Abstract
Water samples from Xikuangshan (China), the world largest antimony (Sb) mine with a Sb mining and smelting history of more than 200 years, were analyzed. These water samples ranged from stream water in the vicinity of the mining and smelting area that received seepage from ore residues to the underground mine-pit drainage. The concentrations of total Sb, Sb (III) and Sb (V) of the samples were determined by HPLC-ICP-MS. In addition, water pH and concentrations of major cations and anions were analyzed. All 18 samples demonstrated total Sb concentrations with ppm levels from 0.33 ppm to 11.4 ppm, which is two to three orders of magnitude higher compared to the typical concentration of dissolved Sb in unpolluted rivers (less than 1 ppb). This is probably the first time that such high Sb contents have been documented with complete environmental information. Distribution of total Sb and Sb species was investigated, taking into account the respective local environment (in the mining area or close to the smelter, etc.). Sb (V) was the predominant valence in all 18 samples. Only trace levels of Sb (III) were detected in 4 of the 18 samples. Geochemical speciation modeling showed the dominant species was Sb(OH) −6 . It is also probably the first time that such high Sb contents have been documented in the natural environment with Sb speciation distribution information. Several potential oxidation pathways are also discussed that might have facilitated the oxidation of Sb (III) in the natural environment. Signs of intoxication were observed among local mine workers with extensive exposure to different forms of Sb for a long period of time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antimony (Sb) is a toxic metalloid that belongs to group 15 of the Periodic Table of the Elements (Gebel 1997; Scheinost et al. 2006). Sb (III) and Sb (V) are the most prevalent oxidation states of Sb in the natural environment and biota (Filella et al. 2002a). Antimony is geochemically categorized as a strong chalcophile, occurring with sulfur and some heavy metals (Titretir et al. 2008). This may contribute to the most common forms of Sb ore in nature such as Sb2S3 (stibnite, antimonite), which is the only form of Sb ore mineral in Xikuangshan—the world’s largest Sb ore deposit. Traditional applications of antimony include manufacture of flame retardants, munitions, medicines for some tropical diseases, batteries, and glasses, with relatively modern uses in the semiconductor industry for diodes, inferred detectors, and Hall-effect devices (Krachler et al. 2001).
Antimony and its compounds are considered as pollutants of priority interest by the Environmental Protection Agency of the United States (USEPA 1979) and the Council of the European Communities (CEC 1976). It is also listed as a hazardous substance under the Basel Convention concerning the restriction of transfer of hazardous waste across borders (UNEP 1999). Yet, probably because of its relatively low abundance in most matrices (0.2–0.5 mg kg−1 in the earth’s crust, less than 0.3–8.4 mg kg−1 in soils, and less than 1 μg l−1 in unpolluted water), and low solubility of most of its compounds, research on antimony, its speciation, concentration, bioavailability, and transformation have long been relatively neglected (Crommentuijn et al. 2000; Filella et al. 2002a; Smith and Huyck 1999). It is commonly assumed that antimony is similar to arsenic in both chemical behavior and toxicity (Fowler et al. 1991).
According to the United States Geological Survey, global Sb production (USGS 2004) amounts to 142,000 tons per year, with most produced in China (88%). The most important mining district in China is Xikuangshan, located near Lengshuijiang City, Hunan province, in southwest China. The city is renowned as the “World Capital of Antimony” with the most abundant antimony deposit in the world: more than 2,000,000 tons proved reserve (CMA 2006). The area belongs to the subtropical monsoon climate zone with affluent precipitation (1,400 mm on average annually) and dense river networks.
Sb mining and smelting have been the pillar industry for local people and which has prospered in Xikuangshan for more than 100 years. Due to the lack of advanced technology, untreated ore tailings, smelter clinkers, and smelting residues have piled up in the open air, and wastewater from mineral separation and smelting ran freely into the environment, leading to an unprecedented Sb-related environmental crisis in this area.
Knowledge of the distribution, speciation, and transformation of Sb in natural water systems is important for understanding its geochemical and biological cycling as well as for the environmental remediation and monitoring of this toxic metalloid. Review papers (Filella et al. 2002a, b) on this topic have also pointed out the urgent need for research on the speciation of antimony in natural waters and the kinetics of Sb (III) oxidation.
Despite the significance of quantitative speciation, data of Sb in freshwater system are sparse, especially those based on reliable analytical methods (Andreae 1983; Andreae et al. 1981; Deng et al. 2000; Hou and Narasaki 1999; Laintz et al. 1992; Mohammad et al. 1990; Mok and Wai 1987; Shieh 1993; Sun et al. 1993; Takayanagi and Cossa 1997; Ulrich 1998; Van der Sloot et al. 1989). Among this published literature, only three show Sb concentrations at the ppm level, with samples that showed Sb at the ppm level all collected from Sb mines or in the vicinity of Sb mines (Laintz et al. 1992; Mohammad et al. 1990; Ulrich 1998). Yet the data provided a very limited number of samples (2–3) and insufficient sampling site information to make a reasonable story.
