Abstract
Mitigation of air pollution is the major global challenge in the current world. Quality of air is undergoing continuous deterioration due to industrial and urban development. The present study aims at the seasonal investigation of air pollution tolerance index (APTI) and anticipated performance index (API) of six commonly found plant species namely Ziziphus jujube (S1), Pithecellobium dulce (Sw.) DC (S2), Celosia argentea L. (S3), Ricinus communis L. (S4), Apluda mutica L. (S5) and Terminalia catappa L. (S6) growing along the industrial area (Chembur) of Mumbai. The air pollution tolerance indices were evaluated by using pH, ascorbic acid, total chlorophyll content, the relative water content in the fresh leaves of plant species growing along the industrial and non-industrial area of Mumbai. The API was determined on combining the APTI values of the plant species with their biological and socio-economic parameters. The APTI of all the industrial zone plant species was found to be higher than the non-industrial zone plant species in all the three seasons. The rise in the mean of the three season’s APTI of S1, S2, S3, S4, S5 and S6 plant species from the polluted industrial zone over the less-polluted non-industrial zone was found to be 60.14, 76.59, 71.98, 82.30, 71.12, 65.52%, respectively. The higher API values obtained for the Pithecellobium dulce (Sw.) DC, Ricinus communis L. and Terminalia catappa L. plant species from polluted zone indicated higher air pollution tolerance and hence are recommended for green belt expansion in the Chembur industrial area. Terminalia catappa L. is an evergreen plant which can be planted in large number to develop green zone throughout the year around the Chembur industrial area. Similar studies can be performed globally to increase green zones around any industrial or residential area which will provide healthy living for the next generations on the earth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A rapid increase in air pollution is a major global issue. In the present scenario, the environment and human well-being are adversely affected due to the industrial progress and expansion of cities. The plantations, local residents and atmosphere are enormously affected by the release of oxides of sulphur, carbon, nitrogen. It is of serious concern to deal with the abatement of air pollution. There are no known physical or chemical methods to upgrade air quality of polluted zone [1]. Researchers are trying to investigate a simple and cheaper solution to ameliorate air quality all over the world. Expansion of green belt is the best and economical solution for the mitigation of air pollution in such industrial zone which is a site-specific activity [2, 3]. The concentration of air pollutants can be reduced by using the plants as a sink [4]. The air pockets of plants efficiently captivate air contaminants. The physiological stress generated on plants by a large number of air pollutants gets reflected on leaves [5]. The observations related to physiological stress on plants are useful in understanding the effect of climate condition, the physico-chemical properties of air pollutants and their dwelling time in the atmosphere [6]. The plant leaves get apparently damaged due to epinasty, chlorosis and necrosis [7]. Plants play an imperative role in monitoring and perpetuating the ecological equilibrium by strongly contributing to the cycling of nutrients and gases like carbon dioxide, oxygen. Provision of the massive leaf area by plants for impingement, captivation and deposition of air pollutants can be the best natural way to clean the atmosphere through the flora [8,9,10,11,12].
The acquaintance of air pollution tolerant plant species plays a key role in the decline of air pollution. The APTI values can be evaluated to study the biochemical, physiological and morphological facets of the plants which are reported to respond more efficiently towards polluted air [4]. The study includes analysis of leaf air pollution impact parameters like RWC, TCh, AA and pH to determine APTI values. Based on these four parameters, APTI values for the plant species can be measured. It indicates the sensitivity and tolerance of the different plant species growing in the same industrial area [13]. The plant species with lower APTI value can be used as bio-indicators, whereas the tolerant ones with higher values can serve as buffers [14, 15]. Combination of APTI and API values is helpful in identification of air pollution tolerant species which will serve as the stepping stone towards enhancement of the environment. Different responses can be exhibited by different plant species under similar ecological conditions or the same plant species under variable climatic conditions [16].
The present study aims at assessing the air pollution tolerance potential and anticipated performance index of six plant species with seasonal variations over a period of 2 years (April 2017 to March 2019). It is anticipated that the results of the present study will be helpful in the identification of tolerant and sensitive species contributing to green belt extension in the industrial area.
