Abstract
Despite the high amphibian biodiversity and increasing pesticide use in tropical countries, knowledge on the sensitivity of tropical amphibians to pesticides remains limited. The aim of this study was to evaluate the acute toxicity of the active ingredients of four of the main herbicides used in Brazilian sugarcane production to tadpoles of two tropical frog species: Physalaemus cuvieri and Hypsiboas pardalis. The calculated 96 h-LC50 (median lethal concentration; in mg a.s./L) values for P. cuvieri and H. pardalis were 4.4 and 7.8 (acetochlor); 15 and <10 (ametryn); 115 and 106 (glyphosate); and 85 and 68 (metribuzin), respectively. These toxicity values demonstrated little interspecies variation and the toxicity of the herbicides appeared to be at least partly related with the respective octanol-water coefficient. Published acute toxicity data of fish and amphibians for herbicides were also compiled from the US-EPA ECOTOX database. These data indicated little difference in herbicide sensitivity between tropical amphibians and both non-tropical amphibians and fish. These findings indicate that temperate (fish and amphibian) herbicide toxicity data are also protective for tropical amphibians. Constraints in such extrapolations and indications for future research are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphibians are important organisms for aquatic and terrestrial ecosystems, playing a key role maintaining ecosystem structure and functioning (Schiesari et al. 2009). In Neotropical ecosystems, for example, amphibians are essential for energy flow and nutrient cycling, in addition to aiding in controlling pest organisms (Valencia-Aguilar et al. 2013).
Declining amphibian populations have raised major concerns over the past decades. The International Union for Conservation of Nature (IUCN), for example, reported that approximately one-third (32%) of all amphibian species worldwide are threatened and that many others have their population numbers declining, which indicates that this situation is likely to continue or even aggravate in the near future (IUCN 2017). Several causes have been identified for this alarming trend, which include habitat loss and fragmentation, invasive species, overharvesting (for consumption), UV-B radiation, climate change, emerging infectious diseases and pollution (Araújo et al. 2014; Whitfield et al. 2016). Among these, pesticides have been considered important stressors (Alza et al. 2016; Ortiz-Santaliestra et al. 2018) and there are some compelling cases linking pesticide contamination from agricultural activities with amphibian population declines (Bionda et al. 2019; Davidson et al. 2002; Hayes et al. 2010; Pollo et al. 2019; Zhelev et al. 2018). Amphibians have indeed been indicated to possess traits that make them especially prone to pesticide stress, such as a highly permeable skin, shell-less eggs, biphasic life cycle with aquatic and terrestrial life stages, and a relatively rudimentary immune system (Bach et al. 2018; Kerby et al. 2010).
Despite their worldwide decline and apparent high sensitivity to pesticides, relatively few studies have been conducted so far evaluating pesticide toxicity to amphibians as compared to other taxonomic groups (Ortiz-Santaliestra et al. 2018; Vasconcelos et al. 2016). This low research effort has been attributed to (1) animal welfare concerns; (2) the few existing standardized protocols for amphibian testing, but predominantly (3) the fact that amphibian testing is generally not required in ecological risk assessments (EFSA 2013; Ortiz-Santaliestra et al. 2018; Vasconcelos et al. 2016). The absence of mandatory toxicity tests with amphibians in pesticide risk evaluations is due to the assumption that the sensitivity of the standard fish test species also covers that of amphibians (EFSA 2018; Vasconcelos et al. 2016). Pesticide sensitivity comparisons between fish and amphibians have indeed demonstrated that toxicity thresholds for fish are typically also protective for amphibians (Fryday and Thompson 2012; Weltje et al. 2013). A recent review, however, indicated that this assumption is based on a very limited dataset and that this may not hold true for all pesticide mode of action groups (Ortiz-Santaliestra et al. 2018). Even though the number of studies into the sensitivity of tropical amphibians to pesticides has increased in the past decade (e.g., Attademo et al. 2016; Bionda et al. 2019; Lajmanovich et al. 2019), they remain underrepresented in sensitivity comparisons between amphibians and fish conducted so far (Egea-Serrano, Solé 2017; Ghose et al. 2014; Méndez et al. 2016; Sánchez-Domene et al. 2018).
The deficiency in research effort with native tropical amphibian species is unfortunate, given that amphibians have greater biodiversity and species decline in tropical regions (EFSA 2018; Kerby et al. 2015; Sánchez-Domene et al. 2018; Schiesari et al. 2007; Whitfield et al. 2016). In addition, tropical countries are among the largest consumers of pesticides in the world, some of which with poor regulation and enforcement, and using compounds that are banned in most temperate countries (Albuquerque et al. 2016; Ghose et al. 2014; Lewis et al. 2016). Life history and other important ecological traits also vary among temperate and tropical amphibians (Schiesari et al. 2007). Besides potentially altering their response to pesticide stress, this also allows Neotropical amphibians to exploit a wide variety of breeding habitats, including those most prone to pesticide contamination (Egea-Serrano and Solé 2017).
