Abstract
We assessed the contamination, dynamics, and health risks of the pesticides cyanazine, simetryn, fenarimol, isoprothiolane, diazinon, irgarol, fenitrothion, and diuron in marine samples (seawater, sediments, plankton, fish, and other edible organisms) at various locations in the Seto Inland Sea in Japan in 2016 and 2017. Pesticide concentrations were highest at sampling sites close to the coastline, and mean concentrations in seawater were slightly higher in surface water than in bottom water. All eight pesticides were detected in plankton. Diazinon concentrations (77–387 ng/g dw) were highest in sediments and cyanazine was the most frequently detected pesticide (88%, n = 17) in sediments. Only cyanazine (2.7–41.9 ng/g dw), simetryn (1.0–34.3 ng/g dw), and diazinon (6.3–308.8 ng/g dw) were detected in fish and other edible marine organisms. Based on the calculated bioconcentration factor, the results showed that plankton, fish, and marine animals bioaccumulated pesticides. The highest hazard quotients were calculated for diazinon in red seabream and greenling, indicating a possible risk to consumers. It is, therefore, imperative to promote strict implementation of pollution control, integrated pest management practices, and policy formulation on pesticides. Usage of diazinon must be controlled and monitored to ensure large residues do not reach aquatic ecosystems and marine coastlines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human and industrial activities have increased the use of chemicals. According to the National Institute of Environmental Studies of Japan (NIES 2017), pesticide use in Japan has increased over the past 20 years. Agrochemical pollution is a global concern because of its longevity in the environment, bioaccumulation, and hazardous effects on humans and other organisms. Cyanazine, simetryn, diazinon, fenarimol, isoprothiolane, diuron, and fenitrothion are triazine, organophosphate, phenyl-urea, pyrimidine, and dithiolane pesticides and listed under the Agricultural Chemical Regulation Law in Japan (FAMIC 2017). These chemicals are used predominantly to control weeds, insects, and fungi, while others are used as marine antifouling compounds (US National Library of Medicine 2016; FAMIC 2017; PPDB 2017; ORCERC 2017). Their environmental concentrations are largely attributable to contamination from agricultural, industrial, and urban activities (US-EPA 2000; Bloomfield et al. 2006; Kaonga et al. 2015a; Chidya et al. 2018). Runoff into aquatic ecosystems contaminates water bodies and can harm benthic and aquatic organisms (Ohyama et al. 1987; Japan Plant Protection Association – JPPA 2000; Bloomfield et al. 2006; Kaonga et al. 2015b; Derbalah et al. 2019a).
The Seto Inland Sea is the largest enclosed area of the Sea of Japan and provides important marine services such as commercial fishing, transportation, and tourism (EMECS 2007). The population of the Seto Inland Sea area numbers approximately 35 million, approximately 28% of the total population of Japan. The coastal areas of the Seto Inland Sea receive runoff from agriculture, local industries, and urban domestic wastewaters that alter the quality of the water and may affect aquatic organisms (Derbalah et al. 2003; Okamura et al. 2003; EMEC 2007). The contaminants flow into the sea from a large network of rivers and streams (EMECS 2007; Kaonga et al. 2015b, 2016, 2017).
Public awareness of pesticide residues in foods has led to a growing concern about food safety. Fish, lobster, prawns, mussels, and oysters are examples of seafood high in protein and other nutrients (Lartey 2017). One of the permanent and clear indicators of a high level of pollution in the aquatic environment in all its food chains is the presence of pesticide residues in fish and sediments (Kafilzadeh 2015). Uptake of chemical compounds by marine organisms may be induced via various pathways and may involve the dermis, gut, pulmonary surfaces, or gills (ECHA 2017; Inoue et al. 2012). Antifouling agents, phenyl herbicides, and organophosphates have all been detected in seawater, sediments, plankton, fish, and marine organisms from the Seto Inland Sea (Okamura et al. 2003; Balakrishnan et al. 2012; Kaonga et al. 2015b). Pesticide residues in the environment can exert adverse effects on both humans and aquatic organisms (EU 2014; Ministry of Environment-Japan 2013).
According to the pesticide database by NIES (2017), about 28, 164, 30, 348, 411, 0.79, and 76 tons were used in Japan in the year 2015 for cyanazine, diuron, simetryn, diazinon, fenitrothion, fenarimol, and isoprothiolane, respectively. Although the general trend in the usage of these pesticides appears to be decreasing, the amounts consumed per annum remain significantly high. Excessive use and exposure of pesticides through direct or indirect ingestion may pose risk to human health and aquatic organisms, hence a need for regular monitoring of the environment. Ecological and human health risk assessments of pesticides in the environment like rivers are significant to ensure the safety of aquatic organisms and humans (NIES 2017; PPDB 2017). Depending on the environmental dynamics and the excessive use of pesticides, it is important to monitor residues in water bodies and marine species. Furthermore, although pesticide residues are present in aquatic environments at low concentrations, they may exert a significant impact on microorganisms and human health through biomagnification (Okaichi and Yanagi 1997; PPDB 2017). Therefore, this work examined the pollution, spatio-temporal variations, dynamics, and health risks of eight pesticides (cyanazine, simetryn, fenarimol, isoprothiolane, diazinon, irgarol, fenitrothion, and diuron) in seawater, marine sediment, plankton, fish, and other marine organisms from the Seto Inland Sea. These pesticides were selected from reports in the literature because of their extensive use in Japan.
Materials and methods
Description of the study area
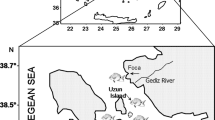
Figure 1 is a map of the study area in the Seto Inland Sea, which is encircled by three islands (EMECS 2007). The Seto Inland Sea and the coastal areas of these three islands form a region known as the Setouchi that serves as a watercourse, joining the Pacific Ocean to the Sea of Japan. The coastal area of the Seto Inland Sea includes tourist destinations and historic sites, and the sea is reported to contain marine species such as sweetfish (Plecoglossus altivelis), horseshoe crabs, the Indo-Pacific finless porpoise (Neophocaena phocaenoides), and the great white shark (Carcharodon carcharias). The sea is also known for the periodic incidence of red tides reportedly caused by an overgrowth of phytoplankton that results in the deaths of large numbers of fish in some coastal areas (EMECS 2007; Okaichi and Yanagi 1997). The region has a moderate climate, with relatively low rainfall, and steady year-round temperatures.