2 Materials and methods
2.1 Study area
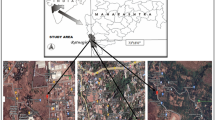
The Chembur area of the present study is a suburb in the north-eastern corner of Mumbai (19.051° N 72.894° E) near the Trombay area about 15 km from Victoria Terminus (presently known as Chhatrapati Shivaji Terminus). The study area is in proximity to Kurla, Deonar, Mahul, Govandi, Chunabhatti and Ghatkopar suburbs. The area under study has been facing pollution problems and was recently ranked 46th in a list of the most polluted industrial clusters in India and first in Mumbai with a Comprehensive Environment Pollution Index (CEPI) of 69.19 [17, 18]. There are already industries like RCF (Rashtriya Chemical & Fertilizers), HPCL (Hindustan Petroleum Corporation Limited), BPCL (Bharat Petroleum Corporation Limited), Tata Power station, Bharat Petroleum Refinery, Indian oil Corporation Limited and Devnar dumping ground which are causing air pollution in Chembur area. The uncontrolled release of ammonia and nitrous oxides from the Rashtriya Chemicals & Fertilizers (RCF) complex is the major problem. Although scrubbing of ammonia is technically possible, the pollution problem seems to arise due to operational failure of equipment used for pollution control and/or working on the urea/ammonia complex above the design capacity without improvement in pollution control techniques. The smoke from the Deonar dumping ground has caused health issues for the residents of Chembur and reported to affect asthma patients [17, 19]. The increased industrial activity in the study area, as well as garbage incinerators in proximity, also raised the level of air pollution in the neighbourhood, earning it the nickname of “Gas Chamber of Mumbai” [17]. The ambient air quality monitoring results during the present study period are shown in Table 1 [20]. The temperature of the area ranges between 13 and 39 °C. Maximum of 747 mm rain was recorded due to the southwest monsoon (June to mid-October) during July. The climate is humid and relative humidity ranges between 29 and 96% [21]. It has a North–South running basalt hill to its South with the marine alluvium type of soil [22].
2.2 Chemicals and reagents
HPLC grade solvents like acetone and methanol, used for the analysis of samples, were redistilled 2 × before use. Analytical grade ascorbic acid (C6H8O6) with 99.0% purity as supplied by F. Hoffmann–La Roche Ltd. Basel, Swiss, was used for the present study. Deionised water was supplied using a Milli-Q system. Analytical grade acetic acid, the sodium salt of 1-hexane sulfonic acid supplied by S. D. Fine-Chem Ltd. and Loba chemicals Ltd. were used for the ascorbic acid analysis.
2.3 Plant species
The most common plant species present in the study area were identified. Fully matured leaves of three replicates of six plant species were collected from the Chembur industrial area as experimental samples (ES). The details about the type and native countries of the plant species selected for the present study are mentioned in Table 2 [23]. The controlled samples (CS) of the same plant species were collected from the botanical garden of Bhavan’s College, Andheri (19.073° N 72.501° E), which was away from industrial and traffic areas. The distance between the Chembur industrial area (industrial site) and Bhavan’s College, Andheri (non-industrial site), is around 11.36 km [24]. The ES and CS were collected at regular intervals in all the three seasons over 2 years.
2.4 Sample collection and analysis
The fully matured leaf samples from six different commonly found plant species in the study area were collected as ES. The leaf samples of similar plant species growing in a less-polluted zone with similar ecological conditions were collected as CS. The entire sampling and study of both CS and ES were done at regular intervals for 2 years during the summer (April), rainy (August) and winter (December) seasons. The biochemical parameters like pH, AA, TCh and the RWC in the fresh leaves of ES and CS plant species were studied. Samples were collected in a cloth/polythene bag, then immediately brought to the laboratory and kept in the refrigerator for further analysis.
2.5 TCh analysis
For analysis, 0.5 g of fresh leaf sample was finely crushed and diluted to 10 ml with distilled water. A 2.5 ml of the solution thus prepared was mixed with 10 ml of acetone and filtered. The absorbance of the resulting solution was measured at λ = 645 nm (A645) and λ = 663 nm (A663). The absorbance of the leaf extract at 645 nm and 663 nm for chlorophyll ‘a’ and chlorophyll ‘b’, respectively was measured using UV–visible spectrophotometer (Elico SL 207 Mini Spec). The total chlorophyll content was calculated from chlorophyll ‘a’ and chlorophyll ‘b’ by Eq. (1) [9].
2.6 AA analysis
Ascorbic acid content was analysed using HPLC instrumentation method. The concentration of ascorbic acid content was investigated using a Shimadzu LC HPLC instrument isocratic system coupled with a diode array detector and flame-photometric detector. Superspher RP-18 (250 × 4.6 mm, 10 µm particle size) was used as a column (λ = 280 nm), and the mobile phase was pumped isocratically at a flow rate of 0.7 mL min−1 at 20 °C. The low column temperature was maintained because of less stability of ascorbic acid at a higher temperature. During the study, the routine analysis of method blanks, spiked blanks and sample duplicates was conducted with field samples. In order to assess the error during sample extraction, samples were analysed in duplicate, and the results showed satisfactory precision. The presence of any target compound was not shown by blanks [25].