Sugarcane plantations in Southeastern Brazil—the most important sugarcane-producing region in the world—have expanded rapidly over the past decade as a result of increasing biofuel demand (Rudorff et al. 2010; CANASAT 2018). The conversion of Atlantic Forest and cerrado to pastures to sugarcane plantations has previously been demonstrated to result in an impoverished amphibian biodiversity (Schiesari and Corrêa 2016), and the conversion of pasture to sugarcane fields involves a variety of land management practices with potential adverse effects on amphibians, including pesticide application. Brazil is the largest consumer of pesticides in the world and sugarcane is the third largest market, responding to 12% of all pesticide sales in the country (Albuquerque et al. 2016; SINDAG 2012).
The aim of the present study was to evaluate the lethal toxicity of the active ingredients of four herbicides (acetochlor, ametryn, glyphosate and metribuzin) to tadpoles of two tropical frog species. These herbicides are among the most used herbicides in Brazilian sugarcane production (Armas et al. 2005; IBAMA 2014). To this end, acute laboratory bioassays were conducted with tadpoles of the native frog species Physalaemus cuvieri and Hypsiboas pardalis. These species are known to occur in or near sugarcane plantations (Schiesari and Corrêa 2016). The generated toxicity data were subsequently compared with published toxicity data for non-tropical amphibians and fish. In addition, published toxicity data were also compiled for other herbicides. This subsequently allowed a comparison of the acute herbicide sensitivity of tropical amphibians with that of non-tropical amphibians and fish for a wider range of compounds and taxa.
Materials and methods
Test species
Three or more egg masses from different parents of Physalaemus cuvieri and Hypsiboas pardalis were collected from ponds at the Estação Biológica de Boracéia in Salesópolis, South-East Brazil (23°37′59”S, 45°31′59”W), which is located within a non-polluted, protected watershed (Verdade et al. 2011). Egg masses were transported in sealed plastic bags containing water from the collection site to the laboratory of the School of Arts, Sciences and Humanities in the University of São Paulo. Hatched larvae were kept in 50-L plastic tanks filled with tap water filtered through an activated carbon granular filter. Tank water was renewed every other day. The temperature in the laboratory was controlled at 25 ± 2 °C with natural photoperiod. Larvae were fed daily with a 3:1 ground mixture of rabbit chow (Purina Mills, LLC, USA; ~16% protein) and Tetra Min Fish Flakes (Tetra Werke, Melle, Germany; ~45% protein) ad libitum until the beginning of the experiments. These conditions (temperature, light and food regime) were established in previous experiments to be adequate to maintain these organisms in healthy conditions in the laboratory (e.g., Jiquiriçá 2010; Schiesari 2004). The bioassays were conducted with Gosner stage 25 (Gosner 1960) tadpoles, which is the most common life stage used in amphibian ecotoxicological tests (Sparling et al. 2010). Only healthy individuals, as judged by external morphology and behavior (Bantle et al. 1991), were selected for the experiments.
Lethality tests
Acute (96 h) bioassays were conducted to evaluate the sensitivity of P. cuvieri and H. pardalis to the pure active ingredients acetochlor (CAS Number 34256-82-1; Purity 96.8%; Sigma-Aldrich), amethryn (CAS Number 834-12-8; Purity 98.5%; Sigma-Aldrich), glyphosate (CAS Number 1071-83-6; Purity 99.2%; Sigma-Aldrich), and metribuzin (CAS Number 21087-64-9; Purity 99.8%; Sigma-Aldrich). A semi-static design was adopted, in which test solutions were renewed 48 h after the start of the experiment. DT50 (detection time 50%; in days) values available in the Pesticide Properties Database (PPDB 2018) indicate that this renewal assured stable test concentrations over the course of the experiment: acetochlor: 40.5; ametryn (no value available but considered stable to hydrolysis and photolysis); glyphosate: 9.9; and metribuzin: 41. To maximize comparability of test results with those obtained from the literature (c.f. sections “species sensitivity distributions” and “relative tolerance calculations”), internationally-adopted protocols for ecotoxicological tests with amphibians were followed (ASTM 2013; OECD 2015).
