Abstract
Multiple interactions between different pollutants in the surface waters can cause unpredictable consequences. The aim of the study was to evaluate the combined effect of two widespread xenobiotics, titanium oxide nanoparticles (TiO2) and bisphenol A (BPA), on freshwater bivalve Unio tumidus. The specimens were exposed for 14 days to TiCl4 (Ti, 1.25 µM), TiO2 (1.25 μM), BPA (0.88 nM), or their combination (TiO2 + BPA). Every type of exposure resulted in a particular oxidative stress response: TiO2 had antioxidant effect, decreasing the generation of reactive oxygen species (ROS) and phenoloxidase (PhO) activity, and doubling reduced glutathione (GSH) concentration in the digestive gland; Ti caused oxidative changes by increasing levels of ROS, PhO and superoxide dismutase; BPA decreased the GSH level by a factor of two. In the co-exposure treatment, these indices as well as lysosomal membrane stability were not affected. All Ti-containing exposures caused elevated levels of metalated metallothionein (Zn,Cu-MT), its ratio to total metallothionein protein, and lactate/pyruvate ratio. Both BPA-containing exposures decreased caspase-3 activity. All exposures, and particularly co-exposure, up-regulated CYP450-dependent oxidation, lipid peroxidation and lipofuscin accumulation, lysosomal cathepsin D and its efflux, as well as alkali-labile phosphates in gonads and caused DNA instability (except for TiO2). To summarize, co-exposure to TiO2 + BPA produced an overlap of certain individual responses but strengthened the damage. Development of water purification technologies using TiO2 requires further studies of the biological effects of its mixtures. U. tumidus can serve as a sentinel organism in such studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modelling the environmental impact on aquatic animals needs to take into account multiple interactions between different pollutants both in the water and inside of the organisms in different unpredictable scenarios (Della Torre et al. 2015; Fang et al. 2015). Within the xenobiotics found in surface waters, titanium oxide nanoparticles (n-TiO2) are arguably among the most worrisome, since they can modify the effects of other pollutants (Baun et al. 2008). They are manufactured worldwide in ever increasing quantities for a wide range of applications including industry, cosmetics (e.g., in sunscreens) and medicine (e.g., in biomedical implants and cancer therapy) (Chen and Mao 2007; Giese et al. 2018). A study of manufactured nanomaterial concentrations in the environment revealed that n-TiO2 concentrations are much higher than those of the other four prevalent manufactured nanomaterials, including the nanonized form of Zinc oxide (ZnO) (Giese et al. 2018). The nanonized nature of the initial n-TiO2 can change, particularly in salt water, through agglomeration (Minetto et al. 2014). However, it was shown that in fresh water n-TiO2 particles are suspended in a water column of humic and humus-poor lake waters for a long time without any remarkable changes in the particle size, and only prone to aggregate and quickly settle in the brackish waters with high salinity (Li et al. 2016). The persistence of stable residual n-TiO2 in aquatic environments for extended periods was demonstrated by Zhang et al. (2017). At the same time, in water, the bulk form of TiO2, derived from TiO2 pigments, can produce nanoparticles (Botta et al. 2011). Similarly, when the presence of n-TiO2 in the surface waters is detected, it is a technical problem to distinguish between the manufactured nanoparticles and those naturally occurring in nano-scale minerals (Gondikas et al. 2018). Due to this variability of sources, it is difficult to precisely prove the nature of the TiO2 particles in the surface waters.
One of the major applications n-TiO2 utilizes its unique photocatalytic activity. It is applied to wastewater purification due to relatively low cost and high stability (Khalilova et al. 2018). Reported effects of n-TiO2 on various organisms are related to the generation of reactive oxygen species (ROS), namely hydroxyl radicals, resulting in oxidative stress and oxidative damage, the destabilization of cell lysosome structure (Barmo et al. 2013; Diniz et al. 2013; Federici et al. 2007; Khene et al. 2017; Reeves et al. 2008). However, biological effects of n-TiO2 are frequently studied in acute exposures (Sureda et al. 2018) or at high concentrations of mg L−1 in the water (D’Agata et al. 2014; Doyle et al. 2016; Federici et al. 2007). On the other hand, the recorded concentrations of n-TiO2 in the surface waters are at the magnitudes of ng L−1 (Sun et al. 2017), and exposures are continuous. Nevertheless, even at 20 g L−1, n-TiO2 was not toxic to Vibrio fischeri bacteria (measured by the luminescence inhibition test) and crustaceans Thamnocephalus platyurus (measures as 24 h mortality) (Heinlaan et al. 2008).
In a polluted environment, n-TiO2 is presumed to cause the degradation of phenol compounds, particularly Bisphenol A (BPA) (Banni et al. 2016; Lu et al. 2013). BPA (2,2-bis(4-hydroxyphenyl)propane) is one of the most widely used chemicals, for example in the production of epoxy resins and polycarbonate plastics. Consequently, it is a common environmental pollutant (Crain et al. 2007). Whereas BPA is structurally similar to synthetic female hormone diethylstilbestrol, the most expected and confirmed sign of its toxicity is endocrine disruption (Balbi et al. 2017; Guo et al. 2019; Rubin 2011). In vertebrate animals, it acts as an activator of the estrogen receptors causing alterations in reproduction, development, metabolism, immune response, and neurobehavior (Gassman 2017). In bivalve mollusks, BPA also causes numerous changes in oxidative stress indices and lysosomal stability, similar to the effects of estrogenic compounds (Canesi et al. 2007). BPA affects the mollusks at low concentrations, in the range of ng L−1 to µg L−1, while in fish most effects were detected at much higher concentrations (Aarab et al. 2006; Canesi et al. 2007; Oehlmann et al. 2009). At the same time, BPA concentrations used in experimental models usually exceed environmentally realistic levels (Aarab et al. 2006; Crain et al. 2007).
Bivalve mollusks are on the first line of impact from nanoparticles and soluble aquatic effluents due to their suspension-feeding and sedentary lifestyle (Canesi and Corsi 2016; Doyle et al. 2016). Populations of freshwater bivalves are declining dramatically all over the world (Geist 2011; Lopes-Lima et al. 2017; Lydeard et al. 2004). In particular, this decline is corroborated by the authors’ own research evaluating the impact of environmental toxicity on the bivalves in the basin of Dnister, the second largest river in Ukraine (Falfushynska et al. 2009; Mischuk and Stoliar 2009). While Unio tumidus is a widely distributed and abundant European bivalve and a keystone species in its ecosystems, the populations of this mollusk are in sustained decline (Weber 2005).
The aim of this study was to compare the effects of TiO2 and BPA in individual and combined exposures of freshwater bivalve mollusk Unio tumidus. Based on the expected biochemical effects of these substances, we selected a broad range of markers to study. They included the markers of oxidation/reduction state that are known to be affected by n-TiO2 (Federici et al. 2007; Reeves et al. 2008). We also evaluated metallothionein concentrations as thiols and metal-buffering molecules (Amiard et al. 2006; Krezel and Maret 2007). Metabolic trends were determined based on the lactate/pyruvate ratio; cathepsin D activity served to estimate the extent of autophagy, which can be induced by metabolic disorders (Benes et al. 2008; Man and Kanneganti 2016; Ursini et al. 2016). Lysosomal membrane stability was studied because of its known vulnerability to the manufactured nanoparticles (Canesi and Corsi 2016; Diniz et al. 2013). We measured vitellogenin-like proteins (alkali-labile phosphates (ALP)) involved in gametogenesis (Gagné and André 2011). The extent of cellular lesions was verified by determination of DNA instability and the activity of key apoptotic executive enzyme caspase-3. We aimed to track the responses caused by individual TiO2 and BPA exposures in the combined exposure effects to determine the mode of the possible interaction.