2.7 Analysis of RWC content
For weighing the leaf samples, the electronic weighing balance of accuracy 0.0001 g was used (Contech, CA 223). From the FW, TW and DW values, the RWC of leaf samples was calculated using Eq. (2) [26].
2.8 pH Determination of leaf extract
The pH of filtrate of the leaf extract was measured on the pH meter (Equiptronics, EQ 614A) which was calibrated using the buffer solution of pH = 4 and pH = 9 [27].
2.9 APTI determination
APTI determination was done using the formula given in Eq. (3) [28].
2.10 Statistical analysis
The explicit parameters to be related to the same species were evaluated by calculating the correlation among the parameters of individual species. The relationship between the individual parameters concerning species, season and site was determined by performing the correlation analysis using univariate Pearson correlation coefficient for individual species. One-way ANOVA method was used for the statistical analysis of the results. In most of the cases, the correlation between individual parameters was significant having P < 0.05. This is an indication that these parameters were related to the same species. IBM SPSS statistics version 20 was used for performing all the statistical calculations. The correlation statistics prevailing between the parameters of individual species for season and site are presented in Table 4.
3 Results and discussion
The biochemical parameter (leaf extract pH, AA, RWC, TCh content and APTI) values obtained in the present study are shown in Table 3.
As shown in Table 4, the positive correlation of most of the parameters was significantly observed through a matrix of correlation coefficients. A comprehensive investigation of the statistics set showed that significant correlations occur amongst most of the parameters with correlation coefficient values recurrently greater than 0.5.
3.1 Leaf extract pH
On analysing the data obtained, it was detected that the leaf extract pH values were lower for the experimental samples (acidic medium) and higher for the controlled samples (alkaline medium). Among the experimental samples, the lower mean value was 4.37 for S1 and the higher mean value was 6.03 for S2 samples. Controlled samples have described the highest value of 7.50 for S2 and the lowest value of 6.62 for S1 samples. S2, S3, S5 reported the higher pH value signifying good tolerance as compared to the other plant species. Experimental S1 sample has reported the lowest value of 3.21 in winter 2018–19, whereas S2 sample has reported a higher value of 7.18 in summer 2018–19. For controlled samples, the lower value was 4.35 for S1 in winter 2018–19 and the higher value was 7.86 for S5 species in winter 2018–19. The occurrence of oxides of sulphur and nitrogen or other acidic pollutants in the industrial discharge present in the ambient air causes an alteration in the pH of leaf latex towards acidic [29]. The good association of the leaf extract’s acidic pH with the sensitivity to air pollution [9]. As shown in Table 4, a good positive correlation exists between pH and AA values in most of the experimental and controlled samples. The highest value was observed for S1 species amongst ES (r = 0.9136) and CS (r = 0.9743) in winter season which indicates that an increase in pH value leads to an increase in pollution resistance mechanism of plants. There also exists a good negative correlation between pH and AA values for ES and CS samples of S3 (r for ES = − 0.8542 and r for CS = − 0.8598), S4 (r for ES = − 0.9454 and r for CS = − 0.9046) and S6 (r for ES = − 0.9746 and r for CS = − 0.9464) plant species in winter season which indicates that an increase in AA value leads to a decrease in pH value of leaf extract. There also exists a strong positive correlation between pH and RWC for S2 species in summer season (r for ES = 0.9637 and r for CS = 0.9995) which indicates that an increase in pH value leads to an increase in water holding capacity of leaves. There exists a good positive correlation between pH and TCh for S5 species (r for ES = 0.9652 and r for CS = 0.9271) in the rainy season which indicates that the rise in pH leads to a reduction in loss of chlorophyll content under pollution stress. This increases the dwelling time of plants. There also exists a very strong positive correlation between pH and APTI for S4 (r for ES = 0.9868 and r for CS = 0.9975) sample in the rainy season which indicates that a plant becomes more tolerant with an increase in pH of its leaf extract.
On comparing the previous studies with the present study as shown in Table 5, it was observed that S2 has showed higher pH value than reported by Singh et al. [26] and S6 has reported higher pH values than reported by Adamsab et al. [30], Anake et al. [31], Ogunkunle et al. [32], Ogunrotimi et al. [33].