In our study, we use a combination of field sample analysis and geochemical modeling to study the distribution and speciation of inorganic Sb species in the mining and smelting area of Xikuangshan, as well as in downstream runoff. A total of 18 fresh water samples collected from related areas of Xikuangshan were analyzed for total Sb, Sb (III), and Sb (V), as well as other major cations and anions. Based on the experimental analysis, we then performed geochemical modeling and further investigated the Sb speciation distribution. Several potential oxidation pathways are also discussed that might have facilitated the oxidation of Sb (III) in the natural environment, as well as the toxicity of antimony related to its oxidation state with relevance to the local mine workers in Xikuangshan. In this way, a profile of Sb distribution, speciation, and transformation behavior in the world’s biggest Sb mining and smelting area can be built up with complete natural environment information.
Materials and methods
Site contamination
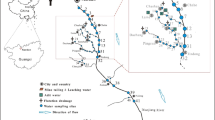
The studied site, the Xikuangshan mining area, is situated between 27.7°N and 111.4°E in Hunan Province, in southwest China (Fig. 1; He 2007). The Sb ore zone, which is about 2 km wide and 9 km long, sits in the northern part of the Xiangzhong Basin, a specific low-temperature mineralization depression zone with rich deposits of mercury, gold, zinc, lead, arsenic, antimony, and coal (Fan et al. 2004). The antimony ore was introduced into the carbonaceous rocks of the Middle Devonian-age Shetian Formation. These rocks contain 15–35 ppm of antimony, compared to the average Sb content of 0.2–0.5 ppm in the earth’s crust, while the rocks of the broad region around Xikuangshan contain about 6 ppm of antimony (Ottens 2007; Smith and Huyck 1999). The deposit also contains trace levels of pyrite, pyrrhotite, and sphalerite with primary gangue minerals such as quartz and calcite, and secondary such as barite and fluorite (Fan et al. 2004).
Study area. Surface water samples were collected from 18 stations in Xikuangshan, Hunan, China (after He 2007)
The Sb mining history in Xikuangshan dates back to the nineteenth century with the first antimony smelter being constructed in 1897 (CMA 2006). Due to the rising demand for antimony in ammunition applications during the World War I, the Sb mining and smelting industry boomed in Xikuangshan with more than 160 family-style plants operating between 1914 and 1918. Mining and smelting were operated by these small private plants until the 1950s when the mines were nationalized and reorganized into one state-owned enterprise, the present Hsikwangshan Twinkling Star company, with more advanced Sb-refining technique.Totals of 55,000 metric tons of Sb ores and 40,000 metric tons of Sb products are produced annually in the Xikuangshan area, mostly by the Hsikwangshan Twinkling Star company (He 2007). However, small plants are still spread all over the mining area with simple, traditional mining and smelting methods dominated by manual labor (CCTV 2008).
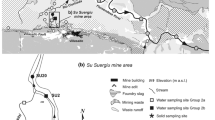
The mining district consists of a relatively historic North Mine and, along the Qing River, approximately 5 km downstream to the southwest, a newer South Mine. Both mines are equipped with a primary smelter (Fig. 1). Ore concentrates are transported in open rail carts for approximately 3 km from the mine to the nearest smelter, where ore sorting, crushing, and concentrating are performed. Most of the processes involve many manual laborers, who are in contact with the ores extensively throughout long periods. In the ore separation and smelting processes, huge amounts of ore-dressing residues, tailing particles, and smelter furnace clinkers are produced. These residues still contain high levels of Sb and other heavy metal contents compared to the uncontaminated environment. They are either piled up untreated in the open air, ultimately releasing high concentrations of Sb and other heavy metals to the adjacent Qing River due to the weathering process (Fig. 2), or washed down with other ore-dressing wastewater via pipelines to a dammed tailing reservoir and occurring as silt- to sand-sized sediments downstream (Fig. 3).
There is no constructed boundary between the mining and residential areas. Land in among the mining area is extensively used for cultivation, with irrigation coming from the adjacent Qing River which accepts the mining and smelting drainage. Food from this farmland supplies most of the local residents. This is a potential addition to the dietary Sb burden, especially when the residential drinking water plant was also built in the mining area (approximately 1 km east of the Ore Tailing Reservoir) with locally supplied source-water contaminated by Sb (Sampling Station 3 is located within the water plant). This also adds to the risk of skin exposure to Sb.
Sampling
Samples were collected in June 2007 for this study. A profile of pH for each sample was obtained on-site with a pH meter. Duplicate samples were collected at each station. The first batch of samples was collected in pre-cleaned polyethylene bottles for analysis of Sb and other anions in water. The second batch was collected in precleaned polyethylene bottles and immediately acidified with HCl for cation analysis. Both batches of samples were kept on dry-ice during the transport to the laboratory in the U.S.A. where they were kept at 4°C until analysis. For antimony speciation measurements, storage at 4°C appears to be appropriate for natural water samples (Florence 1982).
Analytical methods
Antimony total and speciation
The water samples were analyzed under the guidance of the Inductive Coupled Plasma-Mass Spectrometer Method (EPA 3125) for antimony of low concentration (below 50 μg l−1). It is reported as the most favored technique for the determination of antimony in aqueous environmental samples to date (Nash et al. 2000).