The tests were conducted under the same conditions as those described in section “Test species”, except that animals were not fed during the test. Based on the results of range-finding tests, five logarithmically-spaced test concentrations (all in mg a.i./L) were determined:
Acetochlor: 1.0; 1.9; 3.5; 6.5; 12
Amethryn: 10; 13; 16; 20; 25
Glyphosate: 84; 97; 112; 130; 150
Metribuzin: 29; 47; 75; 121; 194
Test concentrations were prepared with stock solutions. Since all active ingredients tested other than glyphosate presented low water solubility, they had to be dissolved in ethanol (ametryn, metribuzin) or acetone (acetochlor). Subsequently, solvent controls were also included at the maximum solvent concentrations used (5 mL acetone/L and 5 mL ethanol/L), besides a culture water control. Each treatment was conducted in quadruplicate, in which each replicate consisted of a glass jar containing 10 tadpoles in 1-L test solution. Every 24 h, water quality parameters (pH, temperature, conductivity, DO) were recorded using a multi-parameter meter (YSI 556), and dead individuals counted and removed.
Species sensitivity distributions
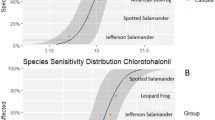
Species sensitivity distributions (SSDs) were constructed to allow a comparison of the 96h-LC50 (median lethal concentration) values derived for the two native tropical frog species with those of fish and other amphibians. To this end, 96h-LC50 values were obtained from the US Environmental Protection Agency (US-EPA) ECOTOX database (https://cfpub.epa.gov/ecotox/). In case that more than one toxicity value was reported for the same compound and species, the geometric mean was calculated and used. SSDs were constructed using the ETX 2.1 software (Van Vlaardingen et al. 2004), by fitting log-normal distribution curves to the toxicity data. Log-normality of the constructed curves was confirmed with the Anderson–Darling test included in the ETX software package, which was evaluated at the 5% significance level. The HC5 and HC50 values (hazard concentration for 5% and 50% of the species assemblage included in the SSD, respectively), and their 95% confidence intervals, were calculated with this software following the method described in Aldenberg and Jaworska (2000).
Relative tolerance calculations
Besides the four herbicides tested, an effort was made to compare the sensitivity of tropical amphibians with that of fish and amphibians from subtropical and temperate regions for a greater range of herbicides. To this end, herbicide toxicity data for fish and amphibians was compiled from the US-EPA database indicated above. Subsequently, amphibians were attributed to a climatic region based on their (native) distribution range as indicated in the IUCN Red List of Threatened Species (IUCN 2017). Only LC50 values with a test duration of 2–21 days were considered and geometric means were taken if more than one toxicity values was available for the same species-compound combination (after Van den Brink et al. 2006; Maltby et al. 2009). To enable grouping toxicity values of different compounds and species, the LC50 values needed to be “normalized”. For example, an LC50 value of a certain herbicide and species can not be directly compared with an LC50 value for a different species exposed to another herbicide. In previous studies, this has been solved by using the relative tolerance (Trel) approach, i.e., by dividing toxicity values of species with the toxicity value of a standard test species. In this way, the Trel approach has successfully been used to compare the sensitivity of aquatic macroinvertebrates and soil invertebrates using respectively Daphnia magna (Wogram and Liess 2001) and Eisenia fetida sensu lato (Daam et al. 2011) as standard test species. In the present study, the relative tolerance of fish and amphibians to herbicides was assessed using the standard fish test species Oncorhynchus mykiss (rainbow trout) as a reference.
Data analysis
The 96h-LC50, LOEC (lowest observed effect concentration) and NOEC (no observed effect concentration) were calculated based on the % mortality rates in the different treatments using the statistical programs PROBIT 1.5 (EPA 2002) and TSK 1.5 (Hamilton et al. 1977). In all cases, the most appropriate statistical test was defined depending on the experimental design and the nature of the available data, following the recommendations of EPA (EPA 2002). To test for interspecies differences in sensitivity, LC50 values for each compound and species were compared with a Z test using the formula proposed by EPA (2002). Analyses of Variance (ANOVA) followed by post hoc tests were employed to test for treatment effects on physical-chemical variables (mean values for each treatment over the experimental period) using the software PAST (Hammer et al. 2001).
Results and discussion
Sensitivity of the two native frog tadpoles to the evaluated herbicides
Survival was 100% in all control treatments except in the ethanol solvent control conducted in the P. cuvieri test, in which one individual died (mortality rate 2.5%). Water quality parameters were comparable in control replicates with a coefficient of variation of less than 4% for all parameters (pH, temperature, conductivity and DO).
