Abstract
Invasive species can exhibit allelopathic effects on native species. Meanwhile, the types of acid deposition are gradually changing. Thus, the allelopathic effects of invasive species on seed germination and growth of native species may be altered or even enhanced under conditions with diversified acid deposition. This study aims to assess the allelopathic effects (using leaves extracts) of invasive plant Solidago canadensis on seed germination and growth of native species Lactuca sativa treated with five types of acid deposition with different SO4 2− to NO3 − ratios (1:0, sulfuric acid; 5:1, sulfuric-rich acid; 1:1, mixed acid; 1:5, nitric-rich acid; 0:1, nitric acid). Solidago canadensis leaf extracts exhibited significantly allelopathic effects on germination index, vigor index, and germination rate index of L. sativa. High concentration of S. canadensis leaf extracts also similarly exhibited significantly allelopathic effects on root length of L. sativa. This may be due to that S. canadensis could release allelochemicals and then trigger allelopathic effects on seed germination and growth of L. sativa. Acid deposition exhibited significantly negative effects on seedling biomass, root length, seedling height, germination index, vigor index, and germination rate index of L. sativa. This may be ascribed to the decreased soil pH values mediated by acid deposition which could produce toxic effects on seedling growth. Sulfuric acid deposition triggered more toxic effects on seedling biomass and vigor index of L. sativa than nitric acid deposition. This may be attributing to the difference in exchange capacity with hydroxyl groups (OH−) between SO4 2− and NO3 − as well as the fertilizing effects mediated by nitric deposition. All types of acid deposition significantly enhanced the allelopathic effects of S. canadensis on root length, germination index, vigor index, and germination rate index of L. sativa. This may be due to the negatively synergistic effects of acid deposition and S. canadensis on seed germination and growth of L. sativa. The ratio of SO4 2− to NO3 − in acid deposition was an important factor that profoundly affected the allelopathic effects of S. canadensis on the seed germination and growth of L. sativa possibly because the difference in exchange capacity with hydroxyl groups (OH−) between SO4 2− and NO3 − as well as the fertilizing effects triggered by nitric deposition. Thus, the allelopathic effects of invasive species on seed germination and growth of native plants might be enhanced under increased and diversified acid deposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the continuing increase in human activities in recent decades, numerous regions have been seriously affected by environmental pollution, such as acid deposition; this phenomenon is especially serious in the three largest acid rain areas in the world (i.e., United States, Europe, and China) (Zhang et al. 2007; Wang and Xu 2009; Zhang and Chang 2012). Acid deposition is a global problem that triggers changes in ecosystem functions, such as the decrease of soil pH (Wang et al. 2010, 2011, 2012a; Lv et al. 2014), reduction in microbial function (Wang et al. 2010, 2011; Lv et al. 2014), alteration of forest species composition (Schaberg et al. 2001), and plant litter decomposition (Wang et al. 2010, 2011, 2012a; Lv et al. 2014). The major sources of acid deposition are sulfur dioxide (SO2) and nitrogen oxides (NO x ), which react with water molecules in the atmosphere to produce acids (Zhang et al. 2007). Since the late 1990s, the proportion of sulfate ion (SO4 2−) in precipitation has shown a decreasing tendency along with emerging policies on controlling and mitigating SO2 emissions and energy structure changes in China (Tu et al. 2005; Liu and Chen 2007; Lv et al. 2014). Meanwhile, the emission of NO x increased because of the enormous increase in the number of motor vehicles and the amount of fertilizer application. The relative contribution of nitrate ion (NO3 −) to acidification has subsequently increased in China (Tu et al. 2005; Liu and Chen 2007; Lv et al. 2014). Thus, the ratio of SO4 2− to NO3 − in precipitation in China has decreased, and the type of acid deposition is changing from acid rain dominated by sulfuric acid rain to acid rain with a mixture of sulfuric and nitric acids (mixed acid rain, MAR), and then to acid rain dominated by nitric acid (NAR) in China (Xu and Ji 2001; Tu et al. 2005; Liu and Chen 2007; Lv et al. 2014). The change in acid deposition types further complicates the ongoing challenge of ecosystem functions (Xu and Ji 2001; Lv et al. 2014). Thus, a single type of acid deposition experiment cannot provide a precise image of its ecological effects.