Standard stock solutions of Sb (III) (1 mg l−1) and Sb (V) (5 mg l−1) were each prepared by adding 10 mg of either Sb (III) oxide (Sigma–Aldrich) or potassium hexa-hydroxo antimonate to 1 l of water and then stirring for 24 h. All standard solutions were calibrated with ICP-MS using a 10-ppm multi-element environmental calibration standard (Agilent, in 5% HNO3) and the National Institute of Standards and Technology 1640 natural water standard reference material. Standards were also speciated using HPLC-ICP-MS. Stocks of the Sb standards were stored at 4°C and diluted on the day of analysis.
All chemicals used for the preparation of the mobile phases were of analytical grade. For calibration and total antimony determination, the carrier solution used was 1% HNO3 with a flow rate of 1 ml min−1 (Optima grade; Fisher Scientific). For speciation, the mobile phase used was 20 mM (Ethylenedinitrilo) tetraacetic acid disodium salt dehydrate (EMD Chemicals) and, 2 mM potassium hydrogen-phthalate (PHP; 99.5%; Sigma–Aldrich) adjusted to pH 4.5 using ammonium hydroxide (20–22%, Optima; Fisher Scientific). All mobile phases were sonicated prior to use. The water was deionized and of high purity (Milli-Q).
The HPLC system consisted of a pump (Perkin Elmer Series 200) and an autosampler (Perkin Elmer Series 200). The detector was an inductively coupled plasma mass spectrometer (Elan DRC Plus) operating in standard mode. PEEK tubing was used to connect the HPLC to the ICP-MS. For the determination of total Sb and calibration of the standards, the HPLC was run without the use of a column to simulate a flow injection setup. The volume delivered by the autosampler was 20 μl. For total Sb analysis, Rh (25 mg l−1) was added as an internal standard, and 1% HNO3 was used as a dilution buffer. Both isotopes of Sb, 121Sb, and 123Sb, were monitored.
Samples were stored at −60°C and portions were thawed at room temperature on the day of analysis. Then, 1 ml of each sample was added to a centrifuge tube and centrifuged (Micro-MB; International Equipment) to remove solids. The supernatant was decanted into a glass HPLC vial (Fisher Scientific). Samples were then diluted prior to analysis.
For speciation analysis, an anion exchange column was used (PRP-X100, 4.1 × 150 mm, 10 μm; Hamilton). The mobile phase used was the aformentioned pH 4.5, 20 mM EDTA, 2 mM PHP with a flow rate of 1 ml min−1. Sample injection size was 20 μl. The ICP-MS was run in standard mode and both 121Sb and 123Sb, were monitored. Samples for speciation were treated in the same manner as for that analysis of total Sb, except Rh was not used as an internal standard. Instead, calibration standards were run in duplicate before, in the middle of, and after running the 18 water samples which were all run in triplicate. No appreciable instrument drift was seen. One sample was spiked with both Sb (III) and Sb (V) to verify the speciation method.
The limits of detection of the instrument in this study were 0.005 μM for total Sb and 0.001 μM for Sb (V) and Sb (III).
Other ions
Cation concentrations were measured using a Perkin–Elmer AAnalysit 800 Atomic Absorption Spectrometer. The analyses were done following the Direct Air-Acetylene Flame Method (EPA3111B) for Ca2+, Mg2+, K+, and Na+. For Si detection, the Direct Nitrous Oxide-Acetylene Flame Method (3111D) was adopted. A standard was run as a start-up and end of the samples and intermittently between every five samples.
Anion concentrations were analyzed using a Dionex IC25 Ion Chromatograph. The analyses were conducted obeying to the Ion Chromatography with Chemical Suppression of Eluent Conductivity (EPA 4110B). The eluent was a carbonate-bicarbonate solution run at a rate of 1 ml min−1 at 30°C. In successive order, tap water, de-ionized water, and standard of 5, 10, and 20 ppm were run before the samples and in the reverse order at the close.
Results and discussion
The sampling stations are shown in Fig. 1. Station 1 was located in a typical slot among the mining area with both industrial and municipal wastewater inputs. Station 2 was in an abandoned mine only around 200 m southwest of the South Smelter. Station 3 was located in the source water pond within the drinking water plant. Stations 6–1 to 7–3 tracked mining and ore-dressing wastewater all the way from the mine to the downstream Tailing Reservoir area. Samples from Stations 8–1 to 8–5 were collected underground at different elevations of a working mine-pit in the North Mine area. Samples from these five stations represented natural water in immediate contact with the Sb ore.
Table 1 summarizes the pH values and concentrations of total Sb, Sb (III), and Sb (V) from each of the 18 sampling sites. Concentrations of other major cations and anions from the samples are presented in Table 2.
Pollution source, other cations, anions, and pH values analysis
The Sb contamination at the studied locations came from ore tailing particles, smelter furnace clinkers, alkali arsenic smelting residues, and wastewater from the mining and smelting processes (see above).
All 18 stations carried slightly basic water with pH values ranging from 7.7 to 8.5. Stations 5–1 and 6–1, which were located in the residential area relatively far from the mining and smelting site, exhibited slightly less alkaline character (pH = 7.88). Stations 8–3, 8–4, and 8–5, which were underground in the mine-pit with direct contact with ores, also presented slightly lower pH values (pH = 7.9, 7.7, and 7.9, respectively). Quartz and calcite are the two primary gangue minerals and are present in 90% of the total Sb reserves of the deposit (Fan et al. 2004). As a consequence, no significant acidification of the water occurs probably due to the immediate neutralization by the abundant calcite present (Singer and Stumn 1968).
