar region: bioindicators of plant pollution response. Environ Geochem Health 37, 441–455 (2015). https://doi.org/10.1007/s10653-014-9657-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9657-9