Eleven prefectures have coastlines on the Seto Inland Sea and Hiroshima, Osaka, and Kobe are the major cities with heavy industrial activity on the coast. The major industries are shipbuilding, steel, chemicals, textiles, vehicle manufacture, and oil refining. Furthermore, the area also serves ports for international and domestic cargo transportation (EMECS 2007).
The sea has a total shoreline of approximately 6,868 km with an average water depth of 38 m (EMECS 2007). The area of the Seto Inland Sea is about 23203 km2 and is located within the coordinates 34°10′0″ N and 133°20′0″ E. The sea receives water and chemical loads from several prefectures through a network of perennial rivers and streams namely Kako (Hyogo), Kurose (Hiroshima), Ashida (Hiroshima), Ota (Hiroshima), Yodo (Shiga), Yoshino (Ehime), Kino (Nara), Saba (Yamaguchi), and Yamato (Nara). These rivers and streams have river mouth, lengths (km), catchment area (km2), and population as follows: Kako river (Harima-nada, 96 km, 1,730 km2 and 0.820 million), Kurose river (Aki-nada, 51 km, 239 km2, 0.300 million), Ashida river (Bingo-Nada, 86 km, 870 km2, 0.273 million), Ota river (Hiroshima bay, 103 km, 1,710 km2, 0.980 million), Yodo river (Osaka bay, 75 km, 8,240 km2, 10.650 million), Yoshino river (Kii Channel, 194 km, 3,750 km2, 0.640 million), Kino river (Kii Channel, 136 km, 1,750 km2, 0.800 million), Saba river (Suou-Nada, 57 km, 432 km2, 0.03 million), and Yamato river (Osaka Bay, 68 km, 1,070 km2, 2.15 million) (https://www.mapsofworld.com/japan/river-map.html).
Sampling sites
The marine samples (seawater, sediments, plankton, and edible marine organisms) were collected during four cruises, conducted on July 4–8 and August 6–7, 2016, and on July 3–7 and August 5–6, 2017, using the Hiroshima University research ship Toyoshio Maru. The total number of sampling sites during the 2016 and 2017 cruises was 19 and 17, respectively.
During the July 2016 cruise, samples (seawater, fish and plankton) were collected from Aki-nada (St. 1–St. 3), Kii Channel (St. 5–St. 7), Osaka Bay (St. 8–St. 11 and St. 16), and Harima-nada (St. 12–St. 15). In August 2016 cruise, four sites were sampled from Hiroshima Bay (St. 1HB–St. 4HB; Fig. 1). In 2016 cruise, the sampling sites had the following characteristics: depth (10–70 m), salinity (27.0–34.41 PSU), EC (4.25–4.91 S/m), temperature (19.77–25.52 °C), pH (7.80–8.14), and DOC (0.69–1.72 mg C/L).
During the July 2017 cruise, marine samples (seawater, plankton, and sediment) were collected from Aki-nada (St. 1–St. 3), Kii Channel (St. 5), Osaka Bay (St. 8–St. 11 and St. 16), Harima-nada (St. 12–St. 15), Bisan Seto (B2), and Hiuchi-nada (B10). In August 2017 cruise, the seawater and plankton samples were collected from four sites in Hiroshima Bay (St. 1HB–St. 4HB). During the 2017 cruise, the sampling sites had the following characteristics: depth (11.8–64 m), salinity (30.84–33.44 PSU), EC (4.44–4.85 S/m), temperature (19.76–23.38 °C), and DOC (0.68–1.45 mg C/L).
Seawater sampling, preparation, and extraction
Seawater was collected using a 10-L Niskin sampler (General Oceanics, USA) by a Rosset system attached to conductivity, temperature, and depth sensors (SBE 9plus, Sea-Bird Scientific, USA). Surface water was collected to a depth of up to 5 m, while bottom water was collected at 10–65 m. After collection, the sample was filtered through glass fiber filter paper (GC-50 pore size 0.45 μm, 47 mm, Advantec MFS, Inc., USA) and transferred to 1-L amber glass bottles for extraction. Sample filtration, analyte extraction, and analysis were conducted following a modification of our previously validated method (Chidya et al. 2018).
Plankton sampling, preparation, and extraction
Plankton was collected with a vertical plankton net (100-μm mesh size, North Pacific Standard-NORPAC, model NXX13) fitted with a flow meter (Rigosha Co. Ltd., Japan). The plankton was transferred into 250-mL plastic bottles and filtered on board using pre-weighed glass fiber filters (GC-50 pore size 0.45 μm, 47 mm), and dried using a freeze-dryer (EYELA, FDU-506, Tokyo, Japan). Next, the filter papers were weighed again, cut into small pieces, transferred into 100-mL volumetric flasks containing methanol (30 mL), and homogenized by a mechanical multi-shaker (EYELA, MMS 310) for 1 h at 254 rpm. The contents were then transferred into a separatory funnel, followed by the addition of 5% sodium chloride (300 mL) and 50 mL of dichloromethane. After gentle shaking of the separatory funnel, the contents were allowed to settle and the organic layer was transferred into conical flasks. Anhydrous sodium sulfate was added to remove water. The organic extract was filtered into a round-bottom flask and concentrated under a rotary evaporator at 40°C to approximately 5 mL. The extract was further concentrated under a stream of nitrogen gas until almost dry, after which the residue was redissolved in acetone (1 mL). Finally, the extract was evaporated to approximately 100 μL and diluted to 2 mL with methanol-ultrapure water (1:1, v/v). The target compounds were analyzed using the same HPLC system used for analyzing seawater.