As one of the major global environmental problems, biological invasion has incurred serious threats to native ecosystems; in particular, changing the structure and/or functions of the ecosystems in which these invasions occur (Powell et al. 2013; Si et al. 2013; Yuan et al. 2013). Numerous studies have suggested that certain plants successfully invade certain environments because such species could release toxic chemicals that have remarkably allelopathic effects on the seed germination and growth of native species (Yang et al. 2007; Abhilasha et al. 2008; Sun and He 2010; Yuan et al. 2013). As one of the most destructive and widespread invasive species, Solidago canadensis is now widely distributed throughout most provinces in China, particularly in Southern China (Abhilasha et al. 2008; Yang et al. 2008; Zhao et al. 2015). Meanwhile, increasing amounts of anthropogenic acid precipitation are also deposited into ecosystems in China (Wang et al. 2007; Zhang et al. 2007; Wang and Xu 2009; Zhang and Chang 2012). As natural atmospheric acid deposition involves various components and the types of acid deposits may change in the future (Xu and Ji 2001; Tu et al. 2005; Liu and Chen 2007; Lv et al. 2014), diversification in the type of anthropogenic acid deposition may complicate the invasion mechanisms of invasive plants. Thus, the increasing degree of plant invasion poses a question of the potential allelopathic effects of the notorious invader S. canadensis on seed germination and growth of native species, especially under different types of acid deposition. Most studies on the effects of acid deposition on the allelopathic effects of invasive plants have focused on the effects of a single acid rain type with different acidity gradients (Wang et al. 2012a, b). However, knowledge on the effects of different types of acid deposition (especially MAR) on the allelopathic effects of invasive plants on the seed germination and growth of native species is limited.
S. canadensis is a herbaceous perennial plant of the Asteracea family. This plant is native to North America. S. canadensis was introduced to Shanghai, China, as a horticultural plant in the early 1930s. At present, the species has become naturalized in most of China and is one of the most destructive and widespread invasive species in the country. S. canadensis poses a serious threat to the diversity and/or abundance of native plants and agricultural productivity in the country (Abhilasha et al. 2008; Yang et al. 2008; Zhao et al. 2015). To date, the species has been established in Europe, large parts of Asia, Australia, and New Zealand (Weber 2001; Lu et al. 2007) and grows mainly in meadows and pastures, along roads, ditches, upland forests, savannas, and limestone glades (Abhilasha et al. 2008). S. canadensis is a highly aggressive plant. Once established, the plant can reduce species diversity or locally out-compete all native plants (Abhilasha et al. 2008). The severe allelopathic effects of S. canadensis on native species is cited as a main reason for its successful invasion (Yang et al. 2007; Abhilasha et al. 2008; Sun and He 2010; Yuan et al. 2013). Meanwhile, Lactuca sativa (Asteracea) seeds are sensitive to allelochemicals (Jefferson et al. 2003; Hao et al. 2007). This study addressed the following hypotheses: (1) S. canadensis exhibit allelopathic effects on seed germination and growth of L. sativa (Yang et al. 2007; Abhilasha et al. 2008; Sun and He 2010; Yuan et al. 2013); (2) acid deposition has negative effects on seed germination and growth of L. sativa (Fan and Wang 2000), and such effects vary with acid deposition types; and (3) the allelopathic effects of S. canadensis on seed germination and growth of L. sativa may be enhanced under acid deposition (Wang et al. 2012a, b), and such effects vary with acid deposition types.
Materials and methods
Materials
In this study, S. canadensis was selected as the invasive species. Leaves of S. canadensis were collected from Zhenjiang (32.20°N, 119.51°E), China. The leaf samples were washed, air dried for 48 h and then stored at 4 °C for further study. L. sativa seeds, which were selected as the native seeds, were bought from a local vegetable market.
Leaf extract preparation
Air-dried leaves of S. canadensis (80 g) were placed in flasks containing 800 mL distilled water and were soaked for 48 h at room temperature. Extracts were strained through cheese cloth and two layers of filter paper to remove solid material. The stock solution (100 g L−1) was stored at 4 °C for further study. Dilutions were made with distilled water prior to use to create a series with gradient contents, namely, CK (control, 0 g L−1), LE1 (25 g L−1), and LE2 (50 g L−1).
