Abstract
The stability and bioavailability of titanium dioxide nanoparticles (TiO2 NPs) suspension could be modified by the physicochemical properties of solution. In the present study, the effect of humic acid (HA) and ionic strength (by adding NaCl) on aggregation and sedimentation of TiO2 NPs suspension were investigated. Accordingly, the sublethal toxicity of TiO2 NPs suspensions with different HA and NaCl concentrations toward zebrafish (Danio rerio) was evaluated by monitoring the changes of superoxide dismutase, catalase, malonaldehyde and glutathione in gill, liver and intestine. The results showed that the aggregations formation and hydrodynamic diameter of TiO2 NPs in suspensions are not essential characteristics to decide toxicity. The varied oxidative stress responses detected in gill, liver and intestine derived from different toxicity mechanisms of TiO2 NPs. Nevertheless, the oxidative stress could be suppressed by the adding of HA and/or the increase of ionic strength, which can decrease the bioavailability of TiO2 NPs in water. The study suggests that the environmental factors, such as HA and ionic strength, are important for the fate (aggregation and sedimentation) and toxicity of nanomaterials in aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoscale materials are rapidly applied in our daily life because of their special physiochemical properties. However, their unique properties can in some case seriously threaten the environment and humans, expressing by the nanoparticles exhibit greater hazard and risk to organisms comparing with their bulk counterparts (Handy et al. 2008; Klaine et al. 2008; Farre et al. 2009; Menard et al. 2011). TiO2 NPs, because of its strong ultraviolet (UV) radiation absorption and photoactive properties, is among the first nanomaterials made readily commercially available in diverse industrial and consumer applications, however, the increasing use of TiO2 NPs has raised equally increasing concerns about their potential to induce adverse health effects.
Despite the massive scientific research on the ecotoxicity of TiO2 NPs to aquatic life, the findings are inconclusive and conflicting, which may arise from the complex environment behavior of NPs (Clemente et al. 2012, 2013). NPs tend to aggregate in the environment and therefore can be eliminated or captured by sedimentation (Zhu et al. 2009; Yu et al. 2011). Thus it is important to understand the behavior and fate of TiO2 NPs in aquatic environments in order to elucidate their toxicology. Apart from the properties of TiO2 NPs, i.e. small size, surface activity, chemical composition and photocatalytic activity, the water chemistry, such as pH, ionic strength and natural organic matters (NOM), are also non-negligible factors to understand the uptake and toxicity of TiO2 NPs to aquatic organisms (Von der Kammer et al. 2010). Previous research report the influence of humic acid (HA) on toxicity of TiO2 NPs toward developing zebrafish, indicating that adsorption of HA increased suspensions stability and decreased TiO2 NPs exposure, and TiO2 NPs showed higher toxicity in the presence of HA (Yang et al. 2013). Moreover, it has been proved that HA can increase the surface negative charges and reduce the aggregation of graphene, and then regulate (Hu et al. 2014). Besides, media ionic strength yields visible toxicity of gold nanoparticles to embryonic zebrafish, suggesting that low ionic media induce morbidity and mortality but high ionic media hardly cause biological response (Truong et al. 2012). These investigations strongly suggest that both HA and ionic strength are important environmental factors that could change the toxicity of nanomaterials. Unfortunately, the effects of HA and ionic strength on TiO2 NPs aggregation, which could help nanoparticles to settle out from suspensions and then reduce toxicity, are still unclear. Accordingly, few researchers have touched the sublethal toxicity of TiO2 NPs to adult fish affected by HA and ionic strength.
Nevertheless, fast aggregation under freshwater conditions could result in exposure of benthic rather than water column species. Some studies suggested that TiO2 NPs showed low acute toxicity toward fishes, and LC50 (96 h) was unable to be determined for zebrafish under standardized experimental conditions (Griffitt et al. 2008), while was indicated as 124.5 mg/L in our previous study (Xiong et al. 2011) and >100 mg/L detected by Oncorhynchus mykiss (Warheit et al. 2007). Similarly, the 96 h exposed zebrafish eggs to up to 500 mg/L of TiO2 NPs did not cause alterations in the survival and hatching rates, or malformations (Zhu et al. 2008). However, water column species are the primary concern in the risk assessment strategy for contaminants (Handy et al. 2012) and then sublethal effects have been observed, mainly related to oxidative stress and inflammation (Federici et al. 2007; Hao et al. 2009; Palaniappan and Pramod 2010; Xiong et al. 2011; Lee et al. 2012). It is likely that a sub-acute or chronic exposure may more acutely reveal the toxicity of TiO2 NPs and be of more ecological importance in environmental risk evaluation. Furthermore, the body distribution, metabolism, and excretion of NPs are poorly understood and hampered by a lack of methods for measuring NPs in tissues. It remains possible that TiO2 NPs could interfere or stimulate stress responses in fish (Menard et al. 2011; Clemente et al. 2013; Yang et al. 2013), which was shown as oxidative response. However, the oxidative response could be caused by direct (primary) or indirect (secondary) effect by NPs or its released ions. Furthermore, NPs could enter into internal organs of fish through different ways, such as sticking on gill or ingestion by fish, leading to various damages on different organs. Therefore, the possible effect of HA and ionic strength on sublethal toxicity of TiO2 NPs to adult fish still need to be investigated.