Sediment sampling, preparation, and extraction
The core sediment samples were collected by the core sampler (HR type, Rigo Saitama, Japan) and surface sediments were collected using a Smith-McIntyre sampler (sampling area 33 × 33 cm2) (Risosha & Co. Ltd., Japan). The samples were immediately transferred into polyethylene bags and preserved at 4°C in the dark. Wet sediment samples were transferred into Petri dishes and dried using a freeze-dryer (EYELA, FDU-506). Each dried sample (10 g) was transferred into a 100-mL flask containing acetonitrile (35 mL) and anhydrous sodium sulfate and homogenized with a mechanical shaker (EYELA, MMS 310) for 1 h. The contents were allowed to settle for 30–60 min. The organic layer was filtered and transferred into a round-bottom flask and pre-concentrated to 5 mL using a rotary evaporator at 40°C. Sample clean-up was performed using preconditioned solid-phase extraction cartridges (Waters OASIS HLB 60 mg/3 cc). The analyte extraction and analysis were also conducted following of our previously validated method (Chidya et al. 2018).
Fish sampling, preparation, and extraction
Edible fish and marine organisms were caught in the Seto Inland Sea during the 2016 cruise. Other seafood samples (unfrozen) were purchased from a local shop at Higashi-Hiroshima Seikyo CO-OP in 2018. The average length and wet weight of the smallest fish were 22 cm and 224 g, respectively. The average length and wet weight of larger fish were 31 cm and 790 g, respectively. The fillet and whole fish samples (20 g wet weight) were cut into small pieces and homogenized using a blender (model 1-800, Blendtec, Orem, USA) after the addition of methanol (100 mL). Organs such as the liver, gills, and viscera were processed without removing their internal contents. Next, the contents were transferred into conical flasks (100 mL) and shaken with a mechanical shaker (EYELA, MMS 310) for 1 h. Contents were then centrifuged for 30 min at 3,000 rpm (KN-70, Kubota Corporation, Japan). The supernatant was filtered into 1-L amber bottles using DISMIC disposable membrane filters (25HP020AN, 0.20 μm; Advantec). Finally, the pesticides were extracted and preconcentrated following a procedure similar to that of extraction from water (Chidya et al. 2018).
Pesticide analysis, recovery, and quality control
Isocratic (HPLC) elution
A reversed-phase HPLC system (LC-10i chromatograph, Shimadzu Corporation, Kyoto, Japan) connected to a UV-Vis light detector (SPD-10A, Shimadzu Corporation) was used for analyzing seawater. The HPLC system was equipped with a chromatographic column (Mightysil RP-18 GP, 250 mm × 4.6 mm internal diameter, particle size 5 μm) and a guard column (Mightysil LC-18, 20 mm × 4.0 mm internal diameter, 5 μm), both from Kanto Chemicals (Tokyo, Japan). A mobile phase of acetonitrile/ultrapure water (ratios 50/50 to 80/20) at 1.0 mL/min was used for the isocratic elution with an injection volume of 50 μL. The column oven temperature was adjusted and maintained at 40°C for the duration of the analysis. Analyte peaks were confirmed by co-injection of standard solutions and spiked samples. A maximal absorbance wavelength of 221 nm was used for fenarimol, cyanazine, and simetryn while for diazion, isoprothiolane, diuron, irgarol 1051, and fenitrothion were 247, 309, 254, 223, and 220 nm, respectively.
The limit of detection and limit of quantification were assessed by the residual standard deviation of the regression line (σ) and the slope (S) of the calibration curves (limit of detection: 3.3 × σ/S; limit of quantification: 10 × σ/S). The limits of detection for cyanazine, simetryn, fenarimol, isoprothiolane, diazinon, diuron, irgarol, and fenitrothion were 2, 6, 3, 6, 8, 4, 7, and 3 ng/L, respectively. The limits of quantification were 7, 19, 10, 20, 22, 12, 23, and 12 ng/L, respectively. Good linearity (r2 ≥ 0.995, n = 8), with an accuracy range of 99.81% ± 4.12% to 100.69% ± 4.2%, was obtained. The percent relative standard deviations of the intra-day (0.54–1.88%), inter-day (2.10–2.75%), and retention time (0.01–0.05%) precisions were satisfactory for the detection of pesticide residues at trace levels. When 0.1 mL of each pesticide (1,000 μg/L) was added to a 1 L seawater sample (n = 3), the recovery rates were 70–122%.
Gradient (HPLC) elution
Plankton and sediment samples were analyzed using a reversed-phase Prominence HPLC gradient system equipped with a degassing unit (DGU-20A5R), column oven (CTO-20A), and SPD-20A Prominence UV-Vis light detector from Shimadzu. The HPLC gradient system used a chromatographic column (COSMOSIL 5C18–MS-II 4.6 mm internal diameter × 250 mm, particle size 5 μm) fitted with a guard column (Mightysil LC-18, 20 mm × 4.0 mm internal diameter, 5 μm), both from Nacalai Tesque (Kyoto, Japan). A mobile phase of acetonitrile/ultrapure water with a gradient elution program was used with a flow rate of 1.0 mL/min and a temperature of 40°C. The operating wavelength of the ultraviolet/visible light detector system was automatically programmed alongside the gradient program. Data acquisition was made using Chromato-PRO software and a USB data acquisition unit (model USB Marina, S/N: MB113328) purchased from Runtime Instruments, Tokyo, Japan.