3.2 AA content
During the analysis of the ascorbic acid content of leaf samples, it was detected that the values were higher in the experimental samples from the industrial zone and lower in the controlled samples from the non-industrial zone. S6 sample has revealed the highest value (10.66 mg g−1), whereas S2 has exhibited the lowest value (9.53 mg g−1) in an industrial zone. S3, S6 have shown the highest value (4.23 mg g−1) and S2 has reported the lowest value (3.84 mg g−1) for the controlled samples. Among the experimental samples, the higher value was observed for S3 (11.53 mg g−1) in rainy 2018–19 and the expressively lower value was observed for S2 (9.02 mg g−1) in winter 2017–18. For controlled samples, the value was higher for S1 (5.18 mg−1) in winter 2017–18. S1, S3, S4, and S6 were found with the higher mean AA values indicating good resistance and others with the lower mean AA values were observed to be less resistant to pollution stress. The higher values of ascorbic acid indicate an efficient increase in the defence system of the plant under polluted stress conditions [34]. The ascorbic acid stimulates resistance to the antagonistic environmental condition, counting air pollution and is observed as an antioxidant found in great quantity in all mounting plant parts [35, 36]. Due to the improved rate of production of reactive oxygen species during photooxidation progression, a pollution load-dependent increase is detected in the ascorbic acid content of all plant species [37]. As indicated in Table 4, there exists a strong positive correlation between AA and RWC for S6 (r for ES = 0.9483 and r for CS = 0.9364) sample in the rainy season which indicates that an increase in ascorbic acid content results in an increase in drought tolerance capacity of the plant. There exists a strong negative correlation between AA and TCh for S6 (r for ES = − 0.9745 and r for CS = − 0.9217) sample in summer season which indicates that a decrease in chlorophyll content due to air pollution leads to an increase in the defence mechanism of plants. There exists a strong positive correlation between AA and APTI for S1 (r = 0.9990) industrial zone species and for S2 (r = 0.9734) species in summer season which indicates that an increase in ascorbic acid content contributes to plant’s tolerance towards pollution stress.
The comparative data of previous and present study shown in Table 5 revealed that the ascorbic acid values of S1 sample in the present study were found to be higher than the values reported by Das et al. [2], Suvarnalakshmi et al. [13], Banerjee et al. [38], Thakar and Mishra [39]. S2 sample in the current study revealed higher ascorbic acid values than reported by Singh et al. [26] and Krishnaveni and Lavanya [40]. S4 sample has reported higher AA values in present study than reported by Singh et al. [26], Choudhary and Banerjee [41]. The present study revealed higher AA value for S6 sample as compared to the values reported by Adamsab et al. [30], Anake et al. [31], Ogunkunle et al. [32], Ogunrotimi et al. [33], Agbaire [42], Krishnaveni et al. [43], Govindaraju et al. [44], Paulsamy and Senthilkumar [45].
3.3 RWC
The current study stated that RWC values varied with the season and sample. Among the experimental samples, the highest amount of mean RWC was observed for S5 (74.33%) followed by S6, S3, S1 and S4 while the least was observed in S2 (69.31%) sample. It also reported that the RWC values were significantly higher for ES than CS due to high exposure to the pollutants. The air pollution stress can be seen on the plant leaves in the form of high transpiration rates which can lead to desiccation of leaves [14]. The high-water content within a plant body will benefit to maintain its physiological equilibrium under stress circumstances such as a revelation to the air pollution. Therefore, conservation of RWC of the plant may resolve the comparative tolerance of plants towards air pollution. The better drought tolerance capacity will be due to the higher RWC in a particular species [46]. In the present study, the plant species S5, S6 and S3 were able to achieve the higher water content in the leaves under pollution stress.
The seasonal analysis of RWC values in the present study has described the highest value for the S4 (91.65%) in the summer season of 2017–18 while the least value of the S6 sample (44.58%) in winter 2018–19 collected from the industrial zone (ES). The CS samples have revealed the higher RWC values for S5 sample (75.91%) in the summer season. The high-water availability during the rainfall leads to higher RWC during the monsoon season [47]. Table 4 indicates that a strong positive correlation exists between RWC and TCh for S4 experimental species (r = 0.9709) in the rainy season and for S2 controlled sample (r = 0.9857) in winter season which indicates that a rise in leaf water content during the rainy and winter season leads to a reduction in loss of chlorophyll content in plants. There exists a strong positive correlation between RWC and APTI for S1 experimental species (r = 0.9888) in the rainy season and for S2 controlled sample (r = 0.9652) in summer season which indicates that an increase in RWC leads to an increase in air pollution tolerance capacity of plants in any season.
