Abstract
Despite the higher diversity of amphibians and the increasing use of agrochemicals in tropical countries, knowledge on the ecotoxicity of such compounds to tropical amphibians remains very limited. The aim of this study was, therefore, to assess the acute lethal toxicity of three nitrogen salts (ammonium sulphate, sodium nitrate and sodium nitrite) to tadpoles of five tropical frog species: Rhinella ornata, Boana faber, B. pardalis, Physalaemus cuvieri, and P. olfersii. The order of sensitivity to the nitrogen salts for all five species was sodium nitrite > ammonium sulphate > sodium nitrate. There was not a single most sensitive species to all three nitrogen salts. However, differences in generated 4-d LC50 values between the most and least sensitive test species were small (a factor 2 to 6). A comparison with published toxicity values does not suggest an intrinsic higher, or lower, sensitivity of the tropical species tested as compared to their temperate counterparts. Reported nitrogen concentrations in sugarcane fields do not indicate a lethal risk to the amphibian species tested. Chronic-exposure and field studies are recommended to evaluate amphibian sensitivity under environmental-realistic multiple-stressor conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphibians play an important role in ecosystem functioning via nutrient cycling, energy flow and pest control (Valencia-Aguilar et al. 2013). Increasing concerns have therefore been raised with worldwide declining amphibian populations, with about one third of species currently under threat of extinction (IUCN 2017). Several underlying reasons for these declines have been identified, including emerging infectious diseases, climate change, invasive species, habitat loss and pollution (Araújo et al. 2014; Whitfield et al. 2016). In rural landscapes, agrochemicals such as pesticides and fertilisers have been suggested to pose a high risk to amphibian communities (Camargo and Alonso 2006; Hedge et al. 2019; Marco et al. 1999; Ortiz-Santaliestra et al. 2018).
Despite of the above, few studies have been conducted so far into the toxicity of agrochemicals to amphibians when compared to other taxonomic groups (Ilha and Schiesari 2014; Ortiz-Santaliestra et al. 2018). This lack in research appears to hold true especially for tropical amphibian species, even though amphibian biodiversity and population declines are higher in the tropics than anywhere else (Sańchez-Domene et al. 2018; Schiesari et al. 2007; Whitfield et al. 2016). In addition, life history traits differ between temperate and tropical amphibians, which dictates that their responses to agrochemical stressors are likely to differ (Schiesari et al. 2007).
Sugarcane plantations in South-eastern Brazil rely on intensive agrochemical inputs and have seen an impoverishment in amphibian biodiversity (Schiesari and Corrêa 2016). In a previous study, we evaluated the acute sensitivity of the four main herbicides used in Brazilian sugarcane production to tadpoles of two indigenous frog species (Daam et al. 2019). In addition, we mined published acute herbicide toxicity data for temperate, subtropical and tropical amphibians. Although this did not indicate a clear difference in herbicide sensitivity between tropical and non-tropical amphibians, differences may exist depending on the test species and compound (Daam et al. 2019). Little remains known, however, on the toxicity of nutrient salts to tropical organisms (Wang and Leung 2015). Brazilian sugarcane relies on an intensive use of fertilisers, with overuse as the rule rather than the exception (Martinelli and Filoso 2008; Schiesari and Corrêa 2016). Nitrogen-based fertilisers may exert several toxicological effects on amphibians (Camargo and Alonso 2006; Hedge et al. 2019; Marco et al. 1999). Nitrogen concentrations in waterbodies embedded in Brazilian sugarcane fields have indeed previously been indicated to potentially reach levels that may cause amphibian tadpole mortality (Ilha and Schiesari 2014).
The aim of the present study was to evaluate the lethal toxicity of inorganic nitrogen (ammonium sulphate, sodium nitrate and sodium nitrite) to tadpoles of five tropical frog species. To this end, acute 4-d laboratory toxicity tests were conducted with tadpoles of these species to determine whether these species are in acute lethal risk of nitrogen concentrations typically encountered in Brazilian sugarcane plantations.