Bioconcentration factors
Bioconcentration and bioaccumulation are commonly assessed using bioconcentration factors (BCFs) in liters per kilogram, octanol-water partition coefficients (KOW), bioaccumulation factors, and biota-sediment accumulation factors. In this study, the number of pesticide residues concentrated by an organism from seawater was derived by calculating the BCF in a steady-state using Eq. 1.
where Corg and Cwater represent the concentration of pesticide residues in the organism (mg/kg) and the concentration of pesticide residues in water (mg/L), respectively (Gobas 2000; Landis et al. 2011). The BCF values obtained were correlated with the KOW of each pesticide to assess their potential to bioaccumulate in plankton and fish. This was used to evaluate the hydrophobicity and hydrophilicity of the organic compounds in the water environment. According to the United States Environmental Protection Agency, under the Toxic Substances Control Act, a substance is not bioaccumulative if it has a BCF below 1,000, bioaccumulative if the BCF is 1,000–5,000, and very bioaccumulative if the BCF is >5,000 (Gobas 2000; ECHA 2017). Similarly, according to the Japanese Chemical Substances Control Law, a highly bioaccumulative substance has a BCF >5,000. Under the regulations of the European Union (http://register.consilium.eu.int), a bioaccumulative substance has a BCF >2,000 and a very bioaccumulative substance has a BCF >5,000 (NITE 2018).
Health risk assessment of pesticides in edible fish and marine organisms
To evaluate the potential health risks posed by the consumption of organisms from the Seto Inland Sea, the estimated daily intake was computed from a commonly used protocol (FAO/WHO 2010; Buah-Kwofie et al. 2018; Akoto et al. 2016) as Eq. 2.
where C is the measured average pesticide concentration (ng/g wet weight); FR is the average daily intake of fish, estimated at 68.5 g day for adults (Global Fish Alliance 2010); and BW is the hypothetical average body weight (60 kg for adults) (IPCS 2002). Consequently, the health risk to consumers from intake of pesticide-contaminated fish via a single pathway was estimated using the hazard quotient (HQ) approach (US EPA 1991). Thus, the HQs were obtained by dividing the estimated daily intake by the acceptable daily intake (US EPA 1991; FAO/WHO 2010) assumed to be a safe concentration for lifetime exposure. For a primary quantifiable risk assessment, an HQ ≤0.2 is considered to indicate negligible adverse health effects of exposure, while HQ values exceeding this threshold require a detailed risk assessment or risk management measures (Health Canada 2004).
Results and discussion
Concentrations and spatial distribution of pesticides in seawater
Tables 1 and 2 list pesticide concentrations in surface and bottom seawater samples collected in 2016 and 2017, respectively. Mean concentrations of the majority of pesticides were slightly higher in surface seawater but not to a significant extent, owing to the continuous mixing of the seawater.
In samples collected in 2016, the maximal concentrations in surface water for cyanazine, simetryn, fenarimol, isoprothiolane, diazinon, diuron, irgarol, and fenitrothion were 27, 29, 8, 3, 72, 65, 35, and 82 ng/L, respectively (Table 1). In bottom water, the maximal concentrations for cyanazine, simetryn, fenarimol, isoprothiolane, diazinon, diuron, irgarol, and fenitrothion were 21, 33, 20, 13, 86, 61, 30, and 21 ng/L, respectively. The highest concentrations were of diazinon recorded at Hiroshima Bay. Fenarimol and isoprothiolane registered the lowest in the 2016 cruise. The frequency of detection was highest for cyanazine and diazinon (100%, n = 38 for surface and bottom seawater), simetryn (95%), and diuron (63%). Isoprothiolane and fenitrothion were the least frequently detected pesticides (19%) where most of the sites registered below detection.
During the 2017 cruise, the maximal concentrations in surface water of cyanazine, simetryn, fenarimol, diazinon, diuron, irgarol, and fenitrothion were 47, 238, 52, 187, 25, 31, and 49 ng/L, respectively (Table 2). Isoprothiolane concentrations were below the detection limits at all sites for both surface and bottom seawater. The maximal concentrations in the bottom water of cyanazine, simetryn, fenarimol, diazinon, diuron, irgarol, and fenitrothion were 37, 172, 18, 181, 21, 23, and 54 ng/L, respectively. Similar to the 2016 findings, diazinon was higher than those of other pesticides in 2017 (mean: 106 and 97 ng/L for surface and bottom waters, respectively). Cyanazine, diazinon, and diuron were the most frequently detected pesticides (100%, total sample size n = 34 for surface and bottom water), followed by irgarol and simetryn (88%). Isoprothiolane and fenitrothion were the least frequently detected pesticides (19%).
Cyanazine concentrations generally decreased with distance from Aki-nada and Harima-nada, but no clear trend for cyanazine was observed at Hiroshima Bay (p > 0.05). Irgarol concentrations also decreased with distance from Aki-nada, indicating that seawater pollution was derived from land sources. In the 2016 cruise, irgarol concentrations were below the detection limit at Hiroshima Bay, attributable to dilution. The concentrations of diuron decreased with distance from the coastal areas of Osaka Bay, an area where we generally measured the highest herbicide concentrations, particularly at site 16 (diuron: 63 ng/L). A report on higher pesticide concentrations in Osaka Bay is consistent with previous findings (Kaonga et al. 2015b; Balakrishnan et al. 2012).
The mean concentrations of diuron (13–19 ng/L), irgarol (12–20 ng/L), and fenitrothion (15–36 ng/L) in both surface and bottom water were similar to those previously reported by Kaonga et al. (2015b). Generally, higher concentrations of cyanazine, simetryn, diazinon, and irgarol were found in 2017 than in 2016. However, isoprothiolane and diuron were slightly higher in 2016 than in 2017. Fenarimol and fenitrothion concentrations did not vary by sampling year (p > 0.05). Isoprothiolane concentrations were below the detection limit at all sites in 2017, and this was consistent with substantially lower use in Japan over the past decade (NIES 2017). Generally, pesticide concentrations exhibited no clear trends in surface and bottom seawater, likely because of mixing.