The comparison of the previous study with the present study shown in Table 5 indicates that the S1 sample has exhibited higher RWC value in the present study as compared to the values reported by Das et al. and Thakar and Mishra [2, 39]. S2 sample has shown higher RWC value than reported by Krishnaveni and Lavanya [40]. S4 sample has revealed higher RWC value as compared to the value reported by Choudhary and Banerjee [41]. S6 sample has shown higher RWC value than reported by Krishnaveni et al. and Paulsamy and Senthilkumar [43, 45].
3.4 TCh content
The present study has revealed the lower TCh for ES and higher for CS. Among the experimental samples, the reduction in mean TCh content reported was S6 (5.90 mg g−1) > S2 > S1 > S4 > S5 > S3 (4.35 mg g−1). CS has exhibited the higher mean TCh for S6 (6.76 mg g−1) and the least for S5 sample (5.43 mg g−1). S1, S2, and S6 were able to maintain TCh values in the polluted zone as compared to S3, S4 and S5 samples. A small difference was observed in the TCh values of experimental and controlled samples. The plants growing in the industrial area were able to maintain their chlorophyll content even under high pollution stress. Diminution in the chlorophyll results in poor vigour of the plants with the reduction in productivity [48]. Therefore, the plants upholding the chlorophyll, even under polluted atmosphere, contribute to APTI. The chlorophyll content displays straight association with the photosynthetic commotion of the plant [49]. The development of the plant is relying on the total chlorophyll content. The consequence of deprivation in the chlorophyll due to loss of magnesium is detected in the form of a significant drop in chlorophyll content value for the plant species rising in an industrial area. It diverges from species to species [14].
Seasonal analysis in the current study has revealed that the highest TCh content was observed in S2 sample (7.18 mg g−1) in summer 2017–18 while the least was observed for S1 sample (3.04 mg g−1) in rainy 2017–18 growing in an industrial area. During the analysis of CS, the highest TCh value was observed for S2 sample (8.87 mg g−1) in summer 2017–18 while the least for S1 sample (3.59 mg g−1) in rainy 2017–18. Table 4 indicates that a strong positive correlation was observed between TCh and APTI for S5 experimental species (r = 0.9603) in the rainy season and S6 (r = 0.9814) controlled species in the winter season. This positive correlation indicates that an increase in TCh value contributes to an increase in air pollution tolerance capacity of plants in any season.
On comparing the data presented in previous and present study given in Table 5, it was observed that S1 sample has reported higher TCh values as compared to those reported by Das et al. [2], Das and Prasad [8], Yan-ju and Hui [12], Banerjee et al. [38], Karthiyayini et al. [50], whereas S2 sample has shown higher TCh values than reported by Krishnaveni and Lavanya [40]. S4 sample in the current study has exhibited higher TCh values than reported by Adamsab et al. [30], Karthiyayini et al. [50]. S6 sample has reported higher TCh values as compared to the values reported by Anake et al. [31], Ogunrotimi et al. [33], Govindaraju et al. [44], Paulsamy and Senthilkumar [45].
3.5 APTI
Experimental samples have shown higher APTI values as compared to controlled samples in Table 3. The plant species display a higher tolerance in the industrial zone than in the non-industrial zone. The highest mean APTI value was exhibited by S2 (18.03), and least mean value was exhibited by S1 (16.59). The minimum difference was observed in highest and lowest mean APTI values. Hence all the six plant species are proved to be comparatively tolerant species. Seasonal analysis has revealed the highest value of 21.60 for S2 sample in summer 2018–19 and the lowest value of 11.73 for S6 sample in rainy 2017–18. An overview of all results obtained from the study reveals that the APTI value of the same plant species varied from season to season. There was a considerable variation in the susceptibility of the plant species towards air pollution [5]. The tolerant plant species described higher APTI while sensitive species indicated lower APTI values. A large number of plant species can be screened for their sensitivity to air pollutants and resolve a simple method of APTI investigation [51].
The comparative data of previous and present study shown in Table 7 revealed that the APTI values observed in the present study were higher for -S1 sample than reported by Das et al. [2], Banerjee et al. [38], S2 sample than reported by Krishnaveni and Lavanya [40], S6 sample than reported by Adamsab et al. [30], Anake et al. [31], Ogunkunle et al. [32], Ogunrotimi et al. [33], Krishnaveni et al. [43], Govindaraju et al. [44], Paulsamy and Senthilkumar [45].