Materials and methods
Test species
Frog and toad egg masses were collected in South-East Brazil from ponds at the Estação Biológica de Boracéia in Salesópolis (23°37′59″S,45°31′59″W), which is located within a protected and non-polluted watershed (Verdade et al. 2011). Only species for which at least three egg masses could be collected were included as test species as to avoid any possible influence of a particular single egg mass. In this way, egg masses of five different species belonging to three families could be collected and taken to the laboratory in sealed plastic bags: (1) Rhinella ornata (Bufonidae); (2) Boana faber (Hylidae); (3) Boana pardalis (Hylidae); (4) Physalaemus cuvieri (Leptodactylidae); and (5) Physalaemus olfersii (Leptodactylidae). All these species are native to South America and their geographic distribution is restricted to Brazil and, depending on the species, Argentina and/or Paraguay (IUCN 2017). In addition, these species have previously been encountered in a wide array of habitats, including sugarcane fields and their surroundings (Schiesari and Corrêa 2016).
In the laboratory, egg masses were hatched in plastic tanks containing 50 L tap water after filtering through an activated carbon granular filter. Every other day, this tank water was renewed. The tank water had the following characteristics: total hardness = 56 mg/L (SM 2340 C-EDTA titrimetric method); total alkalinity = 24 mg/L (SM 2320 B-titration method); turbidity = 0.46 UT (SM 2130 B-nephelometric turbidity method); and total chlorine concentration < 0.01 mg/L (SM 4500 Cl-G-DPD colorimetric method) (APHA 2005).
The laboratory temperature was controlled at 25 ± 2 °C with natural photoperiod. Hatched larvae were fed ad libitum on a daily basis with a 3:1 ground mixture of rabbit chow (Purina Mills, LLC, USA; ~16% protein) and Tetra Min fish food (Tetra Werke, Melle, Germany; ~45% protein) until the start of the bioassays. These laboratory conditions (feeding, temperature and light regime) were noted to be adequate for egg hatching and tadpole development of native frog/toad species in previous experiments (Daam et al. 2019 and references therein). The acute toxicity bioassays were conducted with tadpoles of Gosner stage 25 (Gosner 1960) since this is the most common life stage evaluated in amphibian ecotoxicity tests (Sparling et al. 2010). Only healthy individuals, as judged by behaviour and external morphology (Bantle et al. 1991), were selected for use in the tests.
Acute toxicity bioassays
Acute (96 h) static bioassays were conducted following internationally adopted protocols for amphibian ecotoxicological testing (ASTM 2013; OECD 2015). In these tests, the acute toxicity of ammonium sulphate ((NH4)2SO4; CAS number 7783-20-2), sodium nitrite (NaNO2; CAS number 7632-00-0) and sodium nitrate (NaNO3; CAS number 7631-99-4), (all obtained from Merck KGaA, Darmstadt, Germany; purity > 99%) was evaluated for the five test species indicated in the previous section. These nitrogen salts were selected since previous studies have demonstrated that their toxicity can be essentially attributed to the ammonium, nitrite and nitrate ions, rather than to the sulphate and sodium (Baker and Waights 1994; Schuytema and Nebeker 1999). Given the lack of information on their sensitivity to the test compounds, preliminary range finding tests with the test species were conducted evaluating a wide test concentration range with a limited number of organisms and a single replicate. Based on these preliminary range finding tests, five test concentrations were selected for each of the three test compounds and five test organisms (Table 1), besides an untreated control (culture medium). Unfortunately, it was not possible to conduct chemical analyses of the test compounds due to logistic reasons. In a previous study conducted in our laboratory, the chronic toxicity of the three test compounds was evaluated. In that study, the measured nitrogen concentrations remained within 10% of the respective nominal concentrations in all treatments over the course of the 3-week test period (Ilha and Schiesari 2014). Subsequently, it may be expected that the test compound concentrations in the present study also remained constant over the 4-d test period.
To enable comparing the relative toxicity of the three nitrogen compounds, test concentration units were expressed in mg N/L as calculated from the molar mass of atomic nitrogen contained in the nitrogen salts. Each treatment was conducted in quadruplicate, with each replicate consisting of a glass jar containing 1-L test solution with 10 tadpoles. The tests were carried out under the same conditions as described for egg hatching and tadpole development, except that animals were not fed during the test. After 48 h from the start of the treatment, dead animals were removed from the jars, and median lethal concentrations (LC50) were determined based on the number of dead animals denoted 96 h post start treatment.