In the current study, the effects of various concentrations of HA and ionic strength on aggregation and sedimentation of TiO2 NPs were analyzed. Correspondingly, the changes of antioxidant defense systems in different organs were investigated to assess the sublethal toxicity of TiO2 NPs against zebrafish under different concentrations of HA and ionic strength. In addition, a natural water (from Donghu lake) was used as reference to further reveal the biological potential of TiO2 NPs. The study provides insight into possible mechanism of toxicity as well as information for evaluating the risk of TiO2 NPs to aquatic organisms.
Materials and methods
Nanomaterials and chemicals
TiO2 NPs powders with various particles sizes (20, 30, 50, 100 and 500 nm advertised particle sizes) were purchased from Beijing Dk Nano technology Co., LTD, bulk-TiO2 was obtained from Tianjin Kemiou Chemical Reagent Co. Ltd, China. The particle size was confirmed by scanning electron microscopy (SEM) (Fig. 1). The surfaces of the TiO2 NPs were not modified and the advertised purities of TiO2 NPs and bulk-TiO2 particles were above 99.0 %, respectively. HA was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Commercial kits of all biochemical parameters were purchased from Nanjing Jiancheng Bioengineering Institute (NJBI, China). All chemicals were of analytical reagent grade or better.
Characterization of TiO2 NPs suspensions
The TiO2 NPs suspensions with a concentration of 100 mg/L were prepared by adding the powders of TiO2 NPs into double-distilled water (DD-water). To obtain homogeneous suspensions, the suspensions were dispersed using a bath sonicator (JL-360, 100 W, 40 kHz, Shanghai Jieli Co., Ltd) for 20 min prior to use. The shapes of TiO2 NPs suspensions with different primary particle sizes, i.e., 20, 30, 50, 100 and 500 nm were visualized by transmission electron microscopy (TEM, JEM-100CXII, JEOL, Ltd., Japan), and the hydrodynamic diameter distributions were characterized by a Nano-Zetasizer (1000HS, Malvern Instrument Ltd., UK) which is based on the dynamic light scattering (DLS) technique. The bulk-TiO2 was used as comparison.
In addition, the effect of ionic strength, HA and natural water on the aggregation and sedimentation of TiO2 NPs suspensions was analyzed. During this measurement process, a series of 100 mg/L TiO2 NPs (30 nm) suspensions with different concentrations of ionic strength (0.001, 0.01, 0.05, 0.1 M NaCl) and HA (0.1, 1, 5, 10 mg/L HA) were prepared. The natural water was sampled from the Donghu Lake and its characteristic was shown in Table 3 (supporting information). The hydrodynamic diameter distributions were characterized at 1 and 24 h, and the concentrations of TiO2 in the water was determined at different time point.
Furthermore, the hydrodynamic diameter variations of a 100 mg/L anatase TiO2 NPs (30 nm) and a 100 mg/L rutile TiO2 NPs (30 nm) suspensions, using fish culture water instead of DD-water, were analyzed at 1 and 24 h to reveal the dynamic diameters during the toxic exposure.
Animals
Adult zebrafishes (Danio rerio) with mean age of 120 days, mean length of 3.79 ± 0.36 cm, and mean weight of 0.49 ± 0.18 g were obtained from the department of Molecular Pathology of Fishes (Institute of Hydrobiology, Chinese Academy of Sciences). Fishes were acclimatized in dechlorinated tap water with a natural light/dark cycle (16:8) for 7 days in laboratory before experimentation and fed twice daily with newly hatched brine shrimp (Artemia salina). The water temperature was maintained at 23 ± 2 °C and no fish died prior to experiments. The authors certify that all experiments with live animals were performed in compliance with the relevant laws and the institutional committees have approved our experiments.
Toxic exposure
Aerated DD-water, rather than standard laboratory water, was used to prepare the particle suspensions of 100 mg/L TiO2 NPs (30 nm) and bulk-TiO2 to avoid complications from environmental factors that would influence the toxic effects of TiO2. Test suspensions were prepared followed the same way as described above. Seven fishes were randomly chosen and exposed to each group 96 h in a 2 L glass beaker containing 1.5 L test suspensions. 100 mg/L TiO2 was dissolved in 0, 0.001, 0.01, 0.05 and 0.1 M NaCl as treatment 1, 2, 3, 4 and 5 (see details in Fig. 5) to evaluate the effect of ionic strength on the sublethal toxicity of TiO2 NPs, respectively, and unexposed group was served as control. To study the effect of HA on the sublethal toxicity of TiO2 NPs, 100 mg/L TiO2 was dissolved in 0, 10, 5, 1, and 0.1 mg/L HA as treatment 1, 2, 3, 4 and 5 (see details in Fig. 6), respectively, and unexposed group was served as control.