The increase in pH, AA, TCh, RWC and APTI values for plant samples in the present study as compared to the data reported by other researchers around the world indicates the improved tolerance capacity of plant species of Chembur (Mumbai) industrial area.
3.6 Anticipated performance index (API) and green belt development
The resultant APTI values, some appropriate biological and socio-economic characters (tree habit, canopy structure, type of tree, laminar size, laminar texture and economic importance) were combined to calculate the API values for different plant species. Based on these characters, + or − grades are allotted to the plants. Plants are differentiated using the scored grades [1, 47, 52]. Plant species growing in the industrial area were evaluated for anticipated performance index (API). The API of six plant species was determined as given in Table 6.
With reference to the assessment based on API grade as shown in Table 7, it was observed that the S6 and S2 plant species have shown excellent tolerance, whereas the S4 plant species have exhibited very good tolerance to industrial air pollution. The S3 and S5 plant species have reported good tolerance towards pollution stress conditions. These five plant species can be recommended for plantation in the industrial zone. The S1 plant sample has reported moderate API value which can be planted for aesthetic and economic importance in industrial area.
4 Conclusion
Based on the outcomes of the present study, it can be concluded that the APTI values significantly varied concerning to species, season and zone. Pithecellobium dulce, Celosia argentea, Ricinus communis, Apluda mutica and Terminalia catappa plant species have reported higher API grades amongst six industrial plant species and are recommended for green belt development in the industrial area. Terminalia catappa is an evergreen plant which can be planted on a large scale to develop greenery throughout the year around the industrial zone. With cumulative industrialization and deforestation, there is a collective threat of an increase in air pollution and an adverse human health effect. Hence mitigation of air pollution is the need of the hour. The present study can serve as a simple tool towards the best and cheaper way to ameliorate the air quality in the industrial area. A similar study can be carried out to identify the tolerant plant species in any industrial or residential area of the world. The expansion of green belt around the world can lead to the availability of healthy air and green nature for the next generations.
Abbreviations
- AA:
-
Ascorbic acid
- API:
-
Anticipated performance index
- APTI:
-
Air pollution tolerance index
- ANOVA:
-
Analysis of variance
- BPCL:
-
Bharat Petroleum Corporation Limited
- CEPI:
-
Comprehensive environment pollution index
- CS:
-
Controlled sample
- DW:
-
Dry weight
- ES:
-
Experimental sample
- FW:
-
Fresh weight
- HPCL:
-
Hindustan Petroleum Corporation Limited
- HPLC:
-
High-performance liquid chromatography
- MPCB:
-
Maharashtra Pollution Control Board
- RCF:
-
Rashtriya Chemical & Fertilizers
- RWC:
-
Relative leaf water content
- S:
-
Sample
- SD:
-
Standard deviation
- SPSS:
-
Statistical package for the social sciences
- TCh:
-
Total chlorophyll content
- TW:
-
Turgid weight
References
Walia K, Aggrawal R, Bhardwaj S (2019) Evaluation of air pollution tolerance index and anticipated performance index of plants and their role in development of green belt along national highway-22. Int J Curr Microbiol Appl Sci 8(03):2498–2508. https://doi.org/10.20546/ijcmas.2019.803.296
Das S, Mallick SN, Padhi SK, Dehury SS, Acharya BC, Prasad P (2010) Air pollution tolerance indices (APTI) of various plant species growing in industrial areas of Rourkela. Ind J Environ Prot 30:563–567
Rai P (2014) Environmental magnetic studies of particulates with special reference to biomagnetic monitoring using roadside plant leaves. Atmos Environ 72:113–129
Bakiyaraj R, Ayyapan D (2014) Air pollution tolerance index of some terrestrial plants around an industrial area. Int J Mod Res Rev 2:1–7
Jyothi S, Jaya D (2010) Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvanathpuram, Kerala. J Environ Biol 31:379–386
Wagh N, Shukla P, Tambe S, Ingle S (2006) Biological monitoring of roadside plants exposed to vehicular pollution in Jalgaon city. J Environ Biol 27:419–421
