Abstract
Pentachlorophenol (PCP) is a organochlorine biocide that, unlike most other organochlorines, is still in use as timber preservative. Its water solubility, high toxicity, bioaccumulation potential, and the concentrations reported in estuarine waters (up to 0.1 µg L−1) indicate it may pose a risk in coastal environments. Aquatic environrmental regulations are commonly based on standard freshwater organisms that may not represent the sensitivity of marine species. The present study consists of a water quality criteira reevalutation of PCP in coastal waters based on toxicity tests conducted recording sensitive endpoints of marine species representative of coastal ecosystems, following QA/QC standard procedures. The toxicity thresholds (EC10) found were 4.69 µg L−1 for Paracentrotus lividus sea-urchin embryos, 6.47 µg L−1 for Mytilus galloprovincialis mussel larvae, and 78.4 µg L−1 for Isochrysis galbana cells. Therefore, there is only one order of magnitude between the predicted no-effect concentration (PNEC) for early life stages of bivalves and echinoderms and the maximum concentrations actually recorded in coastal water, which yields a remarkable risk quotient for PCP in these highly productive marine habitats. In addition, we have reviewed the ecotoxicological data on PCP toxicity on marine species representative of the main systematic groups, from algae to chordates, and derived a probabilistic acute saltwater quality criterion of 2.66 µg L−1, intended to protect 95% of the marine species. Lack of adequate protection for marine ecosystems in some current PCP national guidelines has been identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pentachlorophenol (PCP) is a relatively soluble organochlorine biocide that, unlike most other organochlorines, is still in use and widely applied as timber preservative, pesticide and disinfectant. Polychlorinated phenols can be also generated during kraft pulp mill operations when chlorine in the bleaching step reacts with natural phenolic compounds (Newman 2017). PCP interferes with normal reproductive and endocrine function in vertebrates, and PCP metabolites showed genotoxic and mutagenic properties, reviewed by Goodman (2001). Its high toxicity, comparatively high water solubility, bioaccumulation potential in marine organisms with bioconcentration factor (BCF) values between 100 and 1000, and the concentrations reported in estuarine waters (up to 0.1 µg L−1; Muir and Edulijee 1999) indicate it may also pose a risk in coastal environments.
Probabilistic environmental quality criteria obtained from species sensitivity distributions (SSD) allow the protection of a given percentage of the species occurring in an ecosystem (e.g. 95%) with a known confidence level (Aldenberg and Slob 1993). This approach was frequently used in order to derive scientifically sound water quality criteria (WQC) (ANZECC 2000; CCME 2007; Durán and Beiras 2013; Durán and Beiras 2017; EC 2011; OECD 1995; US-EPA 1985). The derivation of probabilistic WQC demands the use of toxicity data for a variety of taxa representative of the communities of interest, covering a wide range of phylogenetic and physiological variability (van Straalen and Denneman 1989). Most standard biological models used in experimental aquatic toxicology are freshwater organisms such as daphnia or salmonid fish. Durán and Beiras (2013) demonstrated that for several trace metals the maximum admissible concentrations reflected in the applicable legislation were above the toxicity thresholds for early life stages of saltwater species with high commercial value, evidencing the need to improve the regulations by taking into account toxicity data from marine species.
The objective of the present work is to derive acute water quality criteria based on ecotoxicological information with representative marine species, which provide a known degree of protection for coastal species, using an alternative method to the classical deterministic approach. With this aim, toxicity tests with Isochrysis galbana microalgae and early life stages of the mussel Mytilus galloprovincialis, and the sea-urchin Paracentrotus lividus were conducted in our laboratory. After a broad literature survey, the data set was completed to include species representative of all major marine taxa: algae, annelids, molluscs, crustaceans, echinoderms and chordates. In order to maximize sensitivity and thus protective value of the resulting criterion, preference was given to sublethal endpoints and early life stages. The derived WQC will be compared to national and international criteria and the degree of protection offered by them will be discussed.
Material and methods
Toxicity data set
Due to the large variability in the sensitivity to a chemical among the different species of an ecosystem, US-EPA (1985) requires the use of 8 different families for the derivation of saltwater criteria, while EC (2011) demands at least 10 species covering a minimum of 8 taxonomic groups. The current study used 20 endpoints obtained from 19 species including at least one representative of each of the main taxonomic groups of marine organisms: algae, annelids, mollusks, crustaceans, echinoderms and chordates. In order to maximize sensitivity, and thus protective value of the WQC, early life stages (embryos, larvae, neonates), sublethal endpoints (algal population growth, size growth, reproduction, embryogenesis and larval development) and, when available, long-term exposures were chosen. Because of lack of sensitivity, data on acute adult mortality were not considered.