The concentrations of other cations and anions showed variation among samples (Table. 2). However, there is generally a correlation between high Sb level and both high cation and anion concentrations (Fig. 4). The consistency between sulfate and Sb concentrations also indicate the source of Sb in the water samples as stibnite (Sb2S3) ores, and the sulfide is oxidized to sulfate during the exposure.
Distribution of total antimony
All 18 samples contained total dissolved Sb with ppm levels from 0.33 ppm to 11.4 ppm, which is two to three orders of magnitude higher compared to the typical concentration of dissolved Sb in unpolluted rivers (less than 1 ppb; Filella et al. 2002a). Table 1 shows that the highest Sb concentrations came from the sample collected in the underground mine-pit (Stations 8–2 and 8–3) or mixed wastewater fromthe ore-dressing process (Station 4–1, located 100 m downstream from the input of ore-dressing water into the river). This indicates the direct contact with stibnite ores as the main pathway of Sb into the natural water system in the study area. As relatively little transformation and migration of Sb have taken place, water in the mine-pit with direct contact with the stibnite ore showed the highest concentrations of Sb among the samples. Stations 7–1, 7–2, and 7–3 were located downstream of the Tailing Dam where huge amounts of seepage from the upper tailing heap entered the river. From the source along the stream, Sb concentrations decrease steadily. The decrease of Sb concentrations in the series of ore-dressing mixed water and tailing seepage mixed water were probably caused by the dilution from background river water and other potential transformation and transport due to the environment, including sediments, soils, and plants. Iron and aluminum hydroxides might form as Fe and Al released from the ore. These hydroxides along with other suspended particles and sediments also have strong abilities of adsorption of the Sb.
Station 3 is located within a local drinking water plant, and water collected is supposed to serve as the source water of the plant. Total Sb concentration is 0.62 ppm at Station 3. Stations 4–1, 4–2, and 4–3, which are in the vicinity of the water plant (within 300 m radial distance from the water plant), showed total Sb concentrations of 9.53, 3.47, and 3.55 ppm, respectively. These concentrations are also three orders of magnitude higher than both USEPA’s maximum contaminant level (6 ppb; USEPA 1999) and EU’s maximum admissible concentration for Sb (5 ppb; CEC 1998) in drinking water and reveal the potential severe Sb contamination of drinking water in the area.
Antimony speciation and mobility
The speciation of solubllized antimony in the nature system is not adequately understood, especially that of Sb (V). Data on antimony is strikingly limited partly because the hydrolization of both Sb (III) and Sb (V) ions in water, thus the difficulty of “free” antimony ions detection in solution except in extreme pH conditions or in highly diluted solution.
In all our samples, Sb (V) is the predominant valence which is consistent with the antimony speciation discussion in oxygenated systems (Filella et al. 2002a). To evaluate the speciation in our samples, geochemical modeling was performed using PHREEQC (Parkhurst and Appello 1999).
In our simulation, antimony sulfide species were excluded as the oxidation process (above) would result in the formation of sulphate ions in the natural water system (Ashley et al. 2003). The database for antimony was built after the evaluation of Filella and May (2003). PHREEQC database (Parkhurst and Appello 1999) was employed for the calculations of other ions in the solutions. The results of Sb (V) speciation simulation are summarized in Fig. 5. Sb(OH) −6 is the predominant species in all water samples with trace levels of Sb(OH)5 and Sb(OH) +4 , which is more than four orders of magnitude lower than the concentrations of Sb(OH) −6 . Our results suggest the relatively high mobility of Sb in natural environment with Sb(OH) −6 as the dominant species, which concurs with Vink’s Eh–pH diagram of Sb (Vink 1996). Previous experts have shown that high pH (e.g., pH = 6–9) is generally unfavorable to the adsorption of Sb(OH) −6 onto amorphous Fe(OH)3 or soils (Tighe et al. 2005).
Trace levels of Sb(III) were found in four samples (Stations 1, 3, 4–1, and 4–2). Station 1 was in the North Mine area with waste mining water and municipal water mixtures. As municipal wastewater typically contained high levels of DOC (dissolved organic carbon) and was depleted in DO (dissolved oxygen; Asano et al. 2007), this might produce a relatively reducing environment for the existence of Sb (III). Stations 4–1 and 4–2 were located close to the input of ore-dressing wastewater (100 and 300 m downstream, respectively). The ore-dressing wastewater probably also contained high levels of DOC such as from the ore-washing process. Another possibility is the relatively short exposure time of the Sb released from minerals into the environment, which limited the oxidation of Sb (III) to Sb (V). The latter reason might play a subsidiary role in this case, as no Sb (III) was detected among Stations 8–1 to 8–5, which were located within the mine and had the shortest exposure time to the environment.
Station 3 was located within the reservoir of the water plant, downstream at the foot of the abandoned ore heap of South Mine. The trace level of Sb (III) might be due to the processing of the water plant. The results also showed the lowest concentration of Sb, 0.62 ppm, among all 18 samples.