Prasad D, Choudhury (1992) Effects of air pollution. In: Misra SG (ed) Environmental pollution air environmental pollution and hazards series. Venus Publishing House, New Delhi, pp 58–60
Das S, Prasad P (2010) Seasonal variation in air pollution tolerance indices and selection of plant species for industrial areas of Rourkela. Ind J Environ Prot 30:978–988
Escobedo F, Wagner J, Nowak D, De Le MC, Rodriguez M, Crane D (2008) Analyzing the cost effectiveness of Santiago, Chiles policy of using urban forests to improve air quality. J Environ Manag 86:148–157
Mahecha G, Bamniya B, Nair N, Saini D (2013) Air pollution tolerance index of certain plant species-a study of Madri industrial area, Udaipur (Raj.), India. Int J Innov Res Sci Eng Tech 2:7927–7929
Varshney C (1985) Role of plant in indicating monitoring and mitigating air pollution. In: Subrahmanium GV, Rao DN, Varshney CK, Viswas DK (eds) Air pollution and plants: a state-of-the-art report. Ministry of Environment and Forest, New Delhi, pp 146–170
Yan-Ju L, Hui D (2008) Variation in air pollution tolerance index of plant near a steel factory: implications for landscape plant species selection for industrial areas. WSEAS Trans Environ Dev 4:24–32
Suvarna Lakshmi P, Lalitha Sravanti K, Srinivas N (2008) Air pollution tolerance index of various plant species growing in industrial areas. Ecosacn 2:203–206
Rawal D, Sijapati J, Rana N, Pradhananga T (2001) Air pollution tolerance index of some tree species of Kathmandu Valley, Nepal. J Sci Technol 3:119–122
Rai PK, Panda LL (2014) Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Qual Atmos Heal 7:93–101. https://doi.org/10.1007/s11869-013-0217-8
Rai P (2014) Study of Air Pollution Tolerance Index of some common road side plant species in an industrial (Rourkela) and non-industrial (Aizawl) area of India: An ecomanagement approach. Issu Tren Wild Conserv Nor East Ind 86–95
Aghor A (2009) Disaster is around the corner for Mumbai. DNA India. Retrieved 10 Oct 2012
Varma G (2009) 43 Industrial clusters imperiled in India, daily news and analysis. Accessed 25 Jan 2011
Times News Network (2012) Chembur residents fume over smoke from Deonar dumping ground. The Times of India (Mumbai). Retrieved 18 May 2012
Maharashtra Pollution Control Board (MPCB) The Energy and Resources Institute (TERI). Annual report on Air Quality Status of Maharashtra 2018–19. Report Submitted to Maharashtra Pollution Control Board (MPCB), June 2019, 140. www.mpcb.gov.in/sites/default/files/focus-area-reports42documents/Air_Quality_Report_2018_19_Eng_11062019.pdf
Verma A, Balachandran S, Chaturvedi N, Patil V (2004) A Preliminary report on the biodiversity of Mahul Creek, Mumbai, India with special reference to Avifauna. Zoos Print J 8:8–9. https://doi.org/10.11609/jott.zpj.1172:1599-1605
Central Ground Water Board Report-Ground Water Information 2009. http://cgwb.gov.in/District_Profile/Maharashtra/Greater%20Mumbai.pdf. Accessed 20 Jan 2011
India Biodiversity Portal. https://indiabiodiversity.org. Accessed 12 Aug 2019
Chembur-Mahul google map. https://www.google.com/maps/place. Accessed 30 May 2020
Mitic S, Kostic D (2011) Rapid and reliable HPLC method for the determination of vitamin C in pharmaceutical sample. Trop J Pharm Res 1:105–111
Singh S, Rao D, Agrawal M, Pandey J, Narayan D (1991) Air Pollution Tolerance Index of Plants. J Environ Manag 32:45–55
Shannigrahi A, Fukushim T, Sharma R (2004) Anticipated air pollution tolerance of some plant species considered for green belt development in and around an industrial/urban area in India: an overview. Int J Environ Stud 61:125–137
Singh S, Rao D (1983) Evaluation of plants for their tolerance to air pollution. In: Proceeding of symposium on air pollution control held at IIT Delhi, pp 218–224
Swami A, Bhatt D, Joshi P (2004) Effects of automobile pollution on sal (Shorearobusta) and rohini (Mallotusphillipinensis) at Asarori, Dehradun. Himal J Environ Zool 18:57–61
Adamsab M, Kousar H, Shwetha D, Sirajuddin M, Ravichandran M (2011) APTI of some selected plants in Shivamogga City, South Asia. In: Proceeding of the international conference on advanced science, engineering and information technology, pp 668–671
Anake Winifred U, Bayode Faith O, Omonhinmin Conrad A, Williams Akan B (2018) Ambient air pollution control using air pollution tolerance index and anticipated performance index of trees. Int J Civ Eng Tech 9(12):417–425