Materials and methods
Chemicals
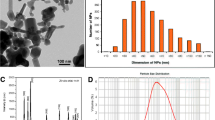
All chemicals were purchased from Sigma Aldrich (St. Louis, USA) and SinbiaS (Ukraina) and were of the Reagent grade or higher. The Titanium (IV) oxide, mixture of rutile and anatase, nanoparticles, <150 nm particle size (volume distribution, DLS), dispersion, 33–35 wt% in H2O (CAS Number: 13463-67-7, EC Number 236-675-5, Sigma Aldrich) was utilized. A stock suspension of 1 g L−1 was prepared by dispersing n-TiO2 powder into deionized water, vortexing of the suspension for 20 s and ultrasonication for about 10 min. The obtained stock suspension was diluted to the final concentration in the experimental medium. The size of nanoparticles in the aquatic medium was confirmed by DLS on DynaPro NanoStar (Wyatt Technology, Santa Barbara, USA) instruments and photon correlation spectra using the non-invasive back scatter (NIBS) technology at 25 °C (S1 Appendix). The samples for DLS measurements were prepared by dissolution of commercial substance in the bi-distilled water, pH 6.5–7.0, samples were 5, 10, 25, 50, 75 and 100 µg L−1. The solutions for DLS study were kept 24 h before the measurement.
Experimental exposures
Bivalve mollusks Unio tumidus (Unionidae) (~6 years old, 8 ± 1 cm length, and 42 ± 5 g weight) were collected in early autumn in the pristine site. This forestry site is located in the upstream portion of river Seret (near the village Ivachiv, 49°49/N, 25°23/E), West Ukraine, where no industrial contamination was expected. Specimens were acclimated to the laboratory conditions for up to seven days in the 80 L aerated tanks. After that they were distributed randomly among five groups (15 specimens each). One group was exposed to the aquarium water only and was considered control (C). Other groups were subjected to 14-day exposure to TiCl4 (Ti, 1.25 µM), TiO2 (1.25 μM that corresponding to 100 µg L−1), BPA (0.88 nM that corresponding to 200 ng L−1), and combined exposure (TiO2 + BPA). These concentrations were approximated to ecologically realistic ones or to those approved in other experiments with bivalves. The concentration for TiO2 was the same that was used by Canesi et al. (2014). Predicted n-TiO2 concentrations in the EU and Switzerland respectively, were 16 and 32 µg L−1 in sewage treatment plant effluents, and 0.53 and 0.67 µg L−1 in surface waters (Sun et al. 2017). Reported concentrations of BPA are less than 21 μg L−1 in stream/river water samples, and less than 17.2 mg L−1 in landfill leachate (Crain et al. 2007).
The utilizing of the exposure to TiCl4 for the comparison of the effect of titanium-contained compounds was motivated by its applying for the chemical treatment of wastewater and surface water with the transformation to TiO2 in aquatic phase (Lee et al. 2009). Low toxicity of TiCl4 in the aquatic environment was indicated with no observed effect concentration to Daphnia magna such high as 100 mg L−1 (Lee et al. 2009).
The exposure time 14 days was chosen as the minimal period for the acclimation in the particular environment. The sufficiency of this period was shown in different studies with aquatic species (Federici et al. 2007; Falfushynska et al. 2015, 2018). No mussel mortality was observed during the experimental exposures. During the trial, water was changed and chemicals were replenished every two days. Mollusks were fed commercial food (“Aquarius”, Ukraine) prior to each water change.
After exposure, mollusks were dissected on ice. The procedure for hemocyte isolation was based on a protocol described in Binelli et al. (2009). For all biochemical traits, except chromatographic analysis, digestive glands, gonads and hemolymph samples were prepared from eight individual mollusks in each experimental group. For the chromatographic analysis, tissue samples of equal size were collected from five individuals in each experimental group, pooled together, and analyzed in triplicate. Each procedure of tissue sampling was carried out at 4 °C. Hemolymph was studied immediately, while all other samples were kept in a freezer (–40 °C) until the time of measurement. Lysosomal membrane stability was determined in hemocytes, levels of ALP—in gonads, and all other characteristics—in the digestive gland. Protein concentration in the samples was measured by the method of Lowry et al. (1951), using bovine serum albumin as the protein standard.
Biomarker assays
Methodology used for each biomarker was described in our previous works (Falfushynska et al. 2015, 2018) and given in detail in S2 Appendix.
Oxidoreductase activities and oxidative lesions assays
Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured according to the method of Beauchamp and Fridovich (1971). The phenoloxidase-like (PhO) activity of tyrosinase (EC 1.14.18.1) was determined by recording the formation of o-quinones (Luna-Acosta et al. 2011). Microsomal CYP450-dependent ethoxyresorufin O-deethylase (EROD) activity was measured in the microsomal pellet obtained by calcium precipitation of the postmitochondrial supernatant (Cinti et al. 1972) by checking the formation of resorufin at 572 nm (Klotz et al. 1984). The rates of oxyradical (ROS) formation in supernatant were determined using a fluorescent dye dihydrorhodamine which is converted by ROS to the fluorescent dye rhodamine-123 (Viarengo et al. 1999). Lipid peroxidation (LPO) was determined by the production of thiobarbituric acid-reactive substances (TBARS) (Ohkawa et al. 1979). Lipofuscin accumulation was determined from the detecting of its fluorescent signal (Manibabu and Patnaik 1997).
Redox balance and metabolic characteristics
Reduced glutathione (GSH) and oxidized glutathione (GSSG) concentrations were quantified by the glutathione reductase recycling assay (Anderson 1985). Redox index of glutathione (RI GSH) was calculated as the ratio of concentrations of GSH/GSSG. Lactate and pyruvate levels were determined spectrophotometrically by the monitoring of changes in NADH concentration in the corresponding incubation mixture (Gawehn 1988). The ratio of the concentrations of Lactate/Pyruvate was calculated. Concentration of the gonad alkali-labile phosphates (ALP) related to the lipophosphoprotein vitellogenin was measured in the gonads according to the protocol of Gagné et al. (2003).
Isolation and quantification of metallothioneins
Metalothioneins (MTs) from the digestive gland were isolated as the thermostable proteins by size-exclusion chromatography on Sephadex G-50 (Roesijadi and Fowler 1991) with necessary adjustments needed to avoid their oxidation. The fractions with high absorbance at 254 nm and high D254/D280 density ratio were identified as putative MTs-containing peaks and pooled (totally 10 mL) for the metal determination. Total concentration of metallothioneins (MT-SH) was assessed in the 1/10 w/v homogenates individually for each specimen by the concentrations of thiols using DTNB reduction method (Viarengo et al. 1997).
Metal determination
The concentrations of zinc and copper (Zn and Cu) were measured in the pooled eluate of metallothionein-containing fractions from the size-exclusion chromatography (10 mL) after the digestion of the samples with HNO3 by the atomic absorption spectrophotometry. Concentration of the metalated metallothioneins (Zn,Cu-MT, μg g−1 FW) was calculated from the concentrations of metals in these samples, considering that one metallothionein molecule with molar weight 7 kg mol−1 binds seven Zn2+ ions or 12 Cu+ ions by its two thiolate domains (Amiard et al. 2006).