Additionally, 20, 50, 100 and 500 nm TiO2 NPs suspensions (100 mg/L) without NaCl or HA were used to test the effect of primary size on toxicity. The control group was provided with DD-water without NPs or bulk particles, and a Donghu Lake water was used as a reference. Each treatment was performed in duplicate and placed under the same conditions with a natural light/dark cycle (16:8) and room temperature of 23 ± 2 °C. To ensure a constant concentration, all the test solutions were changed every 24 h.
Fishes were not fed on the day before or during the experimental period to minimize absorption of the nano and bulk-TiO2 particles in food as well as production of feces to maintain water quality. During the exposure period, the pH and dissolved oxygen content (DO) monitored by pH-meter (YSI 63, Yellow Springs Inc., USA) and DO meter (YSI 85, Yellow Springs Inc., USA), were 6.8–7.2 and no less than 5.10 mg/L, respectively.
At the end of the toxicity tests, superoxide dismutase (SOD) and catalase (CAT) activities, as well as the contents of the reduced glutathione (GSH) and malonaldehyde (MDA) in the zebrafish organs were determined to assess oxidative stress and damage. The test procedure was described as our previous study (Xiong et al. 2011).
Determination of TiO2 NPs concentrations in water and fish
To study the bioaccumulation of TiO2 NPs in zebrafish, low, medium and high exposure concentrations (5, 10 and 20 mg/L TiO2 NPs of 30 nm) were chosen. During a 30 day exposure process, three whole fish from each replicate of every treatment were used as a sample at a 5 day interval. The samples were washed with DD-water triply and weighed at room temperature, then oven dried to a constant weight, digested in concentrated nitric acid, then diluted to 20 ml with ultrapure water (18.2 MΩ). Samples were analyzed for Ti by inductively coupled plasma-mass spectrometer (ICP-MS, Thermo Elemental X7, Thermo Electron Co., USA). The protocol for determining the TiO2 NPs suspended in the solutions was the same with that of fish samples. The results were expressed as milligram TiO2 per liter for water and milligram TiO2 per gram dry weight for fish.
Statistical analysis
For statistical analysis, each of the experimental values was compared to its corresponding control. Results were expressed as mean ± standard deviation (SD). Multi group comparisons of the means were carried out by one-way analysis of variance (ANOVA) test. Dunnett’s test was used to compare the differences between the experimental groups and the control group. The ANOVA was performed using SPSS 17.0 (SPSS, Chicago, IL, USA) and the figures were generated by using OriginLab OriginPro 8.5. Statistical significance for all tests was set at p < 0.05 and extremely significance was set at p < 0.01.
Results
Characteristic of TiO2 NPs
The SEM images of TiO2 NPs in Fig. 1 showed that each powder size of TiO2 NPs presents similar shape, displaying with ultrafine and irregular single nanoparticles, and the primary particle sizes corroborated the manufacturer’s information. However, aggregates existed in TiO2 NPs for different primary sizes (20, 30, 50 and 100 nm) and some of aggregates had been detected sizes over 500 nm. Compared with 500 nm powder sizes of TiO2 NPs and bulk-TiO2, more ultrafine particles were noticed in other 4 kinds of TiO2 NPs (20, 30, 50 and 100 nm).
The average hydrodynamic sizes of anatase TiO2 NPs increased with decreasing initial particle sizes after 1 h dissolution in DD-water (see Table 1), which means the initial particle sizes strongly influence aggregation of TiO2 NPs. Smaller initial particle sizes lead to a higher tendency of aggregation, resulting in a greater hydrodynamic particle size. Unexpectedly, bulk-TiO2 was observed the smallest hydrodynamic sizes compared to other TiO2 NPs. TEM analysis showed the images of TiO2 NPs in aqueous suspensions (Fig. 2), all sizes of TiO2 NPs were collected in aggregates of irregular shape. The main difference between TiO2 NPs and bulk-TiO2 was the combination of the particles, the particles are loosely bound with many pores at the nano-scale, while they are tightly bound and appear as mass at conventional scale.
The effect of humic acid and ionic strength on aggregation and sedimentation of TiO2 NPs
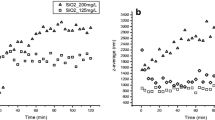
To understand the various dynamic sizes of TiO2 NPs that could be exist in toxicity test, hydrodynamic diameters of TiO2 NPs with different crystal forms, as well as under different ionic strength and HA conditions were detected by DLS. The hydrodynamic sizes of TiO2 NPs varied significantly in different type of water (Table 2), with average diameter ranging from 429.2 nm (10.0 mg/L HA, 1 h) to 2046.7 nm (0.01 mg/L NaCl, 1 h). In DD-water of different ionic strength, all the average hydrodynamic sizes of TiO2 NPs were over 1 μm, without significant increase (in DD-water and 0.001 M NaCl) or decrease (0.01 and 0.1 M NaCl) (p > 0.5) after 24 h. Larger hydrodynamic sizes were obtained in 0.1 mg/L HA at two time points (1 and 24 h). However, similar hydrodynamic sizes of TiO2 NPs were detected in both 1.0 and 10.0 mg/L HA, and they are much smaller than in DD-water (see Table 2). Hydrodynamic sizes, with increasing from 1954.6 nm (1 h) to 10895.8 nm (24 h), grew massively in Donghu lake water compared with in DD-water. In zebrafish-raising water (the biomass density is the same as toxicity test), the anatase TiO2 NPs presented a lager hydrodynamic size (483.5 nm) than its powder size (30 nm) after 1 h dissolution. Moreover, hydrodynamic size increased significantly to 1036.4 nm at 24 h time point. Interestingly, the hydrodynamic size of rutile TiO2 NPs showed slight increase at 24 h time point (477.4 nm vs 567.8 nm).