The oxidation pathways of Sb (III) to Sb (V) in the water system
As mentioned earlier, Sb (V) is the predominant valence present in all 18 samples (Table 1). Sb (V), which has less affinity to Fe and Mn (hydr)oxide surfaces than Sb (III) (Thanabalasingam and Pickering 1990) and forms oxides that are more soluble than oxides of Sb (III) (Sb2O3) (Onishi 1978), solubilizes better than Sb (III) in aqueous system. Since antimony is initially introduced into the environment as stibnite (Sb2S3), oxidation of Sb (III) to Sb (V) plays a critical role in understanding the mobility and fate of Sb in the water system.
Several potential oxidants have been indicated in publications that might facilitate the oxidation of Sb (III) yet no quantitative data has been observed (Belzile et al. 2001; Filella et al. 2007; Leuz 2006; Leuz et al. 2006; Leuz and Johnson 2005; Quentel et al. 2004). Leuz and Johnson (2005) found that O2 as the sole oxidant of Sb(III) appeared to be extremely slow in homogenous aqueous solutions under environmental conditions. No significant oxidation of Sb (III) with O2 was observed within 200 days at pH range of 3.6–9.8, which covers the full pH range of our studies. However, a co-oxidation reaction pathway of Sb (III) with Fe(II) by both O2 and H2O2 was invoked recently (Leuz et al. 2006). The study proved that there are increasing oxidation rate coefficients of Sb (III) in the presence of Fe(II) and O2 with increasing pH. At neutral pH values, 45% of initial Sb (III) was oxidized at a Fe:Sb ratio of 1:1 within 60 min. A hypothetical reaction model dominated by Fenton reaction was first proposed by Leupin and Hug (2005) in the iron-mediated oxidation and removal of arsenic in the groundwater. Leuz et al. (2006) indicated that this model was also applicable to the explanation of the iron-mediated oxidation of Sb, in which protonated Fe(II) species exchange H2O with H2O2 via an inner-sphere electron transfer. Two intermediates {[(H2O)5FeII-O2H2]2+ → INT → OH or [(H2O)4(OH)FeII-O2H2] + → INT-OH → possibly Fe(IV)} are probably formed at nanomolar steady-state concentrations.
According to the compositional analysis of Xikuangshan stibnite ores, the ores generally contained an iron-mineral (mainly pyrite with trace amount of pyrrhotite and sphalerite) composition of 3.2% compared to antimony abundance of 2.43% (Table 3; Fan et al. 2004). The pyrite might have been washed down together with the stibnite and gone through the following oxidation reactions with antimony. In the presence of calcite (above), Reaction (1) was rapidly pushed to the right with the neutralization of acidity, producing Fe(II) in the process (Singer and Stumn 1968). The rate-determining step in pyrite oxidation was Reaction (2). Nevertheless, the oxidation rate of this step could be increased to 10- to 20-fold higher than pure chemical reaction by the possible existence of Fe-oxidizing bacteria (e.g., Thiobacillus ferrooxidans and T. thiooxidans; Battaglia-Brunet et al. 1998; Singer and Stumm 1970). In the slightly alkaline water of our site, the released ferric iron might either hydrolyze and form ferric oxyhydroxide as in Reaction (3) (Brieger et al. 1954), or react as oxidant with additional pyrite as in Reaction (4). Therefore, no matter the existence of sufficient Fe-oxidizing bacteria, both Fe(II) and oxidized iron-oxyhydroxides might have formed during the solubilization (Simon et al. 2001).
The toxicity of antimony species and the health & environmental impacts
Antimony has no known biological function and, like arsenic, it is toxic (Filella et al. 2002a). The toxicity of antimony is closely related to its oxidation state, the solubility of the Sb compounds, and the presence of potential ligands in its surrounding environment (Fowler et al. 1991). Elemental Sb is known to be more toxic than its salts yet this has little environmental relevance as Sb rarely exists in the elemental form in the natural system (Shotyk et al. 2005). Similar to other toxic elements, Sb (III) can bind to sulfhydryl groups of cell constituents to inhibit many metabolic functions. Trivalent species are generally 10 times more toxic than pentavalent forms (Bencze 1994). Upon hydrolysis, [Sb(OH)3] o (aq) and [Sb(OH)6]− are formed respectively. The Sb(III) species, [Sb(OH)3] o (aq), has no electrical charge, which allows it to pass more readily across cell membranes, and it exhibits a higher affinity for sulfhydryl and erythrocytes; these might help to explain why Sb (III) tends to be more toxic (Lewis et al. 2006; Shotyk et al. 2005). Antimony trioxide was listed in the group of substances that are suspected of being carcinogenic in humans by the International Agency for Research on Cancer (IARC; Angere and Schaller 1985). Trivalent antimony was also found to inhibit of enzymes involved in DNA repair (Schaumloffel and Gebel 1998).
There are generally three pathways for antimony to be absorbed by human bodies: inhalation, ingestion, and transcutaneous contact. Animal data showed increased mortality and pulmonary edema following inhalation exposure of rats and Guinea pigs to stibnite. Pulmonary function alternations (airway obstruction, bronchospasm, and hyperinflation), gastrointestinal disorders, and increased blood pressure have also been reportedly caused by occupational exposure to antimony by both inhalation and oral exposure (Brieger et al. 1954; Cooper et al. 1968; Dunn 1928; Potkonjak and Vishnjich 1983).