Figure 2 shows the average usage (tons) of pesticides in 11 prefectures around Seto Inland Sea over the period 2012 to 2016 (NIES 2017). Cyanazine has been highly used in Osaka prefecture (3.6 tons), followed by Hiroshima (3.2 tons) and Fukuoka (2.1 tons). The least amounts of cyanazine (< 0.1 tons) were used in Kagawa, Oita, Yamaguchi, Wakayama, and Tokushima prefectures. For Simetryn, relatively high amounts were used in Hyogo prefecture followed by Hiroshima (0.49 tons) and Okayama (0.19 tons) while the rest of the prefectures registered < 0.04 tons. Comparatively, fenarimol was highly used in Fukuoka (0.04 tons), Wakayama (0.023 tons), Okayama (0.021 tons), and Hiroshima (0.018 tons) prefectures. Okayama (1.79 tons) registered high usage of isoprothiolane followed by Oita (1.46 tons) and Fukuoka (1.30 tons). Notably, diazinon was highly used in Hiroshima prefecture (9.1 tons) followed by Fukuoka (8.70 tons) and Tokushima (7.93 tons) while Kagawa prefecture (0.83 tons) was the least. Fenitrothion was highly used in Fukuoka prefecture (21.85 tons) followed by Hiroshima (13.60 tons) and Wakayama (11.92 tons). Diuron—used both as herbicide and an antifouling agent—was highly used in Hyogo prefecture (3.61 tons) followed by Osaka (2.80 tons). Within this period (2012–2016), fenitrothion was generally the highest used pesticide (1.69–21.85 tons) followed by diazinon (0.83–9.1 tons) and diuron (0.09–3.61 tons). Such distribution and usage of these pesticides may influence the amounts of pesticide residues detected in Seto Inland Sea (Derbalah et al. 2019b).
Pesticide concentrations and BCFs in plankton
Table 3 lists pesticide concentrations in plankton, which ranged from below the limit of detection to 724 ng/g dry weight for diuron in 2016 and below the limit of detection to 2,497 ng/g dry weight for diazinon in 2017. Diuron and irgarol were the most frequently detected pesticides in plankton during both sampling periods, while cyanazine, simetryn, and diazinon were all detected (100%, n = 13) in the 2017 samples. Similar to concentrations in seawater, isoprothiolane was the least frequently detected pesticide in plankton (21% in 2016, n = 19). The highest pesticide concentration in plankton was generally measured in samples from Osaka Bay, followed by Harima-nada, and this is consistent with previous findings (Balakrishnan et al. 2012; Kaonga et al. 2015b). We found a decreasing trend for the concentrations of all pesticides in plankton at both Osaka Bay and Harima-nada.
Table 4 lists the means and ranges of pesticide BCFs in plankton. Mean BCFs were generally lower in 2016 than in 2017. Concerning pesticides in seawater and plankton, the BCF decreased with distance from the coastal areas of Osaka Bay and Harima-nada. Plankton accumulated pesticides from water and dissolved and suspended substances. The BCFs within the order of 103–104 L/kg indicate pesticides with high hydrophobicity, consistent with previous findings (Kaonga et al. 2015b); Balakrishnan et al. 2012). The log-transformed Kow for cyanazine (2.24), simetryn (2.80), fenarimol (3.69), isoprothiolane (2.88), diazinon (3.81), diuron (2.90), irgarol (2.80), and fenitrothion (3.30) did not differ significantly (p > 0.05) (Harino et al. 2007; FAMIC 2017; PPDB 2017). This explains why the BCF ranges were similar and this is consistent with previous findings in plankton from the Seto Inland Sea (Watanabe et al. 2004; Balakrishnan et al. 2012; Kaonga et al. 2015b). Diazinon had the highest BCF, perhaps because of its high log Kow. According to the International EMECS Centre (EMECS 2007) and Balakrishnan et al. (2012), the coastal areas of the Seto Inland Sea receive contaminant runoff from agriculture, domestic use, and local industries, explaining the high pesticide BCFs and levels of dissolved organic carbon in plankton near the coast.
Pesticides concentration in sediment samples
During the 2017 cruise, the maximal concentrations of pesticides in sediments were as follows: cyanazine 15 ng/g dry weight (dw) (at St.10), fenarimol 29 ng/g dw (at St. 5 and St. 8), isoprothiolane 46 ng/g dw (at St. 2HB), and diazinon 387 ng/g dw (at St. 12). Simetryn concentrations were below the limit of detection in sediments at all the sampling sites. Isoprothiolane was only detected at 5 sites namely St. 13 (22 ng/g dw), St. B10 (13 ng/g dw), St. St. 1HB (36 ng/g dw), St. 2HB (46 ng/g dw), and St. 4HB (14 ng/g dw). Cyanazine was the most frequently detected pesticide (88%, n = 17), followed by diazinon and fenarimol (77%). The mean concentrations of diazinon in coastal areas were highest at Osaka Bay (235 ± 19 ng/g dry weight) and Hiroshima Bay (290 ± 16 ng/g dry weight). Diazinon concentrations generally decreased with distance from the shore at Osaka Bay. Similarly, the highest mean concentrations of cyanazine were measured at Osaka Bay (11 ± 3.7 ng/g dry weight) and Hiroshima Bay (7.3 ± 2.4 ng/g dry weight). Herbicides such as diuron and irgarol have previously been reported in core sediments from the Seto Inland Sea (Balakrishnan et al. 2012; Kaonga et al. 2015b; Kaonga et al. (2017), indicating prolonged residence time and potential for accumulation (Kaonga et al. 2017). The fates of diuron, Irgarol 1051, and fenitrothion in the Seto Inland Sea were described by Kaonga et al. (2016). These chemicals can end up in marine organisms, sediment, and the open ocean. The antifouling agents diuron and irgarol have been found in sediment samples obtained from the Seto Inland Sea (Harino et al. 2005, 2007, 2009; Balakrishnan et al. 2012; Kaonga et al. 2015b; Kaonga et al. 2017), including in sediment cores up to 10 cm below the sea bottom (Harino et al. 2009).