Figure 3 indicated sedimentations of TiO2 NPs under conditions of various ionic strength and HA. TiO2 NPs revealed very high sedimentation rate in DD-water, expressing with concentrations decreased sharply from 100 to 30 mg/L after 4 h (Fig. 3a). Only 10.3 and 3.11 mg/L TiO2 NPs were observed after 12 and 24 h, respectively. After 96 h, almost no TiO2 NPs could be detected. Interestingly, compared with in DD-water, a slightly but no significantly fast sedimentation rate were observed in the presence of different ionic strengths. Compared to other high concentrations of HA, remarkable difference of sedimentations of TiO2 NPs in DD-water only and in presence of 0.1 mg/L HA was marked in Fig. 3b. High concentrations of HA (1, 5 and 10 mg/L) suggested comparable sedimentation rate that almost half of TiO2 NPs are settled in the first 2 h and about 22 mg/L of TiO2 NPs were reaming after 96 h. However, a steeper sedimentation rate was found at first 2 h when 0.1 mg/L HA existed (and only 34.85 mg/L of TiO2 NPs was remaining), and the sedimentation reached equilibrium in 48 h.
Sublethal toxicity of different sizes TiO2 NPs to zebrafish
The toxic effect of various primary particle sizes of TiO2 NPs against zebrafish gill, liver and intestine tissues were pointed out in Fig. 4. In gill tissue, SOD activity was repressed extremely significantly in 20 nm TiO2 NPs exposed group (p < 0.01) and significantly in 50 and 100 nm exposed groups (p < 0.05) but not in 500 nm exposed group. Moreover, compared to the 500 nm treated group, the level of SOD activity was 310 % higher when treated with 20 nm. Similarly, the amounts of MDA increased significantly in 20, 50 and 100 nm exposed groups (p < 0.05) but not in 500 nm exposed group. However, TiO2 NPs hardly affected CAT activity and amount of GSH in gill tissue.
Activities of a SOD and b CAT, and concentrations of c MDA and d GSH, in different tissues of zebrafish following exposure to TiO2 suspensions of different primary particle sizes. Values are mean ± SD (n = 2). * and ** indicate statistically significant differences from control values at p < 0.05 and p < 0.01, respectively. While a and b indicate that this treatment is significantly different from the treatment of 20 nm TiO2 NPs at p < 0.05 and p < 0.01, respectively
In liver tissue, no notable difference of SOD activity was detected in exposed groups compared with the control (p > 0.05). Significant difference of MDA and GSH amount were only observed in 500 and 50 nm exposed groups, respectively (p < 0.05). Nevertheless, CAT activity was significantly decreased in 20 and 50 nm exposed groups compared with control. In 100 and 500 nm exposed groups, the extremely significant repression of CAT activity was found (p < 0.01).
In comparison with control, unremarkable differences of SOD and CAT activity and GSH amount in intestine were noticed for each exposed group (p > 0.05). While for the MDA amount, the 50 nm exposed group, the level was significantly higher than that in control and in 20 nm group (p < 0.05).
The effect of ionic strength on the sublethal toxicity of TiO2 NPs
Figure 5 revealed the effect of different ionic strengths on SOD and CAT activities and MDA and GSH amounts in the gill, liver and intestine of zebrafish exposed into 100 mg/L TiO2 NPs. In gill, SOD activity was significantly decreased in 0, 0.001, 0.01 and 0.05 M exposed groups (treatment 1, 2, 3, 4, respectively) compared to control (p < 0.05), but not in 0.1 M exposed group (treatment 5) and 0.1 M NaCl only group (treatment 6). MDA amount had no significant change in each exposed groups except for 0.01 M group (treatment 3). Although the amount of GSH was different between each exposed groups with control, there was no significant difference (p > 0.05).
Activities of a SOD and b CAT, and concentrations of c MDA and d GSH, in different tissues of zebrafish following exposure to TiO2 NPs suspensions of different ionic strengths. Values are mean ± SD (n = 2). * and ** indicate statistically significant differences from control values at p < 0.05 and p < 0.01, respectively. While a and b indicate that this treatment is significantly different from the treatment of 100 mg/L TiO2 only (treatment 1) at p < 0.05 and p < 0.01, respectively
In the liver tissue, only 0 M NaCl exposed group (treatment 1) showed extremely low SOD activity compared with others (p < 0.01). In comparison with control, remarkable difference CAT activity was observed in 0 and 0.001 M exposed groups (treatment 1 and 2, respectively) (p < 0.05), and extremely difference was found in 0.1 M group (treatment 5) (p < 0.01). The GSH amount in liver was essential increased in different exposed groups, but only 0.1 M group (treatment 5) showed significantly increased. Comparably, MDA amount in 0.1 M exposed group (treatment 5) was magnificently lower than in control group (p < 0.01), also significant lower than in treatment 1 (100 mg/L TiO2 NPs only, p < 0.05).