Data analysis
The 96h-LC50 values were calculated based on the % mortality rates in the different treatments using the statistical programmes PROBIT 1.5 (EPA 2002) and TSK 1.5 (Hamilton et al. 1977). In all cases, the most appropriate statistical test was defined depending on the experimental design and the nature of the available data, following the recommendations of EPA (EPA 2002). Differences in LC50 values between species for the same nitrogen salt were evaluated using the Z-test (Zar 1999).
Species sensitivity distributions
Species sensitivity distributions (SSDs) were constructed to visualise differences in 96h-LC50 (median lethal concentration) values derived for the five native tropical frog/toad species and the three nitrogen salts. SSDs were constructed with the ETX 2.1 programme (Van Vlaardingen et al. 2004) by fitting lognormal distribution curves to the toxicity data. The Anderson–Darling test included in the ETX software package was used to confirm lognormality of the curves, which was accepted at the 5% significance level. The HC5 and HC50 values (hazard concentration for 5 and 50% of the species assemblage included in the SSD, respectively), together with their 95% confidence intervals, were also calculated with the ETX software following the method outlined in Aldenberg and Jaworska (2000).
Results and discussion
Differential toxicity of the three nitrogen ions
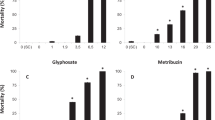
Tadpole survival was 100% in controls for all five species tested. Mortality steadily increased with increasing nitrogen concentrations from low percentages at lower concentrations up to 100% in the higher test concentrations (see examples provided in Fig. 1). Subsequently, LC50 values could be generated for all compound-species combination, except for B. pardalis exposed to ammonium sulphate since the highest test concentration did not exert 50% effect (Table 2). The distribution of these LC50 values were plotted in the SSD provided in Fig. 2. As may be deducted from the SSD curves in this Figure, as well as from their HC5 and HC50 values (Table 3), the overall order of sensitivity was N-nitrite > N-ammonium > N-nitrate. In line with this, Valencia-Castañeda et al. (2019) denoted the same differential toxicity of these three nitrogen ions to juveniles of the shrimp Litopenaeus vannamei. Studies with fish, however, have shown a pattern of higher ammonia toxicity as compared to nitrite and nitrate (Tilak et al. 2002; Luo et al. 2016 and references therein). Ilha and Schiesari (2014) evaluated the differential sensitivity of prolonged exposure (21 days) to sodium nitrite, ammonium sulphate and sodium nitrate to B. faber. At a test concentration of 10 mg N/L, they denoted a slightly greater negative effect on survival of nitrite as compared to ammonium (and no effects of nitrate), which is in line with the results of the present (acute exposure) study. However, relative sublethal sensitivity depended on the test parameter considered: only nitrite had a significant negative effect on body mass, but only ammonium resulted in a significant reduction in activity rates (Ilha and Schiesari 2014). Besides toxic effect measurement, several other factors have been indicated to influence nitrogen speciation and (hence) toxicity to amphibians. The most commonly mentioned factors are pH and temperature (e.g. Casali-Pereira et al. 2015; Wang and Leung 2015; Liu et al. 2019), but also intraspecific (e.g. depending on developmental stage) and interspecific variation evidently plays a great role (Bellezi et al. 2015; Ortiz-Santaliestra et al. 2006; Shinn et al. 2008).
Mortality (in %) of the most sensitive test species to sodium nitrate, ammonium sulphate and sodium nitrate (c.f. Fig. 2) at the end of the 4-d acute lethality tests. Bars represent mean ± SD
Relative toxicity of the test species and other amphibians
Regarding interspecific sensitivity variation, the five species differed in their sensitivity depending on the nitrogen ion: B. pardalis, P. cuvieri and P. olfersii were the most sensitive among the test species to nitrite, ammonium and nitrate, respectively (Fig. 2; Table 2). It should be noted, however, that the difference in LC50 values between the most and least sensitive test species for these compounds was only a factor 6, 4 and 2, respectively. That these factors are relatively low may be exemplified with the fact that the response variability in bioassays with the same species and compound may be a factor 3 or more (Sprague 1985; Baird et al. 1989).