Pesticide concentrations and BCFs in fish and other marine organisms
Only three pesticides (cyanazine, simetryn, and diazinon) were detected in fish and other marine organisms (Table 5). Diazinon was the highest (385 ng/g dw) in red seabream fish tissue (fillet) followed by cyanazine (31.9 ng/g dw) in the same sample. Concentrations were highest in red seabream, suggesting bioaccumulation and supported by the species’ bottom-feeding behavior. This fish inhabits shallow waters (10–200 m in depth) and reefs (Russell et al. 2014), feeding on benthic invertebrates such as echinoderms, worms, mollusks, and crustaceans, as well as on other fish. High concentrations of simetryn (34.3 ng/g dry weight) were detected in greenling. Both fenarimol and isoprothiolane were below the limit of detection in all samples, mirroring our findings in the other matrices sampled. Decapodiformes (Japanese flying squid) is a marine organism inhabiting open oceanic waters (5–27°C) in the upper layers (100–500 m) that generally feeds on phytoplankton, zooplankton, and fish (FAO 1984). Similarly, cuttlefish is a benthic organism that inhabits tropical or temperate ocean waters (shallow sublittoral zone to 200 m in depth) and feeds on crabs, fish, and small shrimp (Buczacki 2002). Japanese horse mackerel (Trachurus japonicus) is a pelagic, oceanodromous species that feed on fish, crustaceans, and cephalopods and is typically found at depths of 100–200 m (Smith-Vaniz et al. 2015). Comparably, the greenling is a bottom-feeding fish found in shallow waters (depth range 139–155 m) that also inhabits rocky coastal areas and artificial reefs (Masuda et al. 1984; FAO 1984). Unsurprisingly, bottom-feeders bioaccumulate pesticides. The fillets generally contained higher concentrations of pesticides than the gills, liver, or intestines.
Health risk assessment of pesticides in seafood
Table 6 gives the HQs of pesticides detected in the organisms sampled. The HQ for cyanazine and simetryn did not exceed the threshold value of 0.20, suggesting that these species were safe for human consumption. The HQs for fenarimol and isoprothiolane were negligible, as their concentrations in the samples were below the limit of detection. However, the risk estimates presented here apply to healthy adults and not to children, the elderly, or individuals with medical conditions. The health hazard index for diazinon (HQ = 0.0034–0.2196) was relatively higher than that of cyanazine and simetryn. The health hazard indices of red seabream (HQ = 0.2196) and greenling (HQ = 0.1762) were close to the threshold value of 0.2, suggesting risk to consumers. The HQs for fenarimol and isoprothiolane were negligible since their concentrations in the fish and marine samples were below detection. However, the risk estimates presented herein do not include the vulnerable groups (like children and the elderly). The health hazard index for diazinon (HQ = 0.0034–0.2196) was relatively higher compared to cyanazine and simetryn. The red seabream (HQ = 0.2196) and greenling recorded (HQ = 0.1762) the highest health hazard index close to the threshold value of 0.2, hence posing a fair risk to consumers. Diazinon concentrations in red seabream and greenling were slightly higher than the maximal residue limits (100 ng/g) set by the Japan Food Chemical Research Foundation (2018) for edible foods or the United States Environmental Protection Agency pesticide upper limit in whole fish (100 ng/g dry weight). Consequently, a detailed risk assessment of these edible fish and marine organisms should be conducted with larger samples to ascertain their safety as food. However, the concentrations of cyanazine and simetryn were below these maximal residue limits. Isoprothiolane concentrations were below the maximal residue limit of 3,000 ng/g set by the Japan Food Chemical Research Foundation (2018). Limitations of the current risk assessment include potential dissimilarities in the quantity of fish consumed by residents and other sources and routes of exposure to these pesticides.
The BCF ranges for cyanazine, simetryn, and diazinon were 184–2,841, 25–858, and 5,540–9,125 L/kg, respectively (Table 6). Slightly higher BCFs were recorded by Kaonga et al. (2015b) in fish collected from the Seto Inland Sea. The differences could be attributed to the specific pesticides tested (the BCFs were determined for fenitrothion, diuron, and irgarol only in the Kaonga et al. 2015a study). In both cases, it is evident that pesticide residues are bioaccumulated in marine organisms. Transport of pesticide residues from aquatic ecosystems to open waters is reported to harm benthic and aquatic organisms (Ohyama et al. 1987; JPPA 2000; Bloomfield et al. 2006). In Japan, irgarol 1051 and diuron—the most frequently used antifouling agents—and other pesticide residues have been previously detected in various environmental and marine samples (Tanabe et al. 2001; Okamura et al. 2003; Harino et al. 2005; Lambert et al. 2006; Tsunemasa and Okamura 2011; Balakrishnan et al. 2012; Kaonga et al. 2015b; Kitada et al. 2008). We found the highest concentrations close to the coastline, in proximity to sources of effluents and wastewater from the household, municipal, and industrial use.
Conclusions
We identified various pesticides in the marine samples, and their concentrations were highest close to the coastlines. The mean concentration in surface water for the majority of the pesticides in seawater was slightly higher than that in the bottom water. The results of this study revealed the accumulation of pesticides in sediment and plankton as well as fish and marine animals, and may pose risk to aquatic organisms and human health, which reflects the need for more marine ecosystem protection and management practices. Pesticide detection in sediments shows that the Seto Inland Sea is an important sink, hence a need for more marine ecosystem protection and management practices. The health hazard index for diazinon indicates the need for a detailed risk assessment of seafood from this area to ascertain its safety for consumption. Periodical monitoring of pesticides in water bodies by advanced analytical methods must be done along with human health and ecotoxicological risk assessment. This is imperative for strict implementation of pollution control, integrated pest management practices, and policy formulation on pesticides. Usage of diazinon must be controlled and monitored to ensure large residues do not reach aquatic ecosystems and marine coastlines.