In the intestine of zebrafish, treatment 1 and treatment 5 expressed significantly (p < 0.05) and extremely significantly (p < 0.01) lower SOD activity than control, respectively. While the intestines of zebrafish exposed in 0.01 M groups (treatment 3) revealed enormous change on MDA and in 0.001 M groups (treatment 2) showed dramatic difference on GSH amount compared to control.
The effect of humic acid on the sublethal toxicity of TiO2 NPs
Significant increase of SOD activities were observed in liver tissue of fish exposed to 0, 5 and 10 mg/L HA groups (treatment 1, 3 and 2, respectively) and in gill tissue in 10 mg/L HA groups (treatment 2) (p < 0.05) (Fig. 6a). CAT activities were strongly motivated in livers in 5 and 10 mg/L HA groups (treatment 3 and 2, respectively) and in intestine in treatment 2 (p < 0.05) (Fig. 6b). Inspection of Fig. 6c, compared to gill and intestine, liver was a sensitive tissue to show the change of MDA amount derived from exposed to TiO2 NPs combined with different concentrations of HA. Furthermore, the dramatically lower amounts of GSH were only noticed in intestine of fish exposed to HA groups (p < 0.01), but not in gill or liver. Interestingly, in intestine of zebrafish exposed to different HA groups, significant difference of SOD and CAT activities and MDA amount were detected in 0.1 mg/L HA group (treatment 5), and GSH amounts were dramatically lower in 0, 0.1, 1 and 10 mg/L HA groups (treatment 1, 5, 4 and 2, respectively) but not in 5 mg/L HA group (treatment 3). Still, compared with control, SOD and CAT activities and MDA and GSH amounts showed essential difference in the gill, liver or intestine of zebrafish exposed to 10 mg/L HA without TiO2 NPs. Especially, SOD and CAT activities and MDA amount in 0.1, 1 and 5 mg/L HA exposure groups (treatment 5, 4 and 3, respectively) were found significant difference in gill, liver or intestine comparison with in treatment 1 (100 mg/L TiO2 NPs only without HA).
Activities of a SOD and b CAT, and concentrations of c MDA and d GSH, in different tissues of zebrafish following exposure to TiO2 NPs suspensions of different HA concentrations. Values are mean ± SD (n = 2). * and ** indicate statistically significant differences from control values at p < 0.05 and p < 0.01, respectively. While a and b indicate that this treatment is significantly different from the treatment of 100 mg/L TiO2 only (treatment 1) at p < 0.05 and p < 0.01, respectively
The toxic effect of TiO2 NPs on zebrafish in Donghu Lake water
TiO2 NPs possessed a relatively low acute toxicity to zebrafish. In DD-water batch, acute lethality to zebrafish was zero after 96 h of exposure to TiO2 NPs suspensions with concentration less than 100 mg/L, while 200 mg/L TiO2 NPs exposure to zebrafish resulted in a lethality of 14.3 % and further increase of concentration would not bring a higher lethality (Data not shown). However, in Donghu lake water batch, the lethality to zebrafish was zero after 96 h of exposure to as high as 200–500 mg/L TiO2 NPs particles suspensions.
In Donghu lake water (Fig. 7), the different concentrations of TiO2 NPs exposure caused molecular biomarker responses in gill, liver and intestine. Expectedly, SOD and CAT activities and GSH amount were dramatically different in gill, liver or intestine of zebrafish exposed to higher concentrations of TiO2 NPs (100 and 200 mg/L). Zebrafish under 50 mg/L of TiO2 NPs exposure showed no serious tissues damage except that SOD activities were repressed greatly in intestine. Although MDA amount in each tissue affected by TiO2 NPs exposure, the effects were not serious (see Fig. 7c).
Activities of a SOD and b CAT, and concentrations of c MDA and d GSH, in different tissues of zebrafish following exposure to different concentrations of TiO2 NPs in Donghu lake water. Values are mean ± SD (n = 2). * and ** indicate statistically significant differences from control values at p < 0.05 and p < 0.01, respectively. While a indicates that this treatment is significantly different from the treatment of 50 mg/L TiO2 NPs at p < 0.05
The bioaccumulation of TiO2 NPs in zebrafish
As can be seen from Fig. 8, bioaccumulation of TiO2 NPs in zebrafish increased with exposure concentrations and duration. Both 5 and 10 mg/L groups showed similar accumulation of TiO2 NPs in fish in the first 15 days, which was lower than 20 mg/L group, then accumulation grew rapidly in 10 mg/L group in 20 days and obtained saturation as the same as in 20 mg/L group.
Discussion
As mentioned above, HA and ionic strength could play critical roles in the toxicity of TiO2 NPs to organisms, thus the effect of HA and ionic strength on formation of aggregation and hydrodynamic diameters of TiO2 NPs in suspensions were investigated. Consequently, the influence of HA and ionic strength on the adverse effect of TiO2 to adult zebrafish were also analyzed.