Ogunkunle C, Suleiman L, Oyedeji S, Awotoye O, Fatoba P (2015) Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agrofor Syst 89:447–454
Ogunrotimi D, Adereti F, Eludoyin A, Awotoye O (2017) Urban air pollution control: selection of trees for ecological monitoring using anticipated performance indices in a medium-size urban area in Southwest Nigeria. Interdiscip Environ Rev 18:40–54
Swami A, Chauhan D (2015) Impact of air pollution induced by automobile exhaust pollution on air pollution tolerance index (APTI) on few species of plants. Int J Sci Res 4:342–343
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J For Pathol 7:338–350
Lima J, Fernandes E, Fawcett W (2000) Mangifera indica and Phaseolus vulgaris in the bioindicator of air pollution in Bahia, Brazil. Ecotoxicol Environ Saf 46:275–278
Tripathi A, Gautam M (2007) Bio-chemical parameters of plants as indicators of air pollution. J Environ Biol 28:127–132
Banerjee S, Banerjee A, Palit D, Roy P (2019) Assessment of vegetation under air pollution stress in urban industrial area for greenbelt development. Int J Environ Sci Technol 16:5857–5870. https://doi.org/10.1007/s13762-018-1963-9
Thakar BK, Mishra PC (2010) Dust collection potential and air pollution tolerance index of tree vegetation around Vedanta aluminium limited, Jharsuguda. Bioscan 3:603–612
Krishnaveni M, Lavanya K (2014) Air pollution tolerance index of plants a comparative study. Int J Pharma Pharmaceut Sci 6:320–324
Choudhury P, Banerjee D (2009) Biomonitoring of air quality in the industrial town of Asansol using the air pollution tolerance index approach. Res J Chem Environ 13:46–51
Agbaire P, Esiefarienrhe E (2009) Air Pollution tolerance indices (apti) of some plants around Otorogun Gas Plant in Delta State, Nigeria. J Appl Sci Environ Manag 13(1):11–14
Krishnaveni M, Harsha R, Santhosh Kumar J (2016) Air pollution tolerance index, antioxidant activity assessment of plants leaves collected at Sago Serve industry, Salem, Tamil Nadu, India. Worl J Pharm Res 5:1562–1577
Govindaraju M, Ganeshkumar R, Muthukumaran V, Visvanathan P (2012) Identification and evaluation of air-pollution-tolerant plants around lignite-based thermal power station for greenbelt development. Environ Sci Poll Res 19:1210–1223
Paulsamy S, Senthilkumar P (2009) Identification of air pollution tolerant tree species for the industrial city, Tirupur, Tamil Nadu. Nat Environ Pollut Technol 8:585–588
Dedio W (1975) Water relations in wheat leaves as screening test for drought resistance. Can J Plant Sci 55:369–378
Sharma B, Bhardwaj S, Sharma S, Nautiyal R, Kaur L, Alam N (2019) Pollution tolerance assessment of temperate woody vegetation growing along the national highway-5 in Himachal Pradesh, India. Environ Monit Assess 191:177
Singh S N, Verma A (2007) Phytoremediation of air pollutants: a review. In: Environmental bioremediation technology. Springer, Berlin, pp 293–314
Agrawal M, Singh B, Rajput M, Marshall F, Bell J (2003) Effect of pollution on peri-urban agriculture: a case study. Environ Poll 126:323–329
Karthiyayini R, Poonammal N, Raguna J (2005) Air pollution tolerance index of certain plants of Coimbatore- ooty highways, near I.T.I. area, Coimbatore, Tamilnadu. Pollut Res 24:801–803
Kuddus M, Kumari R, Ramteke P (2011) Studies on air pollution tolerance of selected plants in Allahabad city, India. E3 J Environ Res Manag 2:42–46
Mondal D, Gupta S, Datta J (2011) Anticipated performance index of some tree species considered for green belt development in an urban area. Int Res J Plant Sci 2(4):099–106
Dwivedi A, Tripathi B (2007) Pollution tolerance and distribution pattern of plants in surrounding area of coal-fired industries. J Environ Biol 28:257–263
Acknowledgement
The authors are thankful to Blatter herbarium of St. Xavier’s College, Mumbai for identification of plant species. The authors are also grateful to Dr. P. S. Ramanathan Advanced Instrumentation Centre (PSRAIC), Ramnarain Ruia College, Matunga, Mumbai, for providing necessary help in instrumental analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singare, P.U., More, S.N. Identification of the most tolerant plant species along the Chembur industrial area of Mumbai: a key step to mitigate global air pollution. SN Appl. Sci. 2, 1663 (2020). https://doi.org/10.1007/s42452-020-03431-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03431-5