Toxicity tests methods
Toxicity tests were carried out under strict quality assurance/quality control following internationally adopted standard methods. Growth inhibition tests with Isochrysis galbana microalgae followed ISO (2006) and OECD (2006). A non-axenic culture of I. galbana was grown in 500 mL flasks in a f/2 medium, and kept in an incubator at 20 °C with 24 h light cycle intensity 60 mE m−2 s−1 using cool daylight lamps (Osram L36W/865; emission spectrum range 380–780 nm). When reaching the exponential growth phase, an intermediate experimental culture, inoculated with the previous culture (density 7000 cells mL−1), was carried out in 5 L Erlenmeyer flask. Experimental solutions were added to 250 mL borosilicate Erlenmeyer flasks in triplicate and three additional flasks as control cultures. No agitation was provided during incubation. Cell density was measured at the beginning and after the 72 h of exposure with a Z2 Coulter Counter particle size analyzer (Beckman-Coulter Particle Count and size analyzer USA). Growth rate (GR) was calculated as:
Acceptability criteria for the test were 16-fold increase in cell density of controls in 72 h, and a coefficient of variation among replicates not exceeding 7% (ISO 2006).
Mature mussels (M. galloprovincialis) and sea-urchins (P. lividus) were collected by scuba divers during the natural spawning season in the outer part of Ría de Vigo (NW Iberian Peninsula). Sea-urchins were maintained in laboratory conditions with circulating sand-filtered seawater at a rate of ca. 9 L min−1, and fed two times per week with macroalgae. Water temperature was 18 ± 2 °C, salinity 34 ± 2 psu and oxygen >5 mg L−1. According to the methods described by Beiras and Bellas (2008) for mussel and Beiras et al. (2012) for sea-urchins, mussel gametes were obtained by thermal induction of spawning, while sea-urchin gametes were obtained by dissection of ripe adults. Mature oocytes of sea-urchins and mussels were transferred to 50 mL measuring cylinders and sperm were added, shaken gently to facilitate fertilization. Fertilized eggs were transferred before the first cleavage into glass vials with airtight teflon-lined caps containing 10 mL of the experimental solutions, at a density of 40 per mL for both species. Four replicates per treatment plus controls were carried out. After 48 h incubation at 20 °C in the dark, vials were fixed with four drops of 40% formalin for ulterior observation under an inverted microscope (Leica DMI 4000B). The endpoints measured were percentage of normal larvae in mussels (n = 100 per vial), and length (maximum linear dimension) in sea-urchins (n = 35 per vial). Mussel larvae were considered abnormal when they did not reach veliger stage, showed irregular shape, convex hinge, and/or protruding mantle (His et al. 1997). Length recordings were made using Leica QWIN image analysis software version 3.4.0 (Leica Microsystems, Germany). Acceptability criteria were percentage of fertilized eggs >98% and size increase in controls >253 μm (Saco-Álvarez et al. 2010) for the sea-urchin test, and control normality > 75% for the mussel test.
Statistical methods
Statistical analyses were conducted using IBM SPSS statistics version 22.0 and DataFit version 9.0 software. I. galbana cell growth, larval normality for mussels and larval size for sea urchin were the endpoints analyzed. All data were corrected by the mean control response. Normal distribution and homoscedasticity of the data was checked using the Shapiro-Wilk’s and Levene’s tests respectively. When significant differences (p < 0.05) among groups were found using ANOVA then each treatment was compared to the control using Dunnett’s post hoc test to calculate the highest no-observed effect concentration (NOEC) and the lowest observed effect concentration (LOEC). Non-parametric tests, Kruskall-Wallis and Mann Whitney U were used when data did not meet the requirements for parametric tests. The LC50 and LC10 values and their 95% confidence intervals were calculated by fitting the data to a modified Weibull dose-response model (Murado et al. 2002), by minimization of the sum of quadratic differences between experimental and model-predicted values using the nonlinear least-squares method provided by the macro Solver of the Microsoft Excel spreadsheet.
The SSD curve for PCF were obtained by fitting for each chemical the cumulative distribution of the toxicity thresholds (TT) obtained for the different species and life stages to a log-logistic model (van Straalen and Denneman 1989) described by the equation:
where Cp is the cumulative probability of the TT, and a and b are fitting parameters. The value of a equals the TT value for a cumulative probability of 0.5, and the value of b is inversely related to the slope of the curve. Non-linear fitting was performed using SigmaPlot (version 10.0) statistical software.