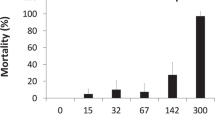
The 96h-LC50 values generated for the four herbicides are presented in Table 1, whereas the mortality levels of the individual treatments for P. cuvieri and H. pardalis are visualized in Figs. 1 and 2, respectively. H. pardalis was significantly more sensitive than P. cuvieri for ametryn, glyphosate and metribuzin and less sensitive to acetochlor (Z test; p < 0.05). However, the absolute difference in toxicity values for these herbicides between the two species was generally small (8–44%), considering that the variability in toxicity values for the same species and compound but generated in different laboratories may reach a factor of three or more (Sprague 1985; Baird et al. 1989). In line with this, the spread (i.e., the ratio of upper and lower limits of the 95% confidence interval; after Brock et al. 2008) in herbicide toxicity values in our dataset (see "Relative tolerance calculations”) for the most tested fish (Oncorhynchus mykiss) and tropical amphibian (Xenopus laevis) were 3.0 ± 0.78 and 3.1 ± 1.9 (mean ± SE), respectively.
Especially for ametryn, H. pardalis appeared to be clearly more sensitive than P. cuvieri (Table 1). For example, whereas the lowest ametryn concentration of 10 mg/L only caused 15% mortality in P. cuvieri (Fig. 1b), the same test concentration led to a 93% mortality of H. pardalis tadpoles (Fig. 2b). Interestingly, ametryn appeared to take a longer time to exert its full toxic action than the other three herbicides. The greatest part of the total tadpole mortality throughout the 96 h tests were elicited within the first 24 h: 95–97% (glyphosate), 79–83% (acetochlor) and 84–80% (metribuzin) for P. cuvieri and H. pardalis, respectively. For ametryn, however, corresponding values were 30–56%. Ametryn, and other triazine herbicides, are known to induce detoxification responses in fish and amphibians (Hostovsky et al. 2014; Moura et al. 2018; Van Der Kraak et al. 2014). Induced detoxification may thus counteract chemical stress until the system is not able anymore to cope with the ametryn-induced stress levels (Moura et al. 2018). In addition, when toxic effects start to emerge on the biochemical level, these do not translate immediately in effects of apical endpoints (Van Der Kraak et al. 2014). This may hence explain the delayed lethal effects observed in the ametryn-exposed tadpoles. In turn, potential differences in defense mechanisms between the two species may also explain the greater vulnerability of H. pardalis. Possibly, P. cuvieri has a better defense mechanism against ametryn toxicity than H. pardalis, which is supported by the discussed lower 24 h toxicity noted for this species (30%) as compared to P. cuvieri (56%).
Both species were more susceptible to acetochlor and ametryn than to metribuzin and glyphosate (Table 1; Figs. 1 and 2). The capacity of organic contaminants to enter the body of aquatic organisms has been demonstrated to increase with increasing hydrophobicity, which can be measured by the octanol–water partition coefficient (Kow; He et al. 2013 and references therein). In line with this, the log(Kow) of acetochlor (4.14) and ametryn (2.63) are considerably higher than those for metribuzin (1.65) and especially glyphosate (−3.2) (PPDB 2018). This may thus at least partly explain the relative sensitivity of the tadpoles to the herbicides. He et al. (2013) noted a significant correlation between Kow and generated LC50 values of the cladoceran Daphnia carinata for three chloroacetanilide herbicides. Although such a correlation also appeared to exist in the present study, this was just not significant (r = 0.71; n = 7; 0.05 < p < 0.1), probably also due to the low number of generated toxicity values.
Relative tolerance of tropical amphibians to herbicides
The SSDs presented in Fig. 3 allow a comparison of the toxicity data of the four tested herbicides generated for the two frog tadpoles with those available for other fish and amphibian taxa. Only for glyphosate, separate SSD curves could be constructed for fish and amphibians. These SSD analyses indicate that both tadpole species were moderately sensitive to the herbicides, especially when compared to the sensitivity of fish. Overall, the standard fish test species O. mykiss (EFSA 2013) was more sensitive than the most sensitive amphibian test species (Fig. 3). Only for metribuzin, H. pardalis (gmLC50 = 68 mg/L) was slightly more sensitive than O. mykiss (gmLC50 = 76 mg/L). The assessment factor of 100 that is commonly applied to the acute LC50 of O. mykiss (e.g., EFSA 2013) would thus be sufficiently protective for all four herbicides tested. As can be deduced from Fig. 4, this also appears to be the case for a larger number of amphibian taxa and herbicide compounds. The range in toxicity data for twelve herbicides evaluating 23 tropical amphibian taxa includes cases where the toxicity of tropical amphibians is greater than O. mykiss and fish in general. However, this difference is generally lower than ten, which confirms the protectiveness of the assessment factor of 100 that is already applied to the acute fish toxicity value for tropical amphibians.