Lysosomal markers
Lysosomal membrane stability was determined by the Neutral Red Retention (NRR) assay, according to a procedure developed for isolated mussel hemocytes and adopted to freshwater mussels (Marchi et al. 2004). Cathepsin D (EC 3.4.23.5) activity was determined with 1% hemoglobin as substrate as described by Dingle et al. (1971). Free (extralysosomal) cathepsin D activity was assessed in the digestive gland tissue homogenate without detergent addition, whereas the total cathepsin D activity was measured after the enzyme release by Triton X100 treatment.
Assays of DNA instability and apoptosis
DNA damage was evaluated by the levels of protein-free DNA strand breaks (DNAsb) in the digestive gland by the alkaline DNA precipitation assay (Olive 1988) using Hoescht 33342 dye as described by Bester et al. (1994). Caspase-3 activity was assayed colorimetrically based on the detection of the colored product of hydrolysis p-nitroaniline (pNA) (Bonomini et al. 2004).
Statistical analysis
The data are presented as means ± standard deviation (SD) unless indicated otherwise. Data were tested for normality and homogeneity of variance by using Kolmogorov–Smirnoff and Levene’s tests, respectively. Whenever possible, data were normalized by Box–Cox common transforming method. For the data that were not normally distributed even after the transformation, non-parametric tests (Kruskall–Wallis ANOVA and Mann–Whitney U-test) were performed. Principal component analysis (PCA) was used to differentiate the individual specimens by the set of their indices. Pearson’s correlation test for the pairs of variables was performed at a 0.05 level of significance. All statistical calculations were performed with Statistica v. 10.0 and Excel for Windows-2010. Differences were considered significant if the probability of Type I error was less than 0.05. All graphics were performed using GraphPad Prism 6.
Results
Oxidoreductase activity and oxidative damage
The exposures affected the oxidative stress indices (Fig. 1). Ti elevated SOD and PhO activities and increased the level of ROS production. Conversely, TiO2 decreased ROS production and PhO activity. EROD activity was substantially elevated in all exposed groups, particularly as a result of TiO2 + BPA co-exposure (by 2.9–7.0 times). The levels of lipofuscin and TBARS formation were also elevated in all exposed groups, up to 1.54 times by nTiO2 + BPA and 2.47 times by Ti correspondingly.
The oxygen-related enzyme activities and indices of oxidative stress in the digestive gland of Unio tumidus exposed to TiCl4 (Ti), n-TiO2, BPA and n-TiO2 + BPA in comparison with the control (C). Data for a superoxide dismutase (SOD) activity; b ROS; c ethoxyresorufin O-deethylase (EROD) activity; d phenoloxidase-like (PhO) activity; e lipofuscin accumulation; f thiobarbituric acid-reactive substances (TBARS) production, presented as mean ± SD (N = 8). If the letters above the bars are the same, this indicates that the values do not differ significantly (P > 0.05)
Thiols and redox state characteristics
The level of GSH in the digestive gland increased twice after the exposure to TiO2 and halved after exposure to BPA (Fig. 2a), while the level of GSSG increased after exposures to Ti, TiO2, and BPA (Fig. 2c). In the group exposed to BPA, these changes resulted in a dramatic decrease of GSH/GSSG ratio (by 3.4 times), however in other exposed groups redox state of GSH was comparable to the control value (Fig. 2e).
Glutathione and metallothionein concentrations in the digestive gland of Unio tumidus exposed to TiCl4 (Ti), n-TiO2, BPA and n-TiO2 + BPA for 14 days, in comparison with the control (C). Data for a GSH; b metallothionein protein (MT-SH); c GSSG; d metalated metallothionein (Zn,Cu-MT); e GSH/GSSG ratio, f MT-SH/Zn,Cu-MT ratio of means, presented as mean ± SD (N = 8 for all indices except Zn,Cu-MT, N = 3 for Zn,Cu-MT sampled from 5 specimens in the group). If the letters above the bars are the same, this indicates that the values do not differ significantly (P > 0.05)
The total level of metallothionein (MT-SH) decreased in all titanium-containing exposures and did not change after exposure to BPA (Fig. 2b). On the other hand, the level of metalated metallothionein (Zn,Cu-MT) increased after the exposures to Ti, TiO2, and TiO2 + BPA, and was similar to control in the BPA-exposed group (Fig. 2d). Consequently, the ratio of MT-SH/Zn,Cu-MT decreased in the mussels exposed to Ti, TiO2 and TiO2 + BPA (Fig. 2f).
All titanium-containing exposures resulted in increased level of lactate and decreased level of pyruvate (Fig. 3a), while the exposure to BPA resulted in substantial increase of pyruvate level (~by twice) (Fig. 3b). As a result, the lactate/pyruvate ratio increased in all groups exposed to titanium compounds, and particularly so after the exposure to TiO2 (by ~three times) and decreased after exposure to BPA (~ by twice) (Fig. 3c).
Lactate a and pyruvate b concentrations and their ratio c in the digestive gland and ALP concentration in gonads d of Unio tumidus, exposed to TiCl4 (Ti), n-TiO2, BPA and n-TiO2 + BPA for 14 days in comparison with the control c. Data presented as mean ± SD (N = 8). If the letters above the bars are the same, this indicates that the values do not differ significantly (P > 0.05)
All exposures caused an increase in ALP level in the mussel gonad, with BPA and TiO2 + BPA co-exposure resulting in the largest increase (in more than three times) (Fig. 3d).
Indices of toxicity
DNA instability increased after all exposures except in the case of TiO2 (Fig. 4a). Caspase-3 activity decreased after exposures to BPA and TiO2 + BPA and was similar to control after other exposures (Fig. 4b).
Characteristics of toxicity in digestive gland a–d and hemocytes e of Unio tumidus, exposed to TiCl4 (Ti), n-TiO2, BPA and n-TiO2 + BPA for 14 days in comparison with the control c. Data for a DNA-strand breaks; b caspase-3 activity; c cathepsin D total activity; d cathepsin D free (outside lysosome) activity; e lysosomal membrane stability, presented as mean ± SD (N = 8). If the letters above the bars are the same, this indicates that the values do not differ significantly (P > 0.05)
Total Cathepsin D activity and its efflux from lysosomes increased compared to the control in all exposures (Fig. 4c, d). A substantial decline in lysosomal membrane stability (by 43%) was detected after exposures to TiO2 and BPA, while the TiO2 + BPA co-exposure did not produce this result (Fig. 4e).
Data integration
Pearson correlation analysis revealed multiple associations among the studied indices (Table 1). All oxidoreductase activities (SOD, PhO, EROD) were interrelated (except for the EROD–PhO pair) and positively correlated with the indices of oxidative damage (ROS, TBARS, Lipofuscin, except ROS–Lipofuscin pair). All abovementioned characteristics of oxidative stress (SOD, EROD, PhO, ROS, TBARS and Lipofuscin) were highly associated with cathepsin D activity (both total and extra lysosomal (free)). The indices of oxidative stress and toxicity (TBARS, Lipofuscin, DNAsb, Cathepsin D total and free, EROD) positively correlated with ALP level in gonads, and some of them (ALP, EROD, Lipofuscin, DNAsb, Cathepsin D free) negatively correlated with the caspase-3 activity.