Amphibians in tropical waters have previously been suggested to be more susceptible to ammonium stress than their temperate counterparts (Wang and Leung 2015; Mooney et al. 2019). Mooney et al. (2019), however, discussed that this is more likely to be related with differences in physico-chemical water quality parameters (e.g. low ionic strength of tropical waters tested), rather than to an intrinsic physiological difference in sensitivity of species from tropical and temperate regions. Schuytema and Nebeker (1999) evaluated the toxicity of ammonium sulphate and sodium nitrate to tadpoles of the high‐latitude Pacific treefrog Pseudacris regilla and the African clawed frog Xenopus laevis, a species native to arid/semiarid regions of Sub-Saharan Africa. The 4d-LC50 values they reported for ammonium (as ammonium sulphate; 115 and 135 mg N/L) were slightly lower than those obtained in the present study, while the 4d-LC50 values for nitrate in that study (as sodium nitrate; 1750 and 1656 mg N/L) were consistent with the highest ones that we obtained (Table 2). Wang et al. (2020) reported 4d-EC50 values of 601 mg N/L and 694 mg N/L for grey treefrog Hyla versicolor and wood frog (Lithobates sylvaticus) larvae. Although these values are lower than those derived in the present study (Table 2), it should be noted that immobility was used as endpoint by Wang et al. (2020) instead of mortality. Finally, the 1d-LC50 of 112 mg N/L for the temperate species Rana temporaria exposed to nitrite (as sodium nitrite; recalculated by the authors from data in Williams and Eddy (1986) is well in the range of the corresponding values of the tropical species tested in the present study (Table 2). There thus does not appear to be an intrinsic higher of lower sensitivity of the tropical tadpoles to the nitrogen salts evaluated as compared to their temperate counterparts.
Implications for the risk of the nitrogen salts and concluding remarks
Ilha and Schiesari (2014) reviewed ammonia, nitrate and nitrite concentrations measured in native, agricultural and urban habitats of the State of São Paulo. Maximum ammonia and nitrate concentrations reported in agricultural (including sugarcane) habitats were below 71 and 9 mg N/L, respectively, whereas nitrite levels were in the 10–100 µg N/L range. Subsequently, no acute lethal effects are to be expected considering the toxicity data derived in the present study (Tables 2, 3; Figs 1, 2). Several indirect and interactive effects of low nitrogen pollution on amphibians, however, have been discussed, which include: (i) a competitive disadvantage with the nitrogen-tolerant invasive bullfrogs (Marco et al. 1999); (ii) increased parasitism (Camargo and Alonso 2006); and (iii) greater toxicity of pesticides at increased nitrogen levels (Hedge et al. 2019). In addition, very few chronic and field studies have been conducted so far with tropical amphibians to elucidate the chain of effects of sublethal nitrogen levels under environmental-realistic conditions (Daam et al. 2019; Mooney et al. 2019).