Due to the well-known weaknesses of the NOEC/LOEC approach, such as dependence on experimental design and statistical power (OECD 1998; Reiley et al. 2003; Vighi et al. 2003), EC10 were preferred for estimation of the TT. In the cases where EC10 data were not available, the TT was estimated using EC50/3, or LOEC, based on the mean ratios between EC10 and the remaining toxicity parameters obtained from the toxicity data base generated in our laboratory for >60 compounds tested with marine species (EC50/EC10 = 3.3, n = 202; EC10/LOEC = 1.1, n = 137).
Following previous consensus (EC 2011; US-EPA 1985; van Straalen and Denneman 1989), the 5th percentile (HC5) of the TT distribution will be used, and in order to take into account the probability that the actual value was lower than the estimate, which would cause under-protection, the WQC will be defined as the lower end of the 95% confidence intervals for the HC5 (Aldenberg and Slob 1993; Durán and Beiras 2013; Durán and Beiras 2017; EC 2011; Smith and Cairns 1993). Therefore, the WQC should protect 95% of the species with a 95% certainty.
Risk quotient (RQ) was calculated according to the expression (Tato et al. 2017):
\(\mathrm{RQ}{\mathrm{ = }}\mathrm{MEC}{\mathrm{/}}\mathrm{NOEC},\)where MEC is the measured environmental concentration and NOEC is the lowest no observed adverse effects concentration of the most sensitive organism tested.
Results and discussion
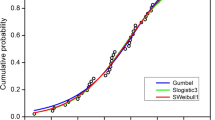
The dose:response curves for the three species tested are shown in Fig. 1, and the EC50 and EC10 values obtained from the curve equations, including their 95% confidence intervals, as long as the NOEC/LOEC values determined by the Dunnett’s test are shown in Table 1. NOEC for the most sensitive species tested was 1 µg L−1. Considering reported maximum environmental concentrations of 0.1 µg L−1 (Muir and Eduljee 1999), this yields a risk quotient, RQ = 0.1, indicating a ‘medium’ level of risk (Tato et al. 2017).
The TT values of PCP for each marine species are compiled in Table 2, and the corresponding SSD curve adjusted to those values is presented in Fig. 2. The fitting parameters, a and b, from the log-logistic model were both significant (p < 0.05), and the curve adjusted well to the compiled TT values (R2 = 0.9797). Parameter a was 24.80 µg L−1 (95% CI 24.12–25.47), and it indicates the median TT value for all marine species. According to this parameter PCP acute toxicity to marine organisms is similar to that of chemicals considered as highly toxic, such as mercury, copper, nonylphenol, pyrene or fluoranthene (Durán and Beiras 2013; Durán and Beiras 2017).
Distribution of the cumulative probability of toxicity thresholds (TT) for marine species for PCP. Dots represent the individual TT values from Table 2
Parameter b was 0.32 (95% CI 0.30–0.33), and it is inversely related to the slope of the curve, and thus provides information on the degree of variability in sensitivity among species. Despite the large taxonomic differences among testing organisms, TT values ranged less than two orders of magnitude, from 4.69 (obtained with the most sensitive species, P. lividus) to 173 µg L−1 (for the least sensitive species, the annelid P. dumerilii). The good fit to a single curve for all species tested and the low value of b, points at a universal mechanism of toxicity for PCP, commonly considered as an uncoupler of mitochondrial oxidative phosphorylation. We have previously reported b values ranging from 0.23 to 0.37 for non-selective organic toxicants (Durán and Beiras 2017).
The 5th percentile (HC5) of the SSD, its 95% confidence intervals (CI), the critical value, and the proposed acute WQC obtained from the lower end of the CI, are summarized in Table 3, which reflects also the standards and criteria stated in different environmental regulations. The EU set the same standards for continental and marine waters, and we can see that the acute value (1 µg L−1) is sufficiently protective at the light of the ecotoxicological information here reviewed. Canada implemented a freshwater criterion that seems suitable for marine waters also (0.5 µg L−1; CCME 2007). In contrast the values adopted by US-EPA (13 µg L−1; US-EPA 2017) and ANZECC (22 µg L−1) are not sufficiently protective for marine ecosystems, since they are above the acute WQC here calculated, and even above the TT experimentally demonstrated for bivalve and sea-urchin larvae.
In conclusion, the acute WQC here derived from marine ecotoxicological tests are higher than those imposed by the EU and Canada, which may be slightly too conservative. In contrast, they suggest an insufficient protection of coastal marine ecosystems if US EPA and ANZECC criteria are applied.