The indices of redox balance (lactate, lactate/pyruvate ratio, GSH, GSH/GSSG ratio) had positive inter-correlations and positive correlations with Zn,Cu-MT levels, while all of them negatively correlated with pyruvate levels and MT-SH/Zn,Cu-MT ratio. Levels of oxidoreductases correlated oxidative damage on the one hand, and redox-related parameters on the other. Thus, lactate positively correlated with EROD, PhO, and TBARS; Zn,Cu-MT: positively correlated with TBARS; MT-SH negatively correlated with TBARS and lipofuscin levels; GSH negatively correlated with SOD, ROS and DNA instability. Lysosome membrane stability (NRR test) correlated negatively with lipofuscin accumulation and positively with PhO, MT-SH and the MT-SH/ Zn,Cu-MT ratio. The largest number of correlations was found for TBARS (13), lactate (11), cathepsin D total (11), and GSH (10). Fewer correlations were uncovered for caspase-3 (4) and GSSG (5).
According to the results of Principal component analysis (Fig. 5), 54.3% of variation of indices was attributed to Factors 1 and 2. All treatment groups were separated from the control along the Factor 1. The locations of Ti and TiO2 + BPA groups overlapped, forming a tight cloud opposite control group along Factor 1. The position of TiO2 and BPA groups was opposite relative to the Factor 2 and most distant from the position of control group.
Discussion
Comparison of the responses to TiO2 and TiCl4 exposures
Titanium is the ninth most abundant element in the Earth’s crust, however there is no known essential role for it in the biology of any organism (Zierden and Valentine 2016). Bioavailability of this metal likely depends on its chemical forms found in the environment. Furthermore, for nanoparticles the bioavailability expected to have common regularities (Christian et al. 2008). However, a study of TiO2 suspensions in various humic acids and NaCl concentrations in sublethal doses on zebrafish Danio rerio did not demonstrate a correlation between aggregation size, hydrodynamic diameter of particles and oxidative stress indices (Fang et al. 2015).
In this study, we found certain common features in the responses of the mussels independent of titanium form in the medium. First, the analysis of metallothionein levels have shown similar responses for all titanium containing exposures, namely the increase in metallothionein metalation (Zn,Cu-MT) combined with the decrease of protein metallothionein (MT-SH) concentration. The increase of metallothionein concentration is a typical sign of metal toxicity (Amiard et al. 2006). However, the data on the effect of titanium compounds on metallothionein concentration is limited and contradictory. In a study of Mutilus galloprovincialis, acute exposure to TiO2-containing sunscreen led to a progressive, dose-dependent increase in metallothionein concentration (MT-SH) in the gills (Sureda et al. 2018). On the other hand, TiO2 brought down MT-SH level in the gills of M. galloprovinciales, previously elevated as a result of cadmium exposure (Della Torre et al. 2015). In the study of D’Agata et al. (2014), significant overexpression of the inducible mt20 gene was detected in the digestive gland of M. galloprovincialis exposed to bulk TiO2, while TiO2 nanoparticles (fresh and aged) did not change the expression of mt genes in this tissue. Moreover, the data on the expression of mt genes in the gills and results of histochemical analysis were inconclusive.
Total and metalated metallothionein concentration assays are rarely combined in the same study (for analysis see Falfushynska et al. 2015). The discrepancies between MT-SH and Zn,Cu-MT levels are usually related to the increase in apo-form (Duncan et al. 2006; Krezel and Maret 2007; Ruttkay-Nedecky et al. 2013; Falfushynska et al. 2015, 2018). The opposite trend, i.e. the hypermetalation of metallothioneins, observed in this study for all titanium-containing treatments, can be explained by a well-known unique protein-binding behavior of titanium (IV): its ability to polymerize through oxo bridges (Rozes et al. 2006), providing the additional metal binding by metallothioneins (Sutherland et al. 2012). This attribute of titanium (IV) is, for instance, utilized in anticancer therapy (Wang et al. 2013). In a recent study, TiO2 was demonstrated to strongly interact with different cellular proteins, selective to specific amino acid side chains (Ranjan et al. 2018).
The elevated lactate levels and lactate/pyruvate ratio were also detected in all groups exposed to titanium containing substances, in contrast to the BPA-exposed group. This suggests the presence of a reduced state within the cells as a result of NADH accumulation (Sies 2015; Ursini et al. 2016) and, consequently, a high redox state of the thiol groups. Importantly, all redox balance indices (the lactate and GSH levels along with lactate/pyruvate and GSH/GSSG ratios) were positively correlated with Zn,Cu-MT and negatively correlated with pyruvate and GSSG levels. This demonstrated that the cells generally sustained the reduced state in all groups exposed to titanium-containing substances independently of the extent of oxidative stress.
The main differences in the effects between the TiO2 and Ti exposures are underscored by the analysis of oxidative stress responses. Induced oxidative injury is the most recognized manifestation of nanoparticle toxicity, including TiO2 nanoparticles (Fu et al. 2014; Kim et al. 2019). The ability of TiO2 to directly induce the production of ROS can be explained by their photocatalytic activity (Zoltan et al. 2016). It was demonstrated in the experimental exposures to UV radiation and several in vitro studies (Geiseler et al. 2012; Moriyama et al. 2018). TiO2-mediated generation of ROS, induction of oxidative stress and oxidative damage were also confirmed in several animal models (Barmo et al. 2013; Della Torre et al. 2015; Diniz et al. 2013; Federici et al. 2007; Khene et al. 2017; Reeves et al. 2008). In particular, TiO2, found in various concentrations in the soil, caused an increase in ROS generation, corresponding changes in antioxidant levels and oxidative damage in the snail Helix aspersa (Khene et al. 2017). In the mussels M. galloprovincialis, lipid peroxidation was a result of acute exposure to sunscreen containing n-TiO2 (Sureda et al. 2018). However, these studies utilized high concentrations of nanoparticles and/or acute exposures.
In contrast, in this study, TiO2 was the sole agent that produced a distinct antioxidant effect, decreasing ROS generation and PhO activity. It was the only treatment that resulted in increased (by the factor of two) GSH concentration. Studies indicate that the genotoxic effect of TiO2 is triggered by ROS production (Trouiller et al. 2009; Petković et al. 2011; El-Said et al. 2014). Therefore, preserved DNA integrity observed in this study only in the TiO2 treatment group confirms the antioxidant effect of TiO2. These results are corroborated by a study of zebrafish Danio rerio larvae, exposed to 100 µg L−1 of TiO2, which also did not induce either ROS generation or DNA damage (Fang et al. 2015).
In mussels, PhO activity is functionally associated with phagocytosis, self-nonself discrimination and cytotoxicity (Luna-Acosta et al. 2011). Similar to the finding of our study, decreased PhO activity and suppressed immune response (reduced transcription of immune-related genes in the digestive gland and decreased phagocytosis in the hemocytes) were demonstrated in response of M. galloprovincialis to a 4-day exposure of low (1–100 µg L−1) TiO2 concentrations (Barmo et al. 2013).
The increase in GSH concentration is likely the factor defining TiO2 antioxidant activity. Supporting this finding, elevated intracellular GSH levels are found to play a critical role in the defense against TiO2 induced DNA damage in the HepG2 human hepatoma cells (Petković et al. 2011). We suggest that antioxidant effect of TiO2 in this study was a result of prolonged exposure allowing the organism to acclimate.
Among the structures most sensitive to the impact of manufactured nanoparticles are lysosome membranes (Barmo et al. 2013; Canesi and Corsi 2016). Indeed, we detected the decrease of lysosomal membrane stability in hemocytes under the exposure to TiO2. This was the main sign of TiO2 toxicity. However, the same effect was observed for the exposure to BPA, and therefore it cannot serve as a distinctive feature of nanoparticle effect. The overall response of the mussels to TiO2 exposure was confirmed by the PCA (Fig. 5).