References
Aldenberg T, Jaworska JS (2000) Uncertainty of hazardous concentrations and fraction affected for normal species sensitivity distributions. Ecotoxicol Environ Saf 46:1–18
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC, USA
ASTM (2013) Standard guide for conducting the frog embryo teratogenesis assay—Xenopus (FETAX). ASTM E1439-12. American Society for Testing and Materials, West Conshohocken, PA, USA
Araújo CVM, Shinn C, Moreira-Santos M, Lopes I, Espindola ELG, Ribeiro R (2014) Copper-driven avoidance and mortality in temperate and tropical tadpoles. Aquat Toxicol 146:70–75
Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM (1989) The Daphnia bioassay: a critique. Hydrobiologia 188(189):403–406
Baker J, Waights V (1994) The effects of nitrate on tadpoles of the tree frog (Litoria caerulea). Herpetol J 4:106–108
Bantle JA, Dumont JN, Finch R, Linder G (1991) Atlas of abnormalities: a guide for the performance of FETAX. Oklahoma State University, Stillwater, USA
Bellezi L, Ilha P, Schiesari L (2015) Ontogenetic variation in the sensitivity of the gladiator frog, Hypsiboas faber, to inorganic nitrogen. Copeia 103:14–21
Camargo JA, Alonso A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32:831–849
Casali-Pereira MP, Daam MA, Resende JC, Vasconcelos AM, Espíndola ELG, Botta CM (2015) Toxicity of Vertimec® 18 EC (active ingredient abamectin) to the neotropical cladoceran Ceriodaphnia silvestrii. Chemosphere 139:558–564
Daam MA, Moutinho MF, Espíndola ELG, Schiesari L (2019) Lethal toxicity of the herbicides acetochlor, ametryn, glyphosate and metribuzin to tropical frog larvae. Ecotoxicology 28:707–715
EPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th edn. Environmental Protection Agency, Washington, DC
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hamilton MA, Russi RC, Thurston RV (1977) Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Hegde G, Krishnamurthy SV, Berger G (2019) Common frogs response to agrochemicals contamination in coffee plantations, Western Ghats, India. Chem Ecol 35:397–407
Ilha P, Schiesari L (2014) Lethal and sublethal effects of inorganic nitrogen on gladiator frog tadpoles (Hypsiboas faber, Hylidae). Copeia 2014:221–230
IUCN (2017) IUCN Red list of threatened species, v 2017. 3. International Union for Conservation of Nature, Gland, Switzerland, http://www.iucnredlist.org
Liu Z, Tai P, Li X, Kong L, Matthews TG, Lester RE, Mondon JA (2019) Deriving site-specific water quality criteria for ammonia from national versus international toxicity data. Ecotoxicol Environ Saf 171:665–676
Luo S, Wu B, Xiong X, Wang J (2016) Short‐term toxicity of ammonia, nitrite, and nitrate to early life stages of the rare minnow (Gobiocypris rarus). Environ Toxicol Chem 35:1422–1427
Marco A, Quilchano C, Blaustein AR (1999) Sensitivity to nitrate and nitrite in pond-breeding amphibians from the Pacific Northwest. Environ Toxicol Chem 18:2836–2839
Martinelli LA, Filoso S (2008) Expansion of sugarcane ethanol production in Brazil: environmental and social challenges. Ecol Appl 18:885–898
Mooney TJ, Pease CJ, Hogan AC, Trenfield M, Kleinhenz LS, Humphrey C, van Dam RA, Harford AJ (2019) Freshwater chronic ammonia toxicity: a tropical-to-temperate comparison. Environ Toxicol Chem 38:177–189
OECD (2015) Test No. 241: the larval amphibian growth and devel- opment assay (LAGDA). Organisation for Economic Cooperation and Development, Paris
Ortiz-Santaliestra ME, Maia JP, Egea-Serrano A, Lopes I (2018) Validity of fish, birds and mammals as surrogates for amphibians and reptiles in pesticide toxicity assessment. Ecotoxicology 27:819–833
Ortiz-Santaliestra ME, Marco A, Fernández MJ, Lizana M (2006) Influence of developmental stage on sensitivity to ammonium nitrate of aquatic stages of amphibians. Environ Toxicol Chem 25:105–111
Sańchez-Domene D, Navarro-Lozano A, Acayaba R, Picheli K, Montagner C, Rossa-Feres DC, da Silva FR, de Almeida EA (2018) Eye malformation baseline in Scinax fuscovarius larvae populations that inhabit agroecosystem ponds in southern Brazil. Amphib–Reptilia 39:325–334