References
Adema DMM, Vink IGJ (1981) A comparative study of the toxicity of 1,1,2-trichloroethane, dieldrin, pentachlorophenol and 3,4 dichloroaniline for marine and fresh water organisms. Chemosphere 10:533–554. https://doi.org/10.1016/0045-6535(81)90255-1
Aldenberg T, Slob W (1993) Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data. Ecotoxicol Environ Saf 25:48–63. https://doi.org/10.1006/eesa.1993.1006
ANZECC (2000) Australian and New Zealand guidelines for fresh and marine water quality, Canberra. https://www.environment.gov.au/water/quality/publications/australian-and-new-zealand-guidelines-fresh-marine-water-quality-volume-1. Accessed 4 Oct 2017
Beiras R, Bellas J (2008) Inhibition of embryo development of the Mytilus galloprovincialis marine mussel by organic pollutants; assessment of risk for its extensive culture in the Galician Rias. Aquaculture 277:208–212. https://doi.org/10.1016/j.aquaculture.2008.03.002
Beiras R, Durán I, Bellas J, Sánchez-Marín P (2012) Biological effects of contaminants: Paracentrotus lividus sea urchin embryo test with marine sediment elutriates. ICES Techniques in Marine Environmental Sciences No. 51, 13pp, International Council for the Exploration of the Sea, Copenhagen, Denmark
Borthwick PW, Schimmel SC (1978) Toxicity of pentachlorophenol and related compounds to early life stages of selected estuarine animals. In: Rao KR (ed) Pentachlorophenol: chemistry, pharmacology, and environmental toxicology. Springer, Boston, MA, pp 141–146
CCME (2007) A protocol for the derivation of water quality guidelines for the protection of aquatic life Canadian Council of Ministers of the Environment 2007. http://ceqg-rcqe.ccme.ca/download/en/220. Accessed 13 Oct 2017
Davis HC, Hidu H (1969) Effects of pesticides on embryonic development of clams and oysters and on survival and growth of the larvae. Fish Bull 67:393–404
Durán I, Beiras R (2013) Ecotoxicologically based marine acute water quality criteria for metals intended for protection of coastal areas. Sci Total Environ 463:446–453. https://doi.org/10.1016/j.scitotenv.2013.05.077
Durán I, Beiras R (2017) Acute water quality criteria for polycyclic aromatic hydrocarbons, pesticides, plastic additives, and 4-Nonylphenol in seawater. Environ Pollut 224:384–391. https://doi.org/10.1016/j.envpol.2017.02.018
EC (2011) Technical guidance for deriving environmental quality standards. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance document n 27. Technical Report-2011-055. European Commission
Euro-Chlor (1999) Euro chlor risk assessment for the marine environment. OSPARCOM Region—North Sea. Pentachlorophenol. 43pp, OSPARCOM Region North-Sea, Brussels, Belgium
Goodman G (2001) Pentachlorophenol. In: Krieger R (ed.) Handbook of pesticide toxicology, vol. 2. 2 edn. Academic Press, San Diego, pp 1481–1509
His E, Seaman MNL, Beiras R (1997) A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Res 31:351–355. https://doi.org/10.1016/S0043-1354(96)00244-8
Hunt JW, Anderson BS, Tudor SL, Stephenson MD, Puckett HM, Palmer FH, Reeve M (1996) Marine bioassay project eighth report: refinement and implementation of four effluent toxicity testing methods using indiginous marine species. California State Water Resources Control Board Report Number 96-4WQ, Sacramento, CA, 109pp
ISO (2006) Water quality. Marine algal growth inhibition test with Skeletonema sp. and Phaeodactylum tricornutum ISO 10253:2006. International Organization for Standardization, Geneva
Muir J, Eduljee G (1999) PCP in the freshwater and marine environment of the European Union. Sci Total Environ 236:41–56. https://doi.org/10.1016/S0048-9697(99)00281-8
Murado MA, González MP, Vázquez JA (2002) Dose–response relationships: an overview, a generative model and its application to the verification of descriptive models. Enzym Microb Technol 31:439–455. https://doi.org/10.1016/S0141-0229(02)00109-6
Newman MC (2017) Fundamentals of ecotoxicology, 4th ed. CRC Press, Boca Raton, 654pp
OECD (1995) Guidance document for aquatic effects assessment. OECD Environment Monographs No 92. Organisation for Economic Co-Operation and Development, Paris
OECD (1998) OECD series on testing and assessment. Number 10: report of the OECD Workshop on Statistical Analysis of Aquatic Toxicity Data. Organisation for Economic Co-Operation and Development, Paris