Of titanium compounds, its nanoform received the most attention as a potential source of toxicity (Kim et al. 2019). However, some studies point to a difference in effects depending on the form. For example, histochemical analysis of M galloprovincialis exposed to ‘bulk’ titanium dioxide showed that it induced enhanced toxicity in comparison with ‘fresh’ or ‘aged’ TiO2 nanoparticles in the concentrations of 10 mg L−1 of each substance (D’Agata et al. 2014). In our study, in the contrast to TiO2, exposure to Ti induced the most severe oxidative stress response, such as up-regulation of ROS, PhO and SOD. It also triggered the highest level of TBARS production. Increased oxidative damage of proteins and lipids was detected in patients with implanted titanium alloy miniplates (Borys et al. 2018; Kim et al. 2019). This noticeable difference in the outcomes of two exposures to titanium compounds confirms that different mechanisms of their bioavailability are in play.
The effect of BPA on mollusks
In this study, we did not detect BPA-induced changes in the oxygen-dependent enzymes and ROS generation, in contrast to what was reported for high micromolar concentrations in the human cells (Gassman 2017). However, BPA caused the oxidative effect through the depletion of GSH and its increased oxidation (Fig. 2). In the BPA only treatment, it also produced the distinct changes of the metabolic activity, such as elevated pyruvate level, which can be a sign of mitochondrial dysfunction described in previous reports (Gassman 2017). The lysosomal disintegration was also indicated as a sign of BPA toxicity, which is the same as for low BPA concentrations in the acute exposure of M. galloprovincialis mussels (Canesi et al. 2007). The main consequence of BPA toxicity in both single and combined exposures was a decrease in caspase-3 level. These findings confirm mollusk sensitivity to BPA even at low nanomolar concentrations (Oehlmann et al. 2009). The anti-apoptotic response of caspase-3 was similar with the results reported in a study of ovarian cancer cells (Ptak et al. 2013). Thus, the decrease in caspase-3 level is of substantial ecotoxicological concern given the wide BPA distribution (Cavalieri and Rogan 2010). The particular effect after individual BPA exposure was confirmed by the PCA (Fig. 5).
Modulations of some particular responses to TiO2 and BPA in the co-exposure
The main difference in the individual responses to TiO2 and BPA was the opposite trend in the changes of GSH and GSSG concentrations. Co-exposure caused mutual cancelation of these responses suggesting the antagonistic relationship between the two substances, TiO2 and BPA, in their biological effects. The validity of this abolishing is confirmed by PCA (Fig. 5). Co-exposure also resulted in normalized lysosomal membrane stability. At the same time, we observed the highest level of DNA strand breaks, cathepsin D efflux from the lysosomes, and ALP level in gonads, as well as a remarkable increase (by seven times) in EROD activity. N-TiO2 can substantially change the behavior and bioavailability of other xenobiotics (Banni et al. 2016; Canesi et al. 2014; Fang et al. 2015). For example, in the larvae of zebrafish Danio rerio, n-TiO2, in a co-exposure, increased pentachlorphenol metabolism and caused oxidative damage and developmental toxicity, in contrast to individual exposure (Fang et al. 2015). N-TiO2 plays a complex role in As (V) toxicity to saltwater zooplankton (Yang et al. 2018). The co-exposure of a marine bivalve M. galloprovincialis to n-TiO2 and dioxin (2,3,7,8-TCDD) produced both synergistic and antagonistic effects in the co-exposure of the marine bivalve (Banni et al. 2016; Canesi et al. 2014). In our study, the synergistic effect of individual substances in the co-exposure was best reflected by the increase in EROD activity (Fig. 1c). It can activate the metabolic transformation of BPA to more reactive substances (Ike et al. 2000; Canesi et al. 2007). Although EROD activity in bivalve mollusks is low in general, its activation by aromatic substances and in the polluted environment was observed in different studies (Siebert et al. 2017).
Nevertheless, the typical features of exposures to both of the titanium containing compounds and BPA were also evident as a result of co-exposure. High Zn,Cu-MT and lactate/pyruvate levels were the most consistent manifestations of all titanium containing exposures both the individual treatments and co-exposure. This finding underscores the importance of detailed analysis of the redox balance (Sies 2015), more precisely of the ‘nucleophilic tone’ (Ursini et al. 2016) caused by Ti-contained exposures. On the other hand, the decrease in caspase-3 activity was a salient sign of the presence of BPA in the medium, both in the individual and combined exposures.
Shared responses to the exposures
While we included ALP in the set of markers to be analyzed, we did not expect to detect an endocrine disrupting effect, well-known for vertebrate (Scott 2013). However, a ALP level increase was previously reported after exposures of mussels to BPA (Rubin 2011). This effect was observed after a three-week exposure of Mytilus edilus females to a concentration of BPA 250 times higher than in our study (50 µg L−1) (Aarab et al. 2006). However, in this study, the elevation of ALP levels in the gonads of U. tumidus cannot be solely attributed to the effect of BPA. It seems to reflect the increased supply of the gonad activity with phospholipoproteins and Zn (Gagné and André 2011) and reflect the common biochemical response strategy to xenobiotics in U. tumidus. This response was also observed after exposures of this species to nano-ZnO and heat (Falfushynska et al. 2018). Additionally, the increase in ALP levels was detected in both female and male sea urchin gonads during the nonreproductive season (Unuma et al. 2011). Low specificity of this response confirms that increase in ALP levels is not valuable biomarker of xenoestrogen effects in the mollusks (Scott 2013; Saìnchez-Marín et al. 2017).
The activation of the oxidative injury (TBARS and lipofuscin accumulation), CYP450-dependent oxidation and cathepsin D in response to all exposures indicates that freshwater bivalves possess high vulnerability to xenobiotic impact. In our study, the numerous correlations between the markers of oxidative damage, ALP and cathepsin D levels conform to the general mode of response to these exposures. Indeed, the substantial losses of lipids and proteins within the cells can explain the autophagy system activation with the involvement of cathepsin D (Turk and Stoka 2007), either as a survival mechanism or an alternative form of programmed cell death (Benes et al. 2008; Man and Kanneganti 2016). Notably, the cathepsin D was among the four proteins highly expressed in the digestive gland of Mytilus edulis exposed to the toxic dinoflagellates (Manfrin et al. 2012).
Conclusion
Our results demonstrate that certain biochemical responses observed after exposures to TiO2 and BPA as single-agent pollutants attenuate in the co-exposure treatment combining these two compounds. However, the indications of cellular injury were elevated in the co-exposure treatment, raising concern about the interaction of TiO2 and BPA in the environment. Consequently, the development of water purification technologies employing n-TiO2 calls for further studies of the effects that n-TiO2 and its mixtures might have on the biological systems. The mollusk U. tumidus could be used as a sentinel organism for this purpose due to its high sensitivity and response to TiO2 and BPA in environmentally realistic exposure.
Data availability
All data analyzed during this study are available via the Mendeley Data (https://doi.org/10.17632/cnhjzb2x49.1).