Geometric means of relative tolerance (gmTrel) values for fish and temperate, subtropical and tropical amphibians based on acute toxicity data available in the US-EPA ECOTOX database. Trel values were calculated by dividing the median effect concentration (EC50) of each species with that of the rainbow trout Oncorhynchus mykiss (after Wogram and Liess 2001; For details, please see text)
Implications for risk assessment and future research
Weltje and Wheeler (2015) compared the sensitivity of tadpoles of the tropical frog Agalychnis callidryas with fish for ten pesticides. Based on their findings, they concluded that fish are suitable surrogates for aquatic tropical amphibian stages and that the standard assessment factors cover the extrapolation to potentially more sensitive species and other uncertainties. In line with this, both the bioassays and the literature toxicity data analysis of the present study did not indicate a consistently large difference in sensitivity of tropical amphibian larvae with temperate amphibian larvae or fish.
Several authors, however, have disputed the use of fish toxicity data to cover for the sensitivity of both temperate and tropical amphibians. In a recent review by Ortiz-Santaliestra et al. (2018), for example, the authors concluded that “there is still much information needed to reduce uncertainties and extract relevant conclusions on the overall protection of amphibians and reptiles by surrogate vertebrates”. From a Legislative perspective, concerns have also been raised that the current risk assessment of pesticides may not sufficiently cover the risk to amphibians (e.g., EFSA 2018).
Such concerns may especially be imperative for the use of temperate toxicity data in tropical risk assessments (Schiesari et al. 2007). Ecological and life history traits (e.g., time to metamorphosis and use of waterbodies as habitat in the adult stage; Babini et al. 2018; Zhelev et al. 2018) as well as genetic characteristics (Pollo et al. 2019) are known to vary greatly among amphibians. This may be an additional factor affecting the way temperate and tropical amphibians are exposed and respond to pollutants (Egea-Serrano and Solé 2017; Méndez et al. 2016; Sparling et al. 2010). In addition, only few tropical taxa have been tested so far, although the diversity of amphibians is known to be greatest in tropical areas (Ghose et al. 2014; Lau et al. 2014; Méndez et al. 2016; Schiesari et al. 2007). This increases the chance of the existence of sensitive, yet untested, amphibian species in tropical areas. In addition, relatively few compounds have been evaluated and almost exclusively in acute laboratory toxicity tests. There is thus also an urgent need for chronic (life-cycle) toxicity tests and more environmentally-realistic (field) experiments with tropical amphibians (Schiesari et al. 2007; Vasconcelos et al. 2016). In-situ toxicity, biomarker and field population and community testing would be very useful to attain insights on the actual impact of laboratory-observed effects in real-word conditions (Attademo et al. 2016; Babini et al. 2018; Bionda et al. 2019; Lajmanovich et al. 2019; Pollo et al. 2019; Zhelev et al. 2018). For example, recent studies have indicated that certain amphibians may survive, adapt and breed despite of chemical-induced morphological changes and serious disorders in many physiological functions (Babini et al. 2018; Zhelev et al. 2018).
Like in the present study, most acute toxicity values in our database (on average about 70% for the four herbicides) were derived with the active ingredients. However, it is known that the toxicity of active ingredients may differ to a great extent from their formulated products. Mann and Bidwell (1999), for example, denoted that the 48h-LC50 values for tadpoles of the glyphosate-based herbicide Roundup® were significantly lower than those of its active ingredient. On the other hand, another formulation (Roundup® Biactive) appeared much less toxic than technical glyphosate (Mann and Bidwell 1999). The same conclusions were reached in follow-up studies by Mann et al. (2003) and Lajmanovich et al. (2011). The more since tadpoles in edge-of-field waterbodies are exposed to formulated products that are applied to agricultural crops, rather than solely their active ingredients, future studies evaluating the toxicity of (different) formulated products are also urgently needed.