As shown in Fig. 2 and Table 1, all of the TiO2 NPs particles with different primary sizes (20, 30, 50, 100 and 500 nm) were aggregated in DD-water, and the smaller the primary size is, the more easily to form aggregation. There is a positive correlation between the size and surface area or surface energy, particles with large surface area or high surface energy usually have high unstable thermodynamic system, which means high tendency to form aggregations. In addition, aggregations easily happen under the van der Waals force and the electrostatic force. Further, aggregations derived from rutile TiO2 NPs show better suspensions stability and dispersibility than those from anatase TiO2 NPs (see Table 2). The result was also noticed previously in deionized water as well as in PBS (Braydich-Stolle et al. 2009; Bolis et al. 2012). This finding confirms the fact that aggregations of TiO2 NPs depend on crystal structures, which is relating to surface charges, atom arrangement and architectural feature of TiO2 NPs. Furthermore, the average hydrodynamic diameter of nanoparticles, which is considered as effective size to cause toxicity, however, different sizes of TiO2 NPs have no difference in hydrodynamic diameters (see Table 1).
Nanomaterials with smaller size are theoretically expected to be more toxic than their bigger counterparts due to the greater surface reactivity and ability to penetrate into and accumulate within cells and organisms (Ispas et al. 2009; Mironava et al. 2010). Unsuccessfully, no discernable correlation between primary particle size and toxic effect were found, thus it was assumed that secondary particle size and particle surface area may be refer to biological potential of nanoparticles although insufficient confirmatory data exist (Menard et al. 2011). According to this hypothesis, TiO2 NPs with larger sizes are supposed to show higher toxicity compared to TiO2 NPs with small sizes, considering the aggregations and hydrodynamic diameters in this research. However, the molecular biomarkers responses detected in zebrafish suggest that small sizes of TiO2 NPs yield stronger hazard than those with larger sizes (Fig. 4). Previous studies indicated that anatase TiO2 NPs induced cell necrosis regardless of sizes (Braydich-Stolle et al. 2009) and aggregation formed through van der Waals force could convert to single nano-crystal which had nano-effect (Jiang et al. 2009). This conversion had been confirmed by that 40 nm nanoparticles were existing in both TiO2 NPs and ZnO NPs aggregations (De Berardis et al. 2010). In present research, TiO2 nanoparticles were also detected existing in aggregations derived from TiO2 NPs powder with small sizes (20, 30, 50 and 100 nm, see Fig. 1), but not in powder of 500 nm TiO2 NPs or bulk-TiO2. It’s worth noting that aggregation occurred does not mean that the nanomaterials are not bioavailable (Handy et al. 2008). Although TiO2 NPs rapidly aggregated to form microscale particles in saltwater, both nauplii and adults were readily accumulated the large aggregates, such as the intestines were filled with particles within 24 h, suggesting that the formation of microscale particles seems non-effective on the bioaccumulation (Ates et al. 2013). Indeed, the bioaccumulation of TiO2 NPs in zebrafish in the current research agrees with this finding. Although the tendency of TiO2 NPs to form aggregation increased with concentrations in solution, the bioaccumulation presented a dose and time-dependent effects rather than aggregation sizes (Fig. 8 and Supplementary Fig. 9). These results indicate that aggregations formation and hydrodynamic diameters of TiO2 NPs are not critical factors to determine their toxicity.
As the TiO2 NPs which existing in suspensions are the functional parts to induce toxic effect, thus the sedimentations of TiO2 NPs are important factor and should be considered to assess their toxicity. In natural water, both various ionic strength and HA could affect the sedimentations of TiO2 NPs (Von der Kammer et al. 2010). The present research notices that different concentrations of ionic strength (NaCl) were slightly associated with sedimentations of TiO2 NPs, showing the similar curves with DD-water (TiO2 NPs only stable in water at first 24 h, as shown in Fig. 3a). These sedimentations are partly dependent on hydrodynamic diameters. Despite sedimentations in 1, 5, and 10 mg/L HA suspensions showed no notable difference (Fig. 3b), the sedimentations are correlated with hydrodynamic diameters of TiO2 NPs in HA suspensions. Still, over half of TiO2 NPs are settling down during the first 24 h. These findings mean that the acute toxicity assessment of TiO2 NPs should be performed shorter than 24 h. Although sedimentations of TiO2 NPs in Donghu lake water are unknown, the sedimentations are supposed strongly, because of the formation of large hydrodynamic diameters and existing of ionic strength and HA (Tables 1, 2). The large hydrodynamic diameters could accelerate sedimentations of TiO2 NPs in Donghu lake water. Moreover, acute toxicity determinations in DD-water reveal that 200 mg/L TiO2 NPs (anatase, 30 nm) only result in 14.3 % of fish death after 96 h, and increasing exposure concentrations don’t increase mortality. In Donghu lake water, 500 mg/L TiO2 NPs produces zero fish death. The possible explanation is that both ions and HA in lake water promote sedimentations of TiO2 NPs, resulting in shortage of TiO2 NPs in solution to elicit fish death.