References
Aarab N, Lemaire-Gony S, Unruh E, Hansen PD, Larsen BK, Andersen OK, Narbonne JF (2006) Preliminary study of responses in mussel (Mytilus edilus) exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether. Aquat Toxicol 78:86–92. https://doi.org/10.1016/j.aquatox.2006.02.021
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their use as biomarkers. Aquat Toxicol 76:160–202. https://doi.org/10.1016/j.aquatox.2005.08.015
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Balbi T, Ciacci C, Grasselli E, Smerilli A, Voci A, Canesi L (2017) Utilization of Mytilus digestive gland cells for the in vitro screening of potential metabolic disruptors in aquatic invertebrates. Comp Biochem Physiol C 191:26–35. https://doi.org/10.1016/j.cbpc.2016.08.009
Banni M, Sforzini S, Balbi T, Corsi I, Viarengo A, Canesi L (2016) Combined effects of n-TiO2 and 2,3,7,8-TCDD in Mytilus galloprovincialis digestive gland: a transcriptomic and immunohistochemical study. Environ Res 145:135–144. https://doi.org/10.1016/j.envres.2015.12.003
Barmo C, Ciacci C, Canonico B, Fabbri R, Cortese K, Balbi T, Marcomini A, Pojana G, Gallo G, Canesi L (2013) In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquat Toxicol 132–133:9–18. https://doi.org/10.1016/j.aquatox.2013.01.014
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17(5):387–395. https://doi.org/10.1007/s10646-008-0208-y
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Benes P, Vetvicka V, Fusek M (2008) Cathepsin D—many functions of one aspartic protease. Crit Rev Oncol Hematol 68:12–28. https://doi.org/10.1016/j.critrevonc.2008.02.008
Bester MJ, Potgieter HC, Vermaak WJ (1994) Cholate and pH reduce interference by sodium dodecyl sulfate in the determination of DNA with Hoechst. Anal Biochem 223:299–305
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009) In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat Toxicol 91:238–244. https://doi.org/10.1016/j.aquatox.2008.11.008
Bonomini M, Dottori S, Amoroso A, Arduini A, Sirolli V (2004) Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Haemost 2:1275–1281. https://doi.org/10.1111/j.1538-7836.2004.00837.x
Borys J, Maciejczyk M, Antonowicz B, Krętowski A, Waszkiel D, Bortnik P, Czarniecka-Bargłowska K, Kocisz M, Szulimowska J, Czajkowski M, Waszkiewicz N, Zalewska A (2018) Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxid Med Cell Longev 2018:3714725. https://doi.org/10.1155/2018/3714725
Botta C, Labille J, Auffan M, Borschneck D, Miche H, Cabie M, Masion A, Rose J, Bottero JY (2011) TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: structures and quantities. Environ Pollut 159:1543–1550. https://doi.org/10.1016/j.envpol.2011.03.003
Canesi L, Borghi C, Ciacci C, Fabbri R, Vergani L, Gallo G (2007) Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol Cell Endocrinol 276(1–2):36–44. https://doi.org/10.1016/j.mce.2007.06.002
Canesi L, Corsi I (2016) Effects of nanomaterials on marine invertebrates. Sci Total Environ 565:933–940. https://doi.org/10.1016/j.scitotenv.2016.01.085
Canesi L, Frenzilli G, Balbi T, Bernardeschi M, Ciacci C, Corsolini S, DellaTorre C, Fabbri R, Faleri C, Focardi S, Guidi P, Kočan A, Marcomini A, Mariottini M, Nigro M, Pozo-Gallardo K, Rocco L, Scarcelli V, Smerilli A, Corsi I (2014) Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat Toxicol 153:53–65. https://doi.org/10.1016/j.aquatox.2013.11.002
Cavalieri EL, Rogan EG (2010) Is bisphenol A a weak carcinogen like the natural estrogens and diethylstilbestrol? IUBMB Life 62:746–751. https://doi.org/10.1002/iub.376
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959. https://doi.org/10.1021/cr0500535
Christian P, Von der Kammer F, Baalousha M, Hofmann T (2008) Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17(5):326–343. https://doi.org/10.1007/s10646-008-0213-1
Cinti DL, Moldeus P, Schenkman JB (1972) Kinetic parameters of drug metabolizing enzymes in Ca2+-sedimented microsomes from rat liver. Biochem Pharm 21:3249–3256. https://doi.org/10.1016/0006-2952(72)90089-5
Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, Le Blanc GA, Guillette LJJ (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24:225–239. https://doi.org/10.1016/j.reprotox.2007.05.008
D’Agata A, Fasulo S, Dallas LJ, Fisher AS, Maisano M, Readman JW, Jha AN (2014) Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels. Nanotoxicology 8:549–558. https://doi.org/10.3109/17435390.2013.807446
Della Torre C, Balbi T, Grassi G, Frenzilli G, Bernardeschi M, Smerilli A, Guidi P, Canesi L, Nigro M, Monaci F, Scarcelli V (2015) Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J Hazard Mater 297:92–100. https://doi.org/10.1016/j.jhazmat.2015.04.072
Dingle JT, Barrett AJ, Weston PD (1971) Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J 123:1–13. https://doi.org/10.1042/bj1230001
Diniz MS, de Matos AP, Lourenço J, Castro L, Peres I, Mendonça E, Picado A (2013) Liver alterations in two freshwater fish species (Carassius auratus and Danio rerio) following exposure to different TiO2 nanoparticle concentrations. Microsc Micro 19:1131–1140. https://doi.org/10.1017/S1431927613013238
Doyle JJ, Ward JE, Mason R (2016) Exposure of bivalve shellfish to titania nanoparticles under an environmental-spill scenario: encounter, ingestion and egestion. J Mar Biol Assoc UK 96:137–149. https://doi.org/10.1017/S0025315415001174.