References
Akoto O, Azuure AA, Adotey KD (2016) Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. Springer Plus 5(1):1849. https://doi.org/10.1186/s40064-016-3544-z
Balakrishnan S, Takeda K, Sakugawa H (2012) Occurrence of diuron and irgarol in seawater, sediments and planktons of Seto Inland Sea, Japan. Geochem J 46(3):169–177. https://doi.org/10.2343/geochemj.1.0163
Bloomfield JP, Williams RJ, Gooddy DC, Cape JN, Guha P (2006) Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—a UK perspective. Sci Total Environ 369(1):163–177. https://doi.org/10.1016/j.scitotenv.2006.05.019
Buah-Kwofie A, Humphries MS, Pillay L (2018) Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso Wetland Park, South Africa. Sci Total Environ 621:273–281. https://doi.org/10.1016/j.scitotenv.2017.11.212
Buczacki S (2002) Fauna Britannica. Common cuttlefish (Sepia officinalis). The living world of molluscs (January, 2003). Hamlyn, London
Chidya RCG, Abdel-dayem SM, Takeda K, Sakugawa H (2018) Spatio-temporal variations of selected pesticide residues in the Kurose River in Higashi-Hiroshima city, Japan. J Environ Sci Health Part B 2018:1–13. https://doi.org/10.1080/03601234.2018.1473972
Derbalah ASH, Nakatani N, Sakugawa H (2003) Distribution, seasonal pattern, flux and contamination source of pesticides and nonylphenol residues in Kurose River water, Higashi-Hiroshima, Japan. Geochem J 37(2):217–232
Derbalah AS, Tahara K, Sakugawa H (2019a) Monitoring sources, discharges, and fluxes of, and assessing the risks from, pesticides in the Kurose and Ashida Rivers, Japan. Int J Environ Sci Technol 17:1035–1050. https://doi.org/10.1007/s13762-019-02525-x
Derbalah A, Chidya R, Jadoon W, Sakugawa H (2019b) Temporal trends in organophosphorus pesticides use and concentrations in river water in Japan, and risk assessment. J Environ Sci (China) 79:135–152. https://doi.org/10.1016/j.jes.2018.11.019
ECHA (2017) European Chemical Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment Chapter R. 11: PBT/vPvB Assessment. ISBN: 978-92-9495-839-6. https://doi.org/10.2823/12862
EMECS (2007) Environmental Conservation of the Seto Inland Sea. In: International EMECS Center. Asahi Print Co., Ltd., Japan
EU (2014) European Union (EU) Drinking Water Regulations (S.I No. 122 of 2104), Dublin
FAMIC (2017) Food and Agricultural Materials Inspection Centre (FAMIC). List of active ingredients, Japan http://www.acis.famic.go.jp/eng/ailist/index.htm. (Accessed July 2017)
FAO (1984) Species catalogue. Cephalopods of the world An Annotated and Illustrated Catalogue of Species of Interest to Fisheries. In: Roper Clyde FE, Sweeney MJ, Nauen CE. FAO Fish Synop 3(125)
FAO/WHO (2010) Pesticide residues in food and feed. In: Acceptable daily intake; Codex Alimentarius Commission. FAO/WHO Food Standards, Rome
Global Fish Alliance (2010) The importance of captured fisheries in Ghana. Available http://www.globalfishalliance.org. Accessed Nov 2017
Gobas FAPC, Morrison HA (2000) Bioconcentration and biomagnification in the aquatic environment. Handbook of property estimation methods for chemicals 189–231
Harino H, Mori Y, Yamaguchi Y, Shibata K, Senda T (2005) Monitoring of antifouling booster biocides in water and sediment from the port of Osaka, Japan. Arch Environ Contam Toxicol 48(3):303–310. https://doi.org/10.1007/s00244-004-0084-2
Harino H, Yamamoto Y, Eguchi S, Kurokawa Y, Arai T, Ohji M, Okamura H, Miyazaki N (2007) Concentrations of antifouling biocides in sediment and mussel samples collected from Otsuchi Bay, Japan. Arch Environ Contam Toxicol 52(2):179. https://doi.org/10.1007/s00244-006-0087-2
Harino H, Iwasaki N, Arai T, Ohji M, Miyazaki N (2009) Occurrence of antifouling biocides and fluorinated alkyl compounds in sediment core from deep sea: Suruga Bay, Tosa Bay, and Nankai Tough, Japan. Arch Environ Contam Toxicol 57(4):661–669. https://doi.org/10.1007/s00244-009-9374-z
Health Canada (2004) Federal Contaminated Site Risk Assessment in Canada PartI: Guidance on Human Health Preliminary Quantitative Risk Assessment (PQRA)
Inoue Y, Hashizume N, Yoshida T, Murakami H, Suzuki Y, Koga Y, Takeshige R, Kikushima E, Yakata N, Otsuka M (2012) Comparison of bioconcentration and biomagnification factors for poorly water-soluble chemicals using common carp (Cyprinus carpio L.). Arch Environ Contam Toxicol 63(2):241–248. https://doi.org/10.1007/s00244-012-9761-8
IPCS (2002) Inventory of IPCS and other WHO Pesticide Evaluations and Summary of Toxicological Evaluations Performed by the Joint Meeting on Pesticide Residues (JMPR) : evaluations through 2002 7th ed. Available: http://apps.who.int/iris/handle/10665/67734. Accessed Feb 2017
Japan Food Chemical Research Foundation (2018) Maximum Residue Limits (MRLs) List of Agricultural Chemicals in Food. Determined bythe Minister of Health, Labour and Welfare. Available online: http://db.ffcr.or.jp/front/. Accessed Nov2017
JPPA (2000) Japan Plant Protection Association (JPPA). Noyaku Yoran, Tokyo
Kafilzadeh F (2015) Assessment of organochlorine pesticide residues in water, sediments and fish from Lake Tashk, Iran. Ach Life Sci 9:107–111. https://doi.org/10.1016/j.als.2015.12.003
Kaonga CC, Takeda K, Sakugawa H (2015a) Diuron, irgarol 1051 and fenitrothion contamination for a river passing through an agricultural and urban area in Higashi-Hiroshima city, Japan. Sci Total Environ 518(519):450–458. https://doi.org/10.1016/j.scitotenv.2015.03.022