References
Albuquerque AF, Ribeiro JS, Kummrow F, Nogueira AJA, Montagner CC, Umbuzeiro GA (2016) Pesticides in Brazilian freshwaters: a critical review. Environ Sci Process Impacts 18:779–787
Aldenberg T, Jaworska JS (2000) Uncertainty of hazardous concentrations and fraction affected for normal species sensitivity distributions. Ecotoxicol Environ Safe 46:1–18
Alza CM, Donnelly MA, Whitfield SM (2016) Additive effects of mean temperature, temperature variability, and chlorothalonil to red-eyed treefrog (Agalychnis callidryas) larvae. Environ Toxicol Chem 35:2998–3004
Araújo CVM, Shinn C, Moreira-Santos M, Lopes I, Espíndola ELG, Ribeiro R (2014) Copper-driven avoidance and mortality in temperate and tropical tadpoles. Aquat Toxicol 146:70–75
Armas ED, Monteiro RTR, Amâncio AV, Correia RML, Guercio MA (2005) Uso de agrotóxico em cana-de-açúcar na bacia de Rio Corumbataí e o risco de poluição hídrica. Quím Nova 28:975–982
ASTM (2013) Standard guide for conducting the frog embryo teratogenesis assay—Xenopus (FETAX). ASTM E1439-12, American Society for Testing and Materials
Attademo AM, Lajmanovich RC, Peltzer PM, Junges CM (2016) Acute toxicity of metaldehyde in the invasive rice snail Pomacea canaliculata and sublethal effects on tadpoles of a non-target species (Rhinella arenarum). Water Air Soil Pollut 227:400
Babini MS, Bionda CL, Salinas ZA, Salas NE, Martino AL (2018) Reproductive endpoints of Rhinella arenarum (Anura, Bufonidae): populations that persist in agroecosystems and their use for the environmental health assessment. Ecotoxicol Environ Safe 154:294–301
Bach NC, Marino DJG, Natale GS, Somoza GM (2018) Effects of glyphosate and its commercial formulation, Roundup® Ultramax, on liver histology of tadpoles of the neotropical frog, Leptodactylus latrans (amphibia: Anura). Chemosphere 202:289–297
Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM (1989) The Daphnia bioassay: a critique. Hydrobiologia 188(189):403–406
Bantle JA, Dumont JN, Finch R, Linder G (1991) Atlas of abnormalities: a guide for the performance of FETAX. Oklahoma State University, Stillwater, USA
Bionda CL, Babini S, Martino AL, Salas NE, Lajmanovich RC (2019) Impact assessment of agriculture and livestock over age, longevity and growth of populations of common toad Rhinella arenarum (anura: Bufonidae), central area of Argentina. Glob Ecol Conserv 14:e00398
Brock TCM, Maltby L, Hickey CH, Chapman J, Solomon KR (2008) Spatial extrapolation in ecological effect management of chemicals. In: Solomon KR, Brock TCM, De Zwart D, Dyer SD, Posthuma L, Richards SM, Sanderson H, Sibley PK, Van den Brink PJ (eds) Extrapolation practice for ecotoxicological effect characterization of chemicals. SETAC Europe Press, Brussels, pp 223–256
CANASAT (2018) Monitoramento da cana-de-açúcar via imagens de satélite. http://www.dsr.inpe.br/laf/canasat/. Accessed 10 Nov 2018
Daam MA, Leitão S, Cerejeira MJ, Sousa JP (2011) Comparing the sensitivity of soil invertebrates to pesticides with that of Eisenia fetida. Chemosphere 85:1040–1047
Davidson C, Shaffer HB, Jennings M (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B and climate change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
EFSA (2013) Guidance on tiered risk assessment for plant protection products for aquatic organisms in the edge-of-field surface waters. EFSA J 11:3290
EFSA (2018) Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA J 16:5125
Egea-Serrano A, Solé M (2017) Effects of insecticides on a phytotelmata-breeding amphibian. Environ Toxicol Chem 36:422–428
EPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5th edn. Environmental Protection Agency, Washington, DC
Fryday S, Thompson H (2012) Toxicity of pesticides to aquatic and terrestrial life stages of amphibians and occurrence, habitat use and exposure of amphibian species in agricultural environments. Supporting Publications 2012:EN-343, EFSA (European Food Safety Authority), Parma, Italy
Ghose SL, Donnelly MA, Kerby J, Whitfield SM (2014) Acute toxicity tests and meta-analysis identify gaps in tropical ecotoxicology for amphibians. Environ Toxicol Chem 33:2114–2119
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hamilton MA, Russi RC, Thurston RV (1977) Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hayes TB, Falso P, Gallipeau S, Stice M (2010) The cause of global amphibian declines: a developmental endocrinologist’s perspective. J Exp Biol 213:921–933
He H, Chen G, Yu J, He J, Huang X, Li S, Guo Q, Yu T, Li H (2013) Individual and joint toxicity of three chloroacetanilide herbicides to freshwater cladoceran Daphnia carinata. Bull Environ Contam Toxicol 90:344–350
Hostovsky M, Blahova J, Plhalova L, Kopriva V, Svobodova Z (2014) Effects of the exposure of fish to triazine herbicides. Neuro Endocrinol Lett 35(Suppl 2):3–25