According to EPA’s Toxics Release Inventory (TRI 1987), the industries that contribute the bulk of releases of antimony particles to the air are those that produce antimony and antimony trioxide. As the world’s biggest manufacturer of antimony, huge amounts of antimony dusts are introduced into the surrounding atmosphere of Xikuangshan during the mining and smelting processes. Most local workers in the mines are observed to come into contact with stibnite ore and airborne antimony extensively through both inhalation and derma exposure without sufficient work-place protection. Local drinking water is severely polluted with antimony, as shown from the analysis above (Stations 4–1, 4–2, and 4–3), and oral exposure to antimony can therefore be expected. Though further clinical tests are needed for further investigation of the damage to other body organs, skin lesions have been observed among local smelter workers in Xikuangshan with prolonged contact with antimony.
In the environment, most antimony compounds generally show low bioavailability in the surface layers of the soil and water (Flynn et al. 2003). Yet some plants, mosses, lichens, and fungi are shown to hold a high level of antimony (Filella et al. 2007). He (2007) reported elevated Sb concentrations in soils near/adjacent to the Xikuangshan mine area. Local terrestrial plants (radish plant) containing high contents of antimony showed a positive correlation with soil Sb concentration (He 2007). Accumulation of antimony was also observed in freshwater algae exposed to high Sb levels and in aquatic plants in the vicinity of the ore mining area (Hozhina et al. 2001; Maeda et al. 1997).
This evidence suggest that the Sb contamination due to mining and smelting operations in the Xikuangshan area might present a severe threat to the environment and to human health if not addressed properly.
References
Andreae, M. O. (1983). The determination of the chemical species of some of the “hydride elements” (arsenic, antimony, tin and germanium) in seawater: Methodology and results. In C. S. Wong, E. B., K. W. Bruland, J. D. Burton, & E. D. Goldberg (Eds.), Trace Metals in Sea Water. New York: Plenum.
Andreae, M. O., Asmode, J. F., Foster, P., & Van ‘t dack, L. (1981). Determination of antimony(III), antimony(V), and methylantimony species in natural waters by atomic absorption spectrometry with hydride generation. Analytical Chemistry, 53, 1766–1771.
Angere, J., & Schaller, K.-H. (1985). Analyses of hazardous substances in biological materials. Weinheim: Wiley-VCH.
Asano, T., Burton, F. L., Leverenz, H. L., Tsuchihashi, R., & Tchobanoglous, G. (2007). Characteristics of municipal wastewater and related health and environmental issues. In M. Eddy (Ed.), Water reuse: Issues, technologies, and applications. New York City: McGraw-Hill.
Ashley, P. M., Craw, D., Graham, B. P., & Chappell, D. A. (2003). Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. Journal of Geochemical Exploration, 77, 1–14.
Battaglia-Brunet, F., d’Hugues, P., Cezac, P., Garcia, J. L., & Morin, D. (1998). The mutual effect of mixed Thiobacilli and Leptospirilli populations on pyrite bioleaching. Mineral Engineering, 11, 195–205.
Belzile, N., Chen, Y.-W., & Wang, Z. (2001). Oxidation of antimony (III) by amorphous iron and manganese oxyhydroxides. Chemical Geology, 174, 379–387.
Bencze, K. (1994). Antimony. In H. G. Seiler, A. Sigel, & H. Sigel (Eds.), Handbook on metals in clinical and analytical chemistry. New York: Marcel Dekker.
Brieger, H., Semisch, C. W., I. I. I., Stasney, J., & Piatnek, D. A. (1954). Industrial antimony poisoning. Industrial Medicine and Surgery, 23, 521–523.
CCTV. (2008). The story of Xikuangshan (in Chinese). China Central Television.
CEC. (1976). Council directive 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. Official Journal L 129, 18/05/1976. Council of the European Communities.
CEC. (1998). Council Directive 98/83/EC of 3 November 1998, Quality of Water Intended for Human Consumption. Official Journal L 330, 05/12/1998. Council of the European Communities.
CMA. (2006). Antimony (in Chinese). Metallic mineral resources. Beijing: China Mining Association (CMA).
Cooper, D. A., Pendergrass, E. P., Vorwald, A. J., Mayock, R. L., & Brieger, H. (1968). Pneumoconiosis among workers in an antimony industry. The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine, 103, 496–508.
Crommentuijn, T., Sijm, D., de Bruijn, J., van den Hoop, M., van Leeuwen, K., & van de Plassche, E. (2000). Maximum permissible and negligible concentrations for metals and metalloids in the Netherlands, taking into account background concentrations. Journal of Environmental Management, 60, 121–143.
Deng, T. L., Chen, Y. W., & Belzile, N. (2000). Antimony speciation at ultra trace levels using hydride generation atomic fluorescence spectrometry and 8-hydroxyquinoline as an efficient masking agent. Analytica Chimica Acta, 432, 293–302.
Dunn, L. C. (1928). A Fifth Allelomorph in the Agouti Series of the House Mouse. Proceedings of the National Academy of Sciences of the United States of America, 14, 816–819.
Fan, D., Zhang, T., & Ye, J. (2003). The Xikuangshan Sb deposit hosted by the Upper Devonian black shale series, Hunan, China. Ore Geology Reviews, 24, 121–133.