As discussed above that acute toxicity tests of TiO2 NPs are visualized inadequately and should be carried out shorter than 24 h, thus the sublethal toxicity test utilizing the antioxidant defense systems was applied in the current research to evaluate toxic effect of TiO2 NPs to different organs in zebrafish. The antioxidant defense systems include a series of antioxidative enzymes and low-molecular non-enzymatic antioxidants, such as SOD activities, CAT activities and contents of MDA and GSH, which were all appropriate for monitoring oxidative stress status of fish after exposure to nano metal oxides (Hao et al. 2009; Xiong et al. 2011; Hao and Chen 2012; Lee et al. 2012; Clemente et al. 2013), in spite of the fact that some of the biomarker responses appear inconsistent because the mechanism is indirect as a consequence of the occlusion of tissue surfaces rather than more readily interpreted dose–response relations. Indeed, the biomarker response varied in different organs and organs varied different sensitivity (detailed below), indicating possibly different toxicity mechanism. Combinations of Fig. 5a–d, increasing ionic strengths decreased toxicity of TiO2 NPs, which could be explained by the ionic strength (NaCl) slightly attribute sedimentations of TiO2 NPs. The illustrations indicated that the SOD activities are rising in gill, liver and intestine at higher concentrations of NaCl. As highest ionic group without TiO2 NPs also repress SOD activities although the repression is not significant (p > 0.05) (Fig. 5), SOD activities are recovering at moderate concentrations of NaCl possibly owing to the toxicity neutralized by ions, and SOD activities are decreasing again at 0.1 mg/L NaCl in liver and intestine due to both the toxicity effect of TiO2 NPs and ions. Still, the comparably low content of MDA and high content of GSH in exposure groups point out that ions help to antioxidant defense and immune system.
Similarly, HA could also prevent toxicity of TiO2 NPs to different organs in zebrafish, showing that SOD and CAT activities and content of MDA and GSH are lower in low concentration of HA exposure group (0.1 mg/L). It can be possibly explained by 0.1 mg/L HA accelerating TiO2 NPs sedimentation then lack of TiO2 NPs in suspension to induce toxicity. As shown in Fig. 6, these molecular biomarkers reveal that HA has no oxidative stress to fish, displaying with SOD and CAT activities and contents of MDA and GSH are lower compared to control group. Contrarily, single exposure TiO2 NPs generates oxidative damage in liver, highlighted by SOD activity and MDA amount are remarkable high (p < 0.01). The biomarkers detected in fish exposed in Donghu lake water also prove that ionic strength and HA repress toxicity of TiO2 NPs, revealed by that oxidative damage is only observed in gill and liver in 200 mg/L exposure group with significantly high content of GSH. The result confirms that no acute toxicity to zebrafish is noticed even increasing the exposure concentration to 500 mg/L of TiO2 NPs.
In principle, TiO2 NPs could vary hazard effect or risk to different organs, relying on different functions of organs and bioavailability of TiO2 NPs in organs. Especially, gill is the primary organ contacting water born TiO2 NPs, and gill could be suffering serious threat when TiO2 NPs sticks to gill surface (Hao et al. 2009; Xiong et al. 2011; Hao and Chen 2012). Absolutely, gill is a more sensitive organ than others to monitor oxidative damage caused by TiO2 NPs exposure, reflected by obvious change of SOD activities and content of MDA. Indeed, Gills are the first organs that contact with ambient water and together with the intestine constitute the main means of metal absorption. Once TiO2 NPs deposited in the gills, it may transfer to other target tissue through different routes and mechanisms (Federici et al. 2007). Furthermore, the gill could be blocked by the TiO2 NPs, and then hypoxia in the blood and alteration in oxidative/reduction status as secondary effect also repress these biomarkers change. This indirect hazard could be underestimated and should be carefully considered. In addition, the intestine seems more sensitive than gill and liver to show the change of GSH content (Figs. 5d, 6d, 7d). This could be explained by nanomaterials entrance into intestine through fish ingestion, and therefore, serious damage could be happened, even the nanomaterials more likely stay within the lumen of the intestine and not actually absorb across epithelial membranes to become truly associated with internal tissues. Once accumulated in the esophagus and intestine, the NPs will possibly shift to the liver and other organs through the circulation (Hao et al. 2009; Hao and Chen 2012; Ramsden et al. 2013). These findings confirmed that TiO2 NPs vary toxicity mechanisms in different fish organs and evaluating the biomarkers at several organs in a given species are needed to fully assess the toxicity of TiO2 NPs.
Conclusion
In summary, aggregations formation and hydrodynamic diameters of TiO2 NPs in suspensions are not critical factors to determine toxicity, but they can accelerate sedimentation of TiO2 NPs in suspensions and partly prevent toxicity induction. Behavior of sedimentation of TiO2 NPs in suspensions and accumulation in zebrafish imply that exposure duration of acute toxicity test of TiO2 NPs should be less than 24 h. Both ionic strength and HA could protect aquatic organisms from oxidative stress of TiO2 NPs exposure through encouraging sediment deposition. While further research will be required to elucidate the mechanism responsible for these findings, our results are consistent with oxidative stress as the cause of TiO2 NPs induced toxicity, and this appearance can be adjusted by the ions and HA. Especially, this study highlights the environmental factors are very important and should be considered when assess the fate and toxicity of nanomaterials in water environment.