OECD (2006) Guidelines for the testing of chemicals No 201. Freshwater alga and cyanobacteria, growth inhibition test. Organisation for Economic Co-operation and Development, París
Onikura N, Nakamura A, Kishi K, Taniguchi K, Yagi M, Oikawa S (2007) Hatching inhibition test using the Japanese whiting Sillago japonica as an acute toxicity test for marine fish species. Aquac Sci 55:293–300. https://doi.org/10.11233/aquaculturesci1953.55.293
Palau-Casellas A, Hutchinson TH (1998) Acute toxicity of chlorinated organic chemicals to the embryos and larvae of the marine worm Platynereis dumerilii (Polychaeta: Nereidae) Environ Toxicol Water Qual 13:149–155. https://doi.org/10.1002/(SICI)1098-2256(1998)13:2149::AID-TOX63.0.CO;2-7
Pérez S, Rial D, Beiras R (2015) Acute toxicity of selected organic pollutants to saltwater (mysid Siriella armata) and freshwater (cladoceran Daphnia magna) ecotoxicological models. Ecotoxicology 24:1229–1238. https://doi.org/10.1007/s10646-015-1489-6
Reiley MC, Stubblefield WA, Adams WJ, di Toro DM, Hodson PV, Erickson RJ, Keating FJ, Jr. (2003) Reevaluation of the state of the science for water-quality criteria development. Proceedings from the Pellston Workshop, Fairmont Hot Springs, Montana, USA, 25–30 June 1998. Society of Environmental Toxicology and Chemistry (SETAC), Pensacola
Saco-Álvarez L, Durán I, Ignacio Lorenzo J, Beiras R (2010) Methodological basis for the optimization of a marine sea-urchin embryo test (SET) for the ecological assessment of coastal water quality. Ecotoxicol Environ Saf 73:491–499. https://doi.org/10.1016/j.ecoenv.2010.01.018
Smith EP, Cairns J (1993) Extrapolation methods for setting ecological standards for water quality: statistical and ecological concerns. Ecotoxicology 2:203–219. https://doi.org/10.1007/BF00116425
Smith S, Furay VJ, Layiwola PJ, Menezes - Filho JA (1994) Evaluation of the toxicity and quantitative structure - activity Relationships (QSAR) of chlorophenols to the copepodid stage of a marine copepod (Tisbe battagliai) and two species of benthic flatfish, the flounder (Platichthys flesus) and sole (Solea solea). Chemosphere 28:825–836. https://doi.org/10.1016/0045-6535(94)90234-8
Tato T, Salgueiro-González N, León VM, González S, Beiras R (2017) Ecotoxicological evaluation of the risk posed by bisphenol A, triclosan, and 4-nonylphenol in coastal waters using early life stages of marine organisms (Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Acartia clausi). Environ Pollut. https://doi.org/10.1016/j.envpol.2017.09.031
US-EPA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. PB85-227049. Office of Research and Development. Environmental Research Laboratories, United States Environmental Protection Agency, Duluth, Minnesota
US-EPA (2017) National recommended water quality criteria - aquatic life criteria Table. https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table, Accessed 11 Oct 2017
van Dijk JJ, van der Meer C, Wijnans M (1977) The toxicity of sodium pentachlorophenolate for three species of decapod crustaceans and their larvae. Bull Environ Contam Toxicol 17:622–630. https://doi.org/10.1007/BF01685988
van Straalen NM, Denneman CAJ (1989) Ecotoxicological evaluation of soil quality criteria. Ecotoxicol Environ Saf 18:241–251. https://doi.org/10.1016/0147-6513(89)90018-3
Vighi M et al. (2003) Water quality objectives for mixtures of toxic chemicals: problems and perspectives. Ecotoxicol Environ Saf 54:139–150. https://doi.org/10.1016/S0147-6513(02)00047-7
Woelke CE (1972) Development of a receiving water quality bioassay criterion based on the 48-hour Pacific oyster (Crassostrea gigas) embryo. Washington Department of Fisheries Technical Report 9, 1–93
Acknowledgements
This work was funded by MINECO (Spanish Government) through the Research Projects PCIN-2015-187-C03-03 and CTM2016-77945-C3-1-R. The authors acknowledge Nuria Trigo and all the staff of ECIMAT for their helpful technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Beiras, R., Tato, T. Marine environmental risk assessment and acute water quality criterion for pentachlorophenol in coastal waters. Ecotoxicology 27, 803–808 (2018). https://doi.org/10.1007/s10646-018-1930-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-1930-8