Duncan KE, Ngu TT, Chan J, Salgado MT, Merrifield ME, Stillman MJ (2006) Peptide folding, metal-binding mechanisms, and binding site structures in metallothioneins. Exp Biol Med 231:1488–1499. https://doi.org/10.1177/153537020623100907
El-Said KS, Ali EM, Kanehira K, Taniguchi A (2014) Molecular mechanism of DNA damage induced by titanium dioxide nanoparticles in toll-like receptor 3 or 4 expressing human hepatocarcinoma cell lines. J Nanobiotechnol 12:48. https://doi.org/10.1186/s12951-014-0048-2
Falfushynska H, Gnatyshyna L, Ivanina A, Sokolova I, Stoliar O (2018) Detoxification and cellular stress responses of unionid mussels Unio tumidus from two cooling ponds to combined nano-ZnO and temperature stress. Chemosphere 193:1127–1142. https://doi.org/10.1016/j.chemosphere.2017.11.079
Falfushynska H, Gnatyshyna L, Yurchak I, Sokolova I, Stoliar O (2015) The effects of zinc nanooxide on cellular stress responses of the freshwater mussels Unio tumidus are modulated by elevated temperature and organic pollutants. Aquat Toxicol 162:82–93. https://doi.org/10.1016/j.aquatox.2015.03.006
Falfushynska HI, Delahaut L, Stolyar OB, Geffard A, Biagianti-Risbourg S (2009) Multi-biomarkers approach in different organs of Anodonta cygnea from the Dnister Basin (Ukraine). Arch Environ Contam Toxicol 57:86–95. https://doi.org/10.1007/s00244-008-9234-2
Fang T, Yu LP, Zhang WC, Bao SP (2015) Effects of humic acid and ionic strength on TiO2 nanoparticles sublethal toxicity to zebrafish. Ecotoxicology 24(10):2054–2066. https://doi.org/10.1007/s10646-015-1541-6
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22:64–75. https://doi.org/10.1016/j.jfda.2014.01.005
Gagné F, André C (2011) New approaches to indirect vitellogenin-like protein evaluations in aquatic oviparous and ovoviviparous organisms. Fresen Environ Bull 20:12–17
Gagné F, Blaise C, Pellerin J, Pelletier E, Douville M, Gauthier-Clerc S, Viglino L (2003) Sex alteration in soft-shell clams (Mya arenaria) in an intertidal zone of the Saint Lawrence River (Quebec, Canada). Comp Biochem Physiol C 134:189–198. https://doi.org/10.1016/S1532-0456(02)00248-X
Gassman NR (2017) Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen 58:60–71. https://doi.org/10.1002/em.22072
Gawehn K (1988) D-(-)-Lactate. Іn: Bergmeyer HU (еd.) Methods of enzymatic analysis, vol. VI, 3rd edn. VCH Publishers (UK) Ltd., Cambridge, UK, pp. 588–592
Geiseler B, Miljevic M, Müller P, Fruk L (2012) Phototriggered production of reactive oxygen species by TiO2 nanospheres and rods. J Nanomater 2012:1–9. https://doi.org/10.1155/2012/708519
Geist J (2011) Integrative freshwater ecology and biodiversity conservation. Ecol Indic 11:1507–1516. https://doi.org/10.1016/j.ecolind.2011.04.002
Giese B, Klaessig F, Park B, Kaegi R, Wigger MH, von Gleich A, Gottschalk F (2018) Risks, release and concentrations of engineered nanomaterial in the environment. Sci Rep 8:1565. https://doi.org/10.1038/s41598-018-19275-4
Gondikas A, von der Kammer F, Kaegi R, Borovinskaya O, Neubauer E, Navratilova J, Praetorius A, Cornelis G, Hofmann T (2018) Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ Sci: Nano 5:313–326. https://doi.org/10.1039/C7EN00952F
Guo Y, Chen L, Wu J, Hua J, Yang L, Wang Q, Zhang W, Lee JS, Zhou B (2019) Parental co-exposure to bisphenol A and nano-TiO2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci Total Environ 650(1):557–565. https://doi.org/10.1016/j.scitotenv.2018.09.007
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316. https://doi.org/10.1016/j.chemosphere.2007.11.047
Ike M, Jin CS, Fujita M (2000) Biodegradation of bisphenol A in the aquatic environment. Water Sci Technol 42(7–8):31–38. https://doi.org/10.2166/wst.2000.0549
Khalilova HK, Hasanova SA, Aliyev FG (2018) Photocatalytic removal of organic pollutants from industrial wastewater using TiO2 catalyst. J Environ Prot 9:691–698. https://doi.org/10.4236/jep.2018.96043
Khene L, Berrebbah H, Yahyaoui A, Bouarroudj T, Zouainia S, Kahli H, Bourayou C (2017) Biomarkers of oxidative stress, lipid peroxidation and ROS production induced by TiO2 microparticles on snails Helix aspersa. Stud Univ “Vasile Goldiş” 27:127–133
Kim KT, Eo MY, Nguyen TTH, Kim SM (2019) General review of titanium toxicity. Int J Implant Dent 5:10. https://doi.org/10.1186/s40729-019-0162-x
Klotz AV, Stegeman JJ, Walsh C (1984) An alternative 7-ethoxyresorufin O-deethylase activity assay: a continuous visible spectrophotometric method for measurement of cytochrome P-450 monooxygenase activity. Anal Biochem 140:138–145
Krezel A, Maret W (2007) Different redox states of metallothionein/thionein in biological tissue. Biochem J 402:551–558. https://doi.org/10.1042/BJ20061044
Lee BC, Kim S, Shon HK, Vigneswaran S, Kim SD, Cho J, Kim IS, Choi KH, Kim JB, Park HJ, Kim JH (2009) Aquatic toxicity evaluation of TiO2 nanoparticle produced from sludge of TiCl4 flocculation of wastewater and seawater. J Nanopart Res 11:2087–2096. https://doi.org/10.1007/s11051-008-9574-x
Li L, Sillanpää M, Risto M (2016) Influences of water properties on the aggregation and deposition of engineered titanium dioxide nanoparticles in natural waters. Environ Pollut 219:132–138. https://doi.org/10.1016/j.envpol.2016.09.080
Lopes-Lima M, Sousa R, Geist J, Aldridge DC, Araujo R, Bergengren J, Bespalaya Y, Bódis E, Burlakova L, Van Damme D, Douda K, Froufe E, Georgiev D, Gumpinger C, Karatayev A, Kebapçi Ü, Killeen I, Lajtner J, Larsen BM, Lauceri R, Legakis A, Lois S, Lundberg S, Moorkens E, Motte G, Nagel KO, Ondina P, Outeiro A, Paunovic M, Prié V, von Proschwitz T, Riccardi N, Rudzīte M, Rudzītis M, Scheder C, Seddon M, Şereflişan H, Simić V, Sokolova S, Stoeckl K, Taskinen J, Teixeira A, Thielen F, Trichkova T, Varandas S, Vicentini H, Zajac K, Zajac T, Zogaris S (2017) Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biol Rev 92:572–607. https://doi.org/10.1111/brv.12244
Lowry OH, Rosebroungh HJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 191:265–275
Lu N, Lu Y, Liu F, Zhao K, Yuan X, Zhao Y, Li Y, Qin H, Zu J (2013) H3PW12O40/TiO2 catalyst-induced photodegradation of bisphenol A (BPA): kinetics, toxicity and degradation pathways. Chemosphere 91:1266–1272. https://doi.org/10.1016/j.chemosphere.2013.02.023
Luna-Acosta A, Thomas-Guyon H, Amar M, Rosenfeld E, Bustamante P, Fruitier-Arnaudin I (2011) Differential tissue distribution and specificity of phenoloxidases from the Pacific oyster Crassostrea gigas. Comp Biochem Physiol B 159:220–226. https://doi.org/10.1016/j.cbpb.2011.04.009
Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark SA, Cummings KS, Frest TJ, Gargominy O, Herbert DG, Hershler R, Perez KE, Roth B, Seddon M, Strong EE, Thompson FG (2004) The global decline of nonmarine mollusks. BioScience 54:321–330. https://doi.org/10.1641/0006-3568(2004)054[0321:TGDONM]2.0.CO;2
Man SM, Kanneganti T-D (2016) Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy 12:2504–2505. https://doi.org/10.1080/15548627.2016.1239679
Manfrin C, De Moro G, Torbol V, Venier P, Pallavicini A, Gerdol M (2012) Physiological and molecular responses of bivalves to toxic dinoflagellates. ISJ 9:184–199
Manibabu PV, Patnaik BK (1997) Lipofuscin concentration of the brain shows a reduction with age in male garden lizard. Comp Biochem Physiol C 117:229–232. https://doi.org/10.1016/S0742-8413(97)00054-6