References
Ates M, Daniels J, Arslan Z, Farah IO (2013) Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: assessment of nanoparticle aggregation, accumulation, and toxicity. Environ Monit Assess 185:3339–3348
Bolis V, Busco C, Ciarletta M, Distasi C, Erriquez J, Fenoglio I, Livraghi S, Morel S (2012) Hydrophilic/hydrophobic features of TiO2 nanoparticles as a function of crystal phase, surface area and coating, in relation to their potential toxicity in peripheral nervous system. J Colloid Interface Sci 369:28–39
Braydich-Stolle LK, Schaeublin NM, Murdock RC, Jiang J, Biswas P, Schlager JJ, Hussain SM (2009) Crystal structure mediates mode of cell death in TiO2 nanotoxicity. J Nanopart Res 11:1361–1374
Clemente Z, Castro VL, Jonsson CM, Fraceto LF (2012) Ecotoxicology of nano-TiO2—an evaluation of its toxicity to organisms of aquatic ecosystems. Int J Environ Res 6:33–50
Clemente Z, Castro VL, Feitosa LO, Lima R, Jonsson CM, Maia AHN, Fraceto LF (2013) Fish exposure to nano-TiO2 under different experimental conditions: methodological aspects for nanoecotoxicology investigations. Sci Total Environ 463:647–656
De Berardis B, Civitelli G, Condello M, Lista P, Pozzi R, Arancia G, Meschini S (2010) Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol Appl Pharmacol 246:116–127
Farre M, Gajda-Schrantz K, Kantiani L, Barcelo D (2009) Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal Bioanal Chem 393:81–95
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17:315–325
Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, Metcalfe C, Steevens JA, Klaine SJ, Koelmans AA, Horne N (2012) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31:15–31
Hao LH, Chen L (2012) Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf 80:103–110
Hao LH, Wang ZY, Xing BS (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). Journal of Environmental Sciences-China 21:1459–1466
Hu XG, Mu L, Kang J, Lu KC, Zhou RR, Zhou QX (2014) Humic acid acts as a natural antidote of graphene by regulating nanomaterial translocation and metabolic fluxes in vivo. Environ Sci Technol 48:6919–6927
Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN (2009) Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol 43:6349–6356
Jiang JK, Oberdorster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Lee BC, Kim KT, Cho JG, Lee JW, Ryu TK, Yoon JH, Lee SH, Duong CN, Eom IC, Kim PJ, Choi KH (2012) Oxidative stress in juvenile common carp (Cyprinus carpio) exposed to TiO2 nanoparticles. Mol Cell Toxicol 8:357–366
Menard A, Drobne D, Jemec A (2011) Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ Pollut 159:677–684
Mironava T, Hadjiargyrou M, Simon M, Jurukovski V, Rafailovich MH (2010) Gold nanoparticles cellular toxicity and recovery: effect of size, concentration and exposure time. Nanotoxicology 4:120–137
Palaniappan PR, Pramod KS (2010) FTIR study of the effect of nTiO(2) on the biochemical constituents of gill tissues of zebrafish (Danio rerio). Food Chem Toxicol 48:2337–2343
Ramsden CS, Henry TB, Handy RD (2013) Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat Toxicol 126:404–413
Truong L, Zaikova T, Richman EK, Hutchison JE, Tanguay RL (2012) Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology 6:691–699
Von der Kammer F, Ottofuelling S, Hofmann T (2010) Assessment of the physico-chemical behavior of titanium dioxide nanoparticles in aquatic environments using multi-dimensional parameter testing. Environ Pollut 158:3472–3481
Warheit DB, Hoke RA, Finlay C, Donner EM, Reed KL, Sayes CM (2007) Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett 171:99–110
Xiong DW, Fang T, Yu LP, Sima XF, Zhu WT (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ 409:1444–1452
Yang SP, Bar-Ilan O, Peterson RE, Heideman W, Hamers RJ, Pedersen JA (2013) Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish. Environ Sci Technol 47:4718–4725
Yu LP, Fang T, Xiong DW, Zhu WT, Sima XF (2011) Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, (OH)-O-center dot production and particle dissolution in distilled water. J Environ Monit 13:1975–1982
Zhu XS, Zhu L, Duan ZH, Qi RQ, Li Y, Lang YP (2008) Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. J Environ Sci Health Part A 43:278–284
Zhu XS, Wang JX, Zhang XZ, Chang Y, Chen YS (2009) The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 20:8532–8536
Acknowledgments
This work was financially supported by grants from the Major Science and Technology Program for Water Pollution Control and Treatment (2012ZX07103-003) and the National Natural Science Foundation of China (21477159).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
We confirm that the study performed was strictly in accordance with acceptable ethical procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, T., Yu, L.P., Zhang, W.C. et al. Effects of humic acid and ionic strength on TiO2 nanoparticles sublethal toxicity to zebrafish. Ecotoxicology 24, 2054–2066 (2015). https://doi.org/10.1007/s10646-015-1541-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1541-6