IBAMA—Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (2014) Relatórios de Comercialização de Agrotóxicos—Boletim Anual de Produção, Importação, Exportação e Vendas de Agrotóxicos no Brasil, Boletim 2013. https://www.ibama.gov.br/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos
IUCN (2017) IUCN Red list of threatened species, v 2017. 3. International Union for Conservation of Nature, Gland, Switzerland. http://www.iucnredlist.org. Accessed 8 Nov 2018
Jiquiriçá PRI (2010) Lethal and sublethal effects of nitrogen pollution on anuran larvae. M.Sc. thesis, University of São Paulo, Brazil
Kerby JL, Richards-Hrdlicka KL, Storfer A, Skelly DK (2010) An examination of amphibian sensitivity to environmental contaminants: are amphibians poor canaries? Ecol Lett 13:60–67
Kerby JL, Whitfield SM, Ghose SL, Donnelly MA (2015) Letters to the editor. Environ Toxicol Chem 34:4–5
Lau ETC, Yung MMN, Karraker NE, Leung KMY (2014) Is an assessment factor of 10 appropriate to account for the variation in chemical toxicity to freshwater ectotherms under different thermal conditions? Environ Sci Pollut Res 21:95–104
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabagna MC (2011) Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: B-esterases and Glutathione S-transferase inhibitors. Arch Environ Contam Toxicol 60:681–689
Lajmanovich RC, Peltzer PM, Martinuzzi CS, Attademo AM, Basso A, Colussi CL (2019) Insecticide pyriproxyfen (Dragón®) damage biotransformation, thyroid hormones, heart rate, and swimming performance of Odontophrynus americanus tadpoles. Chemosphere 220:714–722
Lewis SE, Silburn DM, Kookana RS, Shaw M (2016) Pesticide behavior, fate, and effects in the tropics: an overview of the current state of knowledge. J Agric Food Chem 64:3917–3924
Maltby L, Brock TCM, Van den Brink PJ (2009) Fungicide risk assessment for aquatic ecosystems: importance of interspecific variation, toxic mode of action, and exposure regime. Environ Sci Technol 43:7556–7563
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Mann RM, Bidwell JR, Tyler MJ (2003) Toxicity of herbicide formulations to frogs and the implications for product registration: a case study from Western Australia. Appl Herbitol 1:13–22
Méndez M, Obando P, Pinnock-Branford M, Ruepert C, Castillo LE, Mena F, Alvarado G (2016) Acute, chronic and biochemical effects of chlorothalonil on Agalychnis callidryas, Isthmohyla pseudopuma and Smilisca baudinii tadpoles. Environ Sci Pollut Res 23:21238–21248
Moura MAM, Oliveira R, Jonsson CM, Domingues I, AMVM Soares, AJA Nogueira (2018) The sugarcane herbicide ametryn induces oxidative stress and developmental abnormalities in zebrafish embryos. Environ Sci Pollut Res 25:13416–13425
OECD (2015) Test No. 241: the larval amphibian growth and development assay (LAGDA). Organisation for Economic Cooperation and Development, Paris
Ortiz-Santaliestra ME, Maia JP, Egea-Serrano A, Lopes I (2018) Validity of fish, birds and mammals as surrogates for amphibians and reptiles in pesticide toxicity assessment. Ecotoxicology 27:819–833
Pollo FE, Grenat PR, Otero MA, Babini S, Salas NE, Martino AL (2019) Evaluation in situ of genotoxic and cytotoxic response in the diploid/ polyploid complex Odontophrynus (Anura: Odontophrynidae) inhabiting agroecosystems. Chemosphere 216:306–312
PPDB (2018) Pesticide properties database. https://sitem.herts.ac.uk/aeru/ppdb/en/index.htm. Accessed 5 Nov 2018
Rudorff BFT, Aguiar DA, Silva WF, Sugawara LM, Adami M, Moreira MA (2010) Studies on the rapid expansion of sugarcane for ethanol production in São Paulo State (Brazil) using Landsat data. Remote Sens 2:1057–1076
Sánchez-Domene D, Navarro-Lozano A, Acayaba R, Picheli K, Montagner C, Rossa-Feres DC, da Silva FR, de Almeida EA (2018) Eye malformation baseline in Scinax fuscovarius larvae populations that inhabit agroecosystem ponds in southern Brazil. Amphibia-Reptilia 39:325–334
Schiesari L (2004) Performance tradeoffs across resource gradients in anuran larvae. Ph.D. thesis, University of Michigan, USA
Schiesari L, Corrêa DT (2016) Consequences of agroindustrial sugarcane production to freshwater biodiversity. GCB Bioenergy 8:644–657
Schiesari L, Grillitsch B, Grillitsch H (2007) Biogeographic biases in research and their consequences for linking amphibian declines to pollution. Conserv Biol 21:465–471
Schiesari L, Werner EE, Kling GW (2009) Carnivory and resource-based niche differentiation in anuran larvae: implications for food web and experimental ecology. Freshwater Biol 54:572–586
SINDAG–Sindicato Nacional da Indústria de Produtos para Defesa Agrícola (2012) Vendas de defensivos agrícolas são recordes e vão a US$ 8,5 bi em 2011. SINDAG News.