Experimental design
Experiments were performed by incubation in Petri dishes. L. sativa seeds were surface-sterilized (1 % NaClO for approximately 15 min) and then thoroughly washed thrice with deionized water. Thirty whole seeds of L. sativa were placed in a 9 cm Petri dish and covered with two layers of filter paper. The seeds were treated with 10 mL of S. canadensis leaf extracts or sterile deionized water (CK). In this experiment, we used a full-factorial experimental design with three contents of S. canadensis leaf extracts × five types of simulated acid deposition. Five types of simulated acid deposition were prepared by mixing 0.5 M L−1 H2SO4 and 0.5 M L−1 HNO3 at ratios of 1:0 (sulfuric acid, SA), 5:1 (sulfuric-rich acid, SRA), 1:1 (mixed acid, MAR), 1:5 (nitric-rich acid, NRA), and 0:1 (nitric acid, NA). For MAR, the pH value of basic solution was finally buffered to 5.6 by adding the stock solutions of H2SO4 and HNO3 at a ratio of 1:1. For the four other types of simulated acid deposition, the pH values of basic solution were finally buffered to 5.0 by adding the five stock solutions. In this study, pH 5.6 and the ratio of 5:1 for SO4 2− and NO3 − were the natural values of unpolluted rainfall, and pH 5.0 was the approximate annual average pH value of rainfall in the study site (Wang et al. 2007). The samples were incubated in a climate-controlled incubator at 25 °C with diurnal lighting (light intensity was set at 2200 Lux on a 12:12 h light–dark cycle) for 7 days Five replicates were performed per treatment. During the incubation, deionized water, simulated acid rain, and/or S. canadensis leaf extracts were supplied daily according to the amount of precipitation in the study site. The number of germinated seeds was counted daily at incubation time, and each seed was considered to have germinated when the radicle emerges.
Data measurements
Ten seedlings per Petri dish were selected at random for measurement on the same day. Seedling biomass (BM, fresh weight), root length (RL), seedling height (H), germination rate (GR), germination potential (GP), germination index (GI), vigor index (VI), and germination rate index (GRI) of L. sativa were determined.
BM was determined by using an electronic balance with an accuracy of 0.001 g.
RL and H were measured by using a ruler.
GR was calculated by using the ratio of the final numbers of seed germination to the total numbers of seeds when no new germination occurred after 7 days of incubation.
GP was determined by dividing the numbers of seed germination in the third day by the total numbers of seeds.
GI was calculated by using the following equation: GI = ∑ G i /I, where G i is the number of germination in I [the time after cultivation (day)] (Schmer et al. 2012).
VI was determined by using the following equation: VI = GI × BM (Lin et al. 2000).
Germination rate index (GRI) was calculated using the following equation: GRI = GR × GI (Steinmaus et al. 2000).
Statistical analysis
Data were evaluated to determine the deviations from normality and homogeneity of variance prior to data analysis. Differences among various dependent variables were assessed by using analysis of variance between groups, followed by multiple comparisons by using SPSS (version 17.0). Statistically significant differences were set at P values equal to or lower than 0.05.
Results
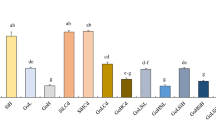
GI, VI, and GRI of L. sativa decreased significantly with increasing concentrations of S. canadensis leaf extracts (Fig. 1, P < 0.05). Meanwhile, RL of L. sativa was also significantly reduced at LE2 treatment (Fig. 1, P < 0.05). While, S. canadensis leaf extracts did not significantly affect BM, H, GR, and GP of L. sativa (Fig. 1, P > 0.05).