Fan, D., Zhang, T., & Ye, J. (2004). The Xikuangshan Sb deposit hosted by the Upper Devonian black shale series, Hunan, China. Ore Geology Reviews, 24, 121–133.
Filella, M., Belzile, N., & Chen, Y. W. (2002a). Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Science Reviews, 57, 125–176.
Filella, M., Belzile, N., & Chen, Y. W. (2002b). Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth-Science Reviews, 59, 265–285.
Filella, M., Belzile, N., & Lett, M.-C. (2007). Antimony in the environment: A review focused on natural waters. III. Microbiota relevant interactions. Earth-Science Reviews, 80, 195–217.
Filella, M., & May, P. M. (2003). Computer simulation of the low-molecular-weight inorganic species distribution of antimony (III) and antimony (V) in natural waters. Geochimica et Cosmochimica Acta, 67, 4013–4031.
Florence, T. M. (1982). The speciation of trace elements in waters. Talanta, 29(5), 345.
Flynn, H. C., Meharg, A. A., Bowyer, P. K., & Paton, G. I. (2003). Antimony bioavailability in mine soils. Environmental Pollution, 124, 93–100.
Fowler, B. A., Goering, P. L., & Merian, E. (1991). Antimony, metals and their compounds in the environment. New York: Weinheim.
Gebel, T. (1997). Arsenic and antimony: Comparative approach on mechanistic toxicology. Chemico-Biological Interactions, 107, 131–144.
He, M. (2007). Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environmental Geochemistry and Health, 29, 209–219.
Hou, H. B., & Narasaki, H. (1999). Differential determination of antimony(III) and antimony(V) in river water by hydride-generation inductively coupled plasma mass spectrometry. Analytical Sciences, 15, 911–914.
Hozhina, E. I., Khramov, A. A., Gerasimov, P. A., & Kumarkov, A. A. (2001). Uptake of heavy metals, arsenic, and antimony by aquatic plants in the vicinity of ore mining and processing industries. Journal of Geochemical Exploration, 74, 153–162.
Krachler, M., Emons, H., & Zheng, J. (2001). Speciation of antimony for the 21st century: Promises and pitfalls. TrAC Trends in Analytical Chemistry, 20, 79–90.
Laintz, K. E., Shieh, G. M., & Wai, C. M. (1992). Simultaneous determination of arsenic and antimony species in environmental samples using bis(trifluoroethyl)dithiocarbamate chelation and supercritical fluid chromatography. Journal of Chromatographic Science, 30, 120–123.
Leupin, O. X., & Hug, S. J. (2005). Oxidation and removal of arsenic (III) from aerated groundwater by filtration through sand and zero-valent iron. Water Research, 39, 1729–1740.
Leuz, A.-K. (2006). Redox reactions of antimony in the aquatic and terrestrial environment. Diploma thesis, Carl von Ossietzky University of Oldenburg, Oldenburg, Germany.
Leuz, A.-K., Hug, S. J., Wehrli, B., & Johnson, C. A. (2006). Iron-mediated oxidation of antimony(III) by oxygen and hydrogen peroxide compared to arsenic(III) oxidation. Environmental Science and Technology, 40, 2565–2571.
Leuz, A.-K., & Johnson, C. A. (2005). Oxidation of Sb(III) to Sb(V) by O2 and H2O2 in aqueous solutions. Geochimica et Cosmochimica Acta, 69, 1165–1172.
Lewis, R. G., Flomenbaum, N., Hoffman, R. S., Howland, M. A., Lewin, N. A., & Nelson, L. S. (2006) Antimony, Goldfrank’s toxicologic emergencies. New York: McGraw-Hill.
Maeda, S., Fukuyama, H., Yokoyama, E., Kuroiwa, T., Ohki, A., & Naka, K. (1997). Bioaccumulation of Antimony by Chlorella vulgaris and the Association Mode of Antimony in the Cell. Applied Organometallic Chemistry, 11, 393–396.
Mohammad, B., Ure, A. M., Reglinski, J., & Littlejohn, D. (1990). Speciation of antimony in natural waters: The determination of Sb(III) and Sb(V) by continuous flow hydride generation—atomic absorption spectrometry. Chemical Speciation Bioavailability, 3, 117–122.
Mok, W. M., & Wai, C. M. (1987). Simultaneous extraction of trivalent and pentavalent antimony and arsenic species in natural waters for neutron activation analysis. Analytical Chemistry, 59, 233–236.
Nash, M. J., Maskall, J. E., & Hill, S. J. (2000). Methodologies for determination of antimony in terrestrial environmental samples. Journal of Environmental Monitoring, 2, 97–109.
Onishi, H. (1978). Antimony. In K. H. Wedepohl (Ed.), Handbook of Geochemistry. Berlin: Springer.
Ottens, B. (2007). Chinese Stibnite: Xikuangshan, Lushi, Wuning and other localities. Mineralogical Record, 38 (1), 3–17.
Parkhurst, D. L., & Appello, A. A. J. (1999). User’s guide to PHREEQC (version 2)-a computer program for speciation, batch-reaction, one dimensional transport, and inverse geochemical modeling. U.S. Geological Survey.