Marchi B, Burlando B, Moore MN, Viarengo A (2004) Mercury- and copper-induced lysosomal membrane destabilisation depends on [Ca2+] dependent phospholipase A2 activation. Aquat Toxicol 66:197–204. https://doi.org/10.1016/j.aquatox.2003.09.003
Minetto D, Libralato G, Volpi Ghirardini A (2014) Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: an overview. Environ Int 66:18–27. https://doi.org/10.1016/j.envint.2014.01.012
Mischuk YV, Stoliar OB (2009) Peculiarities of metallothioneins of the bivalve mollusk Anodonta cygnea L. in the natural and laboratory living conditions. Hydrobiol J 45:63–71. https://doi.org/10.1615/HydrobJ.v45.i5.70
Moriyama A, Yamada I, Takahashi J, Iwahashi H (2018) Oxidative stress caused by TiO2 nanoparticles under UV irradiation is due to UV irradiation not through nanoparticles. Chem Biol Inter 294:144–150. https://doi.org/10.1016/j.cbi.2018.08.017
Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR (2009) A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B 364:2047–2062. https://doi.org/10.1098/rstb.2008.0242
Ohkawa H, Onishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Olive PL (1988) DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen 11:487–495. https://doi.org/10.1002/em.2850110409
Petković J, Žegura B, Filipič M (2011) Influence of TiO2 nanoparticles on cellular antioxidant defense and its involvement in genotoxicity in HepG2 cells. J Phys Conf Ser 304:012037. https://doi.org/10.1088/1742-6596/304/1/012037
Ptak A, Rak-Mardyła A, Gregoraszczuk EL (2013) Cooperation of bisphenol A and leptin in inhibition of caspase-3 expression and activity in OVCAR-3 ovarian cancer cells. Toxicol Vitr 27:1937–1943. https://doi.org/10.1016/j.tiv.2013.06.017
Ranjan S, Dasgupta N, Sudandiradoss C, Ramalingam C, Kumar A (2018) Titanium dioxide nanoparticle-protein interaction explained by docking approach. Int J Nanomed 13(T-NANO 2014 Abstracts):47–50. https://doi.org/10.2147/IJN.S125008
Reeves JF, Davies SJ, Dodd NJ, Jha AN (2008) Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res 640:113–122. https://doi.org/10.1016/j.mrfmmm.2007.12.010
Roesijadi G, Fowler BA (1991) Purification of invertebrate metallothioneins. Methods Enzym 205:263–273. https://doi.org/10.1016/0076-6879(91)05106-6
Rozes L, Steunou N, Fornasieri G, Sanchez C (2006) Titanium-oxo clusters, versatile nanobuilding blocks for the design of advanced hybrid materials. Mon Chem 137:501–528. https://doi.org/10.1007/s00706-006-0464-6
Rubin BS (2011) Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 127:27–34. https://doi.org/10.1016/j.jsbmb.2011.05.002
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14:6044–6066. https://doi.org/10.3390/ijms14036044
Saìnchez-Marín P, Fernaìndez-Gonzaìlez LE, Mantilla-Aldana L, Diz AP, Beiras R (2017) Shotgun proteomics analysis discards alkali-labile phosphate as a reliable method to assess vitellogenin levels in Mytilus galloprovincialis. Environ Sci Technol 51:7572–7580. https://doi.org/10.1021/acs.est.7b01734
Scott AP (2013) Do mollusks use vertebrate sex steroids as reproductive hormones? II. Critical review of the evidence that steroids have biological effects. Steroids 78:268–281. https://doi.org/10.1016/j.steroids.2012.11.006
Siebert MN, Mattos JJ, Piazza CE, de Lima D, Gomes CHA, de Melo CM, Bainy AC (2017) Characterization of ethoxyresorufin O-deethylase activity (EROD) in oyster Crassostrea brasiliana. Comp Biochem Physiol B 203:115–121. https://doi.org/10.1016/j.cbpb.2016.10.002
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Sun TY, Mitrano DM, Bornhöft NA, Scheringer M, Hungerbühler K, Nowack B (2017) Envisioning nano release dynamics in a changing world: using dynamic probabilistic modeling to assess future environmental emissions of engineered nanomaterials. Environ Sci Technol 51:2854–2863. https://doi.org/10.1021/acs.est.6b05702
Sureda A, Capó X, Busquets-Cortés C, Tejada S (2018) Acute exposure to sunscreen containing titanium induces an adaptive response and oxidative stress in Mytillus galloprovincialis. Ecotoxicol Environ Saf 149:58–63. https://doi.org/10.1016/j.ecoenv.2017.11.014
Sutherland DE, Summers KL, Stillman MJ (2012) Noncooperative metalation of metallothionein 1a and its isolated domains with zinc. Biochemistry 51:6690–6700. https://doi.org/10.1021/bi3004523
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69:8784–8789. https://doi.org/10.1158/0008-5472.CAN-09-2496
Turk B, Stoka V (2007) Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett 581:2761–2767. https://doi.org/10.1016/j.febslet.2007.05.038
Unuma T, Sawaguchi S, Yamano K, Ohta H (2011) Accumulation of the major yolk protein and zinc in the agametogenic sea urchin gonad. Biol Bull 221:227–237. https://doi.org/10.1086/BBLv221n2p227
Ursini F, Maiorino M, Forman HJ (2016) Redox homeostasis: The Golden Mean of healthy living. Redox Biol 8:205–215. https://doi.org/10.1016/j.redox.2016.01.010
Viarengo A, Burlando B, Cavaletto M, Marchi B, Ponzano E, Blasco J (1999) Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am J Phys 277:R1612–R1619. https://doi.org/10.1152/ajpregu.1999.277.6.R1612
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic Molluscs. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Wang J, Hou Y, Ma J (2013) Titanium(IV) intake by apotransferrin. In: Kretsinger RH, Uversky VN, Permyakov EA (еds) Encyclopedia of metalloproteins. Springer, New York, NY
Weber E (2005) Population size and structure of three mussel species (Bivalvia: Unionidae) in a northeastern German river with special regard to influences of environmental factors. Hydrobiologia 537:169–183. https://doi.org/10.1007/s10750-004-2839-1
Yang F, Zeng L, Luo Z, Wang Z, Huang F, Wang Q, Drobne D, Yan C (2018) Complex role of titanium dioxide nanoparticles in the trophic transfer of arsenic from Nannochloropsis maritima to Artemia salina nauplii. Aquat Toxicol 198:231–239. https://doi.org/10.1016/j.aquatox.2018.03.009
Zhang C, Lohwacharin J, Takizawa S (2017) Properties of residual titanium dioxide nanoparticles after extended periods of mixing and settling in synthetic and natural waters. Sci Rep 7:9943. https://doi.org/10.1038/s41598-017-09699-9
Zierden MR, Valentine AM (2016) Contemplating a role for titanium in organisms. Metallomics 8:9–16. https://doi.org/10.1039/c5mt00231a
Zoltan T, Rosales MC, Yadarola C (2016) Reactive oxygen species quantification and their correlation with the photocatalytic activity of TiO2 (anatase and rutile) sensitized with asymmetric porphyrins. J Environ Chem Eng 4:3967–3980. https://doi.org/10.1016/j.jece.2016.09.008
Acknowledgements
This work was funded by the grants from the Ministry of Education and Science of Ukraine for O. Stoliar (Projects M/4-2013; M/70-2017, 132B). We thank Dr. Sci. Olexandr Zaichenko and Dr. Sci. Natalia Mitina (Lviv Polytechnic National University, Ukraine) for their assistance with the physical–chemical characterization of the nanoparticles. The authors are grateful Dr. Inna Birchenko and Proof-Reading-Service.com for the scientific editing, linguistic and phraseological improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
All authors have approved this version of the work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gnatyshyna, L., Falfushynska, H., Horyn, O. et al. Biochemical responses of freshwater mussel Unio tumidus to titanium oxide nanoparticles, Bisphenol A, and their combination. Ecotoxicology 28, 923–937 (2019). https://doi.org/10.1007/s10646-019-02090-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02090-6