Kaonga CC, Takeda K, Sakugawa H (2015b) Antifouling agents and fenitrothion contamination in seawater, sediment, plankton, fish and selected marine animals from the Seto Inland Sea, Japan. Geochem J 49(1):23–37. https://doi.org/10.2343/geochemj.2.0327
Kaonga CC, Takeda K, Sakugawa H (2016) Concentration and degradation of alternative biocides and an insecticide in surface waters and their major sinks in a semienclosed sea, Japan. Chemosphere 145:256–264. https://doi.org/10.1016/j.chemosphere.2015.11.100
Kaonga CC, Takeda K, Sakugawa H, Yamazaki H (2017) Pesticides and heavy metals in sediment core samples from a coastal area in Japan. Geochem J 51(7):525–536. https://doi.org/10.2343/geochemj.2.0489
Kitada Y, Kawahata H, Suzuki A, Oomori T (2008) Distribution of pesticides and bisphenol A in sediments collected from rivers adjacent to coral reefs. Chemosphere 71(11):2082–2090. https://doi.org/10.1016/j.chemosphere.2008.01.025
Lambert SJ, Thomas KV, Davy AJ (2006) Assessment of the risk posed by the antifouling booster biocides irgarol 1051 and diuron to freshwater macrophytes. Chemosphere 63(5):734–743
Landis WG, Sofield RM, Yu MH (2011) Introduction to environmental toxicology: molecular structures to ecological landscapes, 4th edn. Boca Raton, CRC Press, pp 117–162 ISBN 978-1-4398-0410-0
Lartey A (2017) Seafood as Part of a Healthy Diet. A Presentation at the Conference on Seafood and Health September 14-15, 2017 (Bergen, Norway)
Masuda HK, Amaoka C, Araga T, Uyeno T, Yoshino (1984) The fishes of the Japanese Archipelago, 1st edn. Tokai University Press, Tokyo, p 437 (text). (Ref. 559)
Ministry of Environment-Japan (2013) Environmental risk initial assessment of chemical substances. Chemical Reports for Diazinon, Isoprothiolane, Fenarimol, Simetryn and Cyanazine. Available: https://www.env.go.jp/chemi/report/h15-01/. Accessed March 2017
NIES (2017) National Institute of Environmental Studies (NIES). Agricultural Chemicals Database, Japan Available: https://www.nies.go.jp/kisplus/. Accessed March 2017
NITE (2018) National Institute of Technology and Evaluation (NITE). Chemical Management. Available: https://www.nite.go.jp/en/chem/qsar/cscl_data.html
Ohyama T, Jin K, Katoh Y, Chiba Y, Inoue K (1987) Fate and behavior of herbicides, butachlor, CNP, chlomethoxynil, and simetryn in river water, shellfish, and sediments of the Ishikari River. Bull Environ Contam Toxicol 39(4):555–562
Okaichi T, Yanagi T (eds) (1997) Sustainable development in the Seto Inland Sea, Japan – from the viewpoint of fisheries. Terra Scientific Publishing Company, Tokyo
Okamura H, Aoyama I, Ono Y, Nishida T (2003) Antifouling herbicides in the coastal waters of western Japan. Mar Pollut Bull 47(1-6):59–67. https://doi.org/10.1016/S0025-326X(02)00418-6
ORCERC (2017) Organization for Research and Communication on Environmental Risk of Chemicals. Japan. Available online: http://www.ecochemi.jp/PRTR.html. Accessed Feb 2017
PPDB (2017) Pesticide Properties Database (PPDB). University of Hertfordshire, Hertfordshire Available: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1295.htm. Accessed Feb 2017
Russell B, Pollard D, Mann BQ, Carpenter KE, Buxton CD, Liao W (2014) Pagrus major. The IUCN Red List of Threatened Species 2014: e.T170167A1286175. https://doi.org/10.2305/IUCN.UK.2014-3.RLTS.T170167A1286175.en. Accessed March 2017
Smith-Vaniz WF, Sidibe A, Nunoo F, Lindeman K, Williams AB, Quartey R, Camara K, Carpenter KE, Montiero V, de Morais L, Djiman R, Sylla M, Sagna A (2015) Trachurus trachurus. The IUCN Red List of Threatened Species
Tanabe A, Mitobe H, Kawata K, Yasuhara A, Shibamoto T (2001) Seasonal and spatial studies on pesticide residues in surface waters of the Shinano River in Japan. J Agric Food Chem 49(8):3847–3852. https://doi.org/10.1021/jf010025x
Tsunemasa N, Okamura H (2011) Effects of organotin alternative antifoulants on oyster embryo. Arch Environ Contam Toxicol 61(1):128–134. https://doi.org/10.1007/s00244-010-9598-y
US EPA (1991) Risk Assessment Guidance for Superfund Volume 1: Human Health Evaluation Manual (Part B), Development of Risk-Based Preliminary Remediation Goals(No. EPA/540/R-92/003)
US. National Library of Medicine (2016) Compound Summary for Diazinon, Isoprothiolane and Fenarimol. NCBI National Center for Biotechnology Information
US-EPA (2000) Drinking water standards and health advisories. U.S. Environment Protection Agency (US-EPA). Office of Water 4304. EPA 822-B-00-001
Watanabe M, Nakashima E, Nakatani N, Yamazaki H, Sakugawa H (2004) Distribution, behavior and historical trend of nonylphenol in Hiroshima Bay, Japan. Chikyukagaku (Geochemistry) 38:17–27
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was financed by the Japan Society for the Promotion of Science (JSPS) (KAKENHI Grant No. 16KT0149). We also recognize the Japanese Government Ministry of Education, Culture, Sports, Science and Technology–(MEXT) for the doctoral scholarship to Dr. Russel C.G. Chidya.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, methodology, data collection, and formal analysis were performed by Russel Chidya, Sakugawa Hiroshi, Sherif M. Abdel-Dayem, Aly Derbalah, Hiroaki Tsuji, Kazuhiko Takeda, and Chikumbusko C. Kaonga. The first draft of the manuscript was written by Russel Chidya and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chidya, R., Derbalah, A., Abdel-Dayem, S. et al. Contamination, dynamics, and health risk assessment of pesticides in seawater and marine samples from the Seto Inland Sea, Japan. Environ Sci Pollut Res 29, 67894–67907 (2022). https://doi.org/10.1007/s11356-022-20617-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20617-z