Sparling DW, Linder G, Bishop C, Krest S (2010) Ecotoxicology of amphibians and reptiles. SETAC/Taylor & Francis, Boca Raton, FL
Sprague JB (1985) Factors that modify toxicity. In: Rand GM, Petrocelli SR (eds) Fundamentals of aquatic toxicology. Hemisphere, Washington, DC, pp 124–163
Valencia-Aguilar A, Cortés-Gómez AM, Ruiz-Agudelo CA (2013) Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. Int J Biodivers Sci Ecosyst Serv Manage 9:257–272
Van den Brink PJ, Blake N, Brock TCM, Maltby L (2006) Predictive value of species sensitivity distributions for effects of herbicides in freshwater ecosystems. Hum Ecol Risk Assess 12:645–674
Van Der Kraak GJ, Hosmer AJ, Hanson ML, Kloas W, Solomon KR (2014) Effects of atrazine in fish, amphibians, and reptiles: an analysis based on quantitative weight of evidence. Crit Rev Toxicol 44:1–66
Van Vlaardingen P, Traas TP, Wintersen AM, Aldenberg T (2004) ETX 2.0. A program to calculate hazardous concentrations and fraction affected, based on normally distributed toxicity data. RIVM Report No. 601501028/2004, National Institute of Public Health and the Environment (RIVM), Bilthoven, the Netherlands
Vasconcelos AM, Daam MA, dos Santos LRA, Sanches ALM, Araújo CVM, Espíndola ELG (2016) Acute and chronic sensitivity, avoidance behavior and sensitive life stages of bullfrog tadpoles exposed to the biopesticide abamectin. Ecotoxicology 25:500–509
Verdade VK, Carnaval AC, Rodrigues MT, Sciesari LC, Pavan D, Bertoluci JA (2011) Decline of amphibians in Brazil. In: Heatwole H, Wilkinson JW (eds) Amphibian biology. Status of decline of amphibians: Western Hemisphere. Part 2: Uruguay, Brazil, Ecuador and Colombia. Surrey Beatty and Sons, Chipping Norton, Australia, p 85–127
Weltje L, Simpson P, Gross M, Crane M, Wheeler J (2013) Comparative acute and chronic sensitivity of fish and amphibians: a critical review of data. Environ Toxicol Chem 32:984–994
Weltje L, Wheeler J (2015) Letters to the editor. Environ Toxicol Chem 34:2–3
Whitfield SM, Lips KR, Donnelly MA (2016) Amphibian decline and conservation in central America. Copeia 104:351–379
Wogram J, Liess M (2001) Rank ordering of macroinvertebrate species sensitivity to toxic compounds by comparison with that of Daphnia magna. Bull Environ Contam Toxicol 67:360–367
Zhelev Z, Tsonev S, Georgieva K, Arnaudova D (2018) Health status of Pelophylax ridibundus (Amphibia: Ranidae) in a rice paddy ecosystem in Southern Bulgaria and its importance in assessing environmental state: haematological parameters. Environ Sci Pollut Res 25:7884–7895
Acknowledgements
We thank ICMBio (ICMBio 17559) and the Museu de Zoologia da Universidade de São Paulo for collection permits, São Paulo Research Foundation (FAPESP) for a scholarship to M. Moutinho (2011/05280-6), and FAPESP Bioenergy Research Program (Young Researcher Award 2008/57939-9) and FAPESP Global Climate Change Research Program (Thematic Project 2015/18790-3) for funding this research. This work was also supported by the Portuguese government (FCT) through a postdoc grant for M. Daam (SFRH/BPD/109199/2015) and the research unit UID/AMB/04085/2019 (CENSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The permit for collection, transport and storage of the test animals used in this study was provided by IBAMA/ICMBio (permit number 17559-1) and the tests conducted were approved by the ethical Commission of the Instituto de Biociências da Universidade de São Paulo (Protocol 039/2007).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Daam, M.A., Moutinho, M.F., Espíndola, E.L.G. et al. Lethal toxicity of the herbicides acetochlor, ametryn, glyphosate and metribuzin to tropical frog larvae. Ecotoxicology 28, 707–715 (2019). https://doi.org/10.1007/s10646-019-02067-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02067-5