Schiesari L, Corrêa DT (2016) Consequences of agroindustrial sugarcane production to freshwater biodiversity. GCB Bioenergy 8:644–657
Schiesari L, Grillitsch B, Grillitsch H (2007) Biogeographic biases in research and their consequences for linking amphibian declines to pollution. Conserv Biol 21:465–471
Schuytema GS, Nebeker AV (1999) Comparative effects of ammonium and nitrate compounds on Pacific treefrog and African clawed frog embryos. Arch Environ Contam Toxicol 36:200–206
Shinn C, Marco A, Serrano L (2008) Inter- and intra-specific variation on sensitivity of larval amphibians to nitrite. Chemosphere 71:507–514
Sparling DW, Linder G, Bishop C, Krest S (2010) Ecotoxicology of amphibians and reptiles. SETAC/Taylor & Francis, Boca Raton, FL
Sprague JB (1985) Factors that modify toxicity. In: Rand GM, Petrocelli SR (eds.) Fundamentals of aquatic toxicology. Hemi-sphere, Washington, DC, p 124–163
Tilak KS, Lakshmi SJ, Susan TA (2002) The toxicity of ammonia, nitrite and nitrate to the fish, Catla catla (Hamilton). J Environ Biol 23:147–149
Valencia-Aguilar A, Corteś-Gómez AM, Ruiz-Agudelo CA (2013) Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. Int J Biodivers Sci Ecosyst Serv Manag 9:257–272
Valencia-Castañeda G, Frías-Espericueta MG, Vanegas-Pérez RC, Chávez-Sánchez MC, Páez-Osuna F (2019) Toxicity of ammonia, nitrite and nitrate to Litopenaeus vannamei juveniles in low-salinity water in single and ternary exposure experiments and their environmental implications. Environ Toxicol Pharmacol 70:103193
Van Vlaardingen P, Traas TP, Wintersen AM, Aldenberg T (2004) ETX 2.0. A program to calculate hazardous concentrations and fraction affected, based on normally distributed toxicity data. RIVM Report No. 601501028/2004. National Institute of Public Health and the Environment (RIVM), Bilthoven, the Netherlands
Verdade VK, Carnaval AC, Rodrigues MT, Sciesari LC, Pavan D, Bertoluci JA (2011) Decline of amphibians in Brazil. In: Heatwole H, Wilkinson JW (eds.) Amphibian biology. Status of decline of amphibians: Western Hemisphere. Part 2: Uruguay, Brazil, Ecuador and Colombia. Surrey Beatty and Sons, Chipping Norton, Australia, p 85–127
Wang N, Dorman RA, Ivey CD, Soucek DJ, Dickinson A, Kunz BK, Steevens JA, Hammer EJ, Bauer CR (2020) Acute and chronic toxicity of sodium nitrate and sodium sulfate to several freshwater organisms in water‐only exposures. Environ Toxicol Chem 39:1071–1085
Wang Z, Leung KMY (2015) Effects of unionised ammonia on tropical freshwater organisms: implications on temperate-to-tropic extrapolation and water quality guidelines. Environ Pollut 205:240–249
Whitfield SM, Lips KR, Donnelly MA (2016) Amphibian decline and conservation in central America. Copeia 104:351–379
Williams EM, Eddy FB (1986) Chloride uptake in freshwater teleosts and its relationship to nitrite uptake and toxicity. J Comp Physiol B 156:867–87
Zar J (1999) Biostatiscal analysis. Upper Saddle River Prentice Hall, New Jersey, USA
Acknowledgements
We thank ICMBio (ICMBio 17559) and the Museu de Zoologia da Universidade de São Paulo for collection permits, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a scholarship to PI, and Conselho Nacional de Desenvolvimento Tecnológico (CNPq Edital Universal Projeto 483801/2007-0) and the São Paulo Research Foundation (FAPESP Young Researcher Award 2008/57939-9, FAPESP Thematic Project 2015/ 18790-3) for funding this research. This work was also supported by the Portuguese government (FCT) through the research unit UIDB/04085/2020 (CENSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The permit for collection, transport and storage of the test animals used in this study was provided by IBAMA/ICMBio (permit number 17559-1) and the tests conducted were approved by the ethical Commission of the Instituto de Biocien̂cias da Universidade de São Paulo (Protocol 039/2007).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daam, M.A., Ilha, P. & Schiesari, L. Acute toxicity of inorganic nitrogen (ammonium, nitrate and nitrite) to tadpoles of five tropical amphibian species. Ecotoxicology 29, 1516–1521 (2020). https://doi.org/10.1007/s10646-020-02247-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02247-8