Potkonjak, V., & Vishnjich, V. (1983). Antimoniosis: A particular form of pneumoconiosis. II. Experimental investigation. International Archives of Occupational and Environmental Health, 51, 299–303.
Quentel, F., Filella, M., Elleouet, C., & Madec, C. L. (2004). Kinetic Studies on Sb(III) Oxidation by hydrogen peroxide in aqueous solution. Environmental Science and Technology, 38, 2843–2848.
Schaumloffel, N., & Gebel, T. (1998). Heterogeneity of the DNA damage provoked by antimony and arsenic. Mutagenesis, 13, 281–286.
Scheinost, A. C., Rossberg, A., Vantelon, D., Xifra, I., Kretzschmar, R., Leuz, A.-K., et al. (2006). Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochimica et Cosmochimica Acta, 70, 3299–3312.
Shieh, G. M. (1993). Analytical techniques for arsenic and antimony speciation in interstitial water of river sediments. PhD Thesis. University of Idaho, Moscow, ID, USA, 155 pp.
Shotyk, W., Krachler, M., & Chen, B. (2005). Anthropogenic impacts on the biochemistry and cycling of antimony. In A. Sigel, H. Sigel, & R. K. O. Sigel (Eds.), Metal ions in biological systemsBiogeochemistry, Vol. 44. Availability, and transport of metals in the environment. . Boca Raton: Taylor & Francis.
Simon, M., Martin, F., Ortiz, I., Garcia, I., Fernandez, J., Fernandez, E., et al. (2001). Soil pollution by oxidation of tailings from toxic spill of a pyrite mine. Science of the Total Environment, 279, 63–74.
Singer, P. C., & Stumm, W. (1970). Acidic mine drainage: The rate-determining step. Science, 167, 1121–1123.
Singer, P. C., & Stumn, W. (1968). Kinetics of the oxidation of ferrous iron In Second symposium on coal mine research. Pittsburgh, PA: Mellon Institute.
Smith, K. S., & Huyck, H. L. O. (1999). An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. Reviews in Economic Geology, 6A, 29–70.
Sun, Y. C., Yang, J. Y., Lin, Y. F., Yang, M. H., & Alfassi, Z. B. (1993). Determination of antimony(III, V) in natural waters by coprecipitation and neutron activation analysis. Analytica Chimica Acta, 276, 33–37.
Takayanagi, K., & Cossa, D. (1997). Vertical distributions of Sb(III) and Sb(V) in Pavin Lake, France. Water Research, 31, 671–674.
Thanabalasingam, P., & Pickering, W. F. (1990). Specific sorption of antimony (III) by the hydrous oxides of Mn, Fe and Al. Water Air and Soil pollution, 49, 175–185.
Tighe, M., Lockwood, P., & Wilson, S. (2005). Adsorption of antimony(V) by floodplain soils, amorphous iron(III) hydroxide and humic acid. Journal of Environmental Monitoring, 7, 1177–1185.
Titretir, S., Kendüzler, E., Arslan, Y., Kula, I., BakIrdere, S., & Ataman, O. Y. (2008). Determination of antimony by using tungsten trap atomic absorption spectrometry. Spectrochimica Acta Part B Atomic Spectroscopy, 63, 875–879.
TRI. (1987). Toxics release inventory. Environmental Protection Agency. http://www.epa.gov/TRI/.
Ulrich, N. (1998). Speciation of antimony(III), antimony(V) and trimethylstiboxide by ion chromatography with inductively coupled plasma atomic emission spectrometric and mass spectrometric detection. Analytica Chimica Acta, 359, 245–253.
UNEP. (1999). Basel convention on the control of transboundary movements of hazardous wastes and their disposal (with amended Annex I and two additional Annexes VIII and IX, adopted at the fourth meeting of the Conference of the Parties in 1998). SBC No. 99/001. Geneva: United Nations Environmental Program.
USEPA. (1979). Water related fate of the 129 priority pollutants, vol. 1. USEPA, Washington, DC, USA, EP-440/4-79-029A. United States Environmental Protection Agency.
USEPA. (1999). Integrated Risk Information System (IRIS) on Antimony. Washington, DC: United States Environmental Protection Agency, National Center for Environmental Assessment, Office of Research and Development.
USGS. (2004). Open-File Report 03-019: Mineral Commodity Profiles: Antimony. In: U.S. Department of the Interioir, U.S.G.S. (Eds.).
Van der Sloot, H. A., Hoede, D., & Wijkstra, J. (1989). Trace oxyanions and their behaviour in the rivers Porong and Solo, the Java Sea and the adjacent Indian Ocean. Netherlands Journal of Sea Research, 23, 379–386.
Vink, B. W. (1996). Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams. Chemical Geology, 130, 21–30.
Acknowledgments
Faye Liu wishes to acknowledge scholarship from China Scholarship Council and fellowship from the Center for Environmental Research at Indiana University, and wishes to thank Dr. Lisa Pratt for advice and supervision during Dr. Chen Zhu’s sabbatical leave. Dr. Chen Zhu wishes to acknowledge a Fulbright Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Le, X.C., McKnight-Whitford, A. et al. Antimony speciation and contamination of waters in the Xikuangshan antimony mining and smelting area, China. Environ Geochem Health 32, 401–413 (2010). https://doi.org/10.1007/s10653-010-9284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9284-z