Indices of L. sativa with different treatments. Data with different letters indicate a significant difference (P < 0.05). BM Seedling biomass (fresh weight) (g), RL root length (cm), H seedling height (cm), GR germination rate, GP germination potential, GI germination index, VI vigor index, GRI germination rate index
All types of acid deposition exhibited significantly negative effects on RL, H, and VI of L. sativa (Fig. 1, P < 0.05). Meanwhile, all types of acid deposition except NA exhibited significantly negative effects on BM of L. sativa (Fig. 1, P < 0.05). In addition, all types of acid deposition except SA also exhibited significantly negative effects on GI and GRI of L. sativa (Fig. 1, P < 0.05). All types of acid deposition did not exert significant effects on GR and GP of L. sativa (Fig. 1, P > 0.05). BM and VI of L. sativa under NA treatment were significantly higher than those under SA treatment (Fig. 1, P < 0.05). However, the other germination and seedling growth indices of L. sativa were not significantly different between NA treatment and SA treatment (Fig. 1, P > 0.05). GI of L. sativa under NRA treatment was significantly lower than that under SRA treatment (Fig. 1, P < 0.05). Nevertheless, the difference in the other germination and seedling growth indices of L. sativa between NRA treatment and SRA treatment was not significant (Fig. 1, P > 0.05).
All types of acid deposition significantly enhanced the allelopathic effects of S. canadensis leaf extracts on RL, GI, VI, and GRI of L. sativa (Fig. 1, P < 0.05). SA, SRA, and NRA treatments significantly enhanced the allelopathic effects of S. canadensis leaf extracts on BM of L. sativa compared with LE2 treatment (Fig. 1, P < 0.05). Similarly, SA treatment significantly enhanced the allelopathic effects of S. canadensis leaf extracts on BM of L. sativa compared with LE1 treatment (Fig. 1, P < 0.05). SA, NRA, and NA treatments significantly enhanced the allelopathic effects of S. canadensis leaf extracts on H of L. sativa compared with LE2 treatment (Fig. 1, P < 0.05). NRA and NA treatments also significantly enhanced the allelopathic effects of S. canadensis leaf extracts on GR of L. sativa compared with LE2 treatment (Fig. 1, P < 0.05). Similarly, NRA treatment significantly enhanced the allelopathic effects of S. canadensis leaf extracts on GR of L. sativa compared with LE1 treatment (Fig. 1, P < 0.05). All types of acid deposition did not significantly affect the allelopathic effects of S. canadensis leaf extracts on GP of L. sativa (Fig. 1, P > 0.05).
Discussion
As one of the most notorious invasive species in China, S. canadensis showed strong allelopathic effects on the growth of native species, and this characteristic served a key function in its successful invasion (Yang et al. 2007; Abhilasha et al. 2008; Sun and He 2010; Yuan et al. 2013). The results of this study also showed that S. canadensis leaf extracts significantly inhibited seed germination and growth of L. sativa. The result was consistent with the first hypothesis and previous studies (Yang et al. 2007; Abhilasha et al. 2008; Sun and He 2010; Yuan et al. 2013). Thus, according to the results, invasive plants could release allelochemicals into the invaded ecosystems and then trigger allelopathic effects on the seed germination and growth of native species to facilitate its further invasion process.
A previous investigator discovered that acid deposition could exert negative effects on seed germination and growth of plants (Fan and Wang 2000). The results of this study also showed that acid deposition depressed seed germination and the growth of L. sativa evidently. The inhibited seed germination and growth of L. sativa mediated by acid deposition may be ascribed to the decreased soil pH values which could produce toxic effects on plant growth, thereby accelerating the leaching of nutrients from plants (Zhang et al. 2007; Pabian et al. 2012). Meanwhile, the negative effects of acid deposition on seed germination and growth of L. sativa varied with the acid deposition types. In particular, sulfuric acid deposition triggered more toxic effects on seedling biomass and vigor index of L. sativa than nitric acid deposition. The result may be due to the difference in exchange capacity with hydroxyl groups (OH−) between SO4 2− and NO3 − (Christ et al. 1995; Lv et al. 2014). The phenomenon may also be ascribed to nitric deposition possibly exerting a fertilizing effect (Jacobsen et al. 1990). By contrast, sulfuric deposition does not exert a fertilizer effect (Jacobsen et al. 1990). The result was consistent with the second hypothesis, implying that the ratio of SO4 2− to NO3 − in acid deposition was an important factor that profoundly affected seed germination and growth of L. sativa. Previous studies also found that the ratio of SO4 2− to NO3 − in acid deposition could exert pronounced effects on ecological processes, such as soil microbial biomass (Neuvonen and Suomela 1990) and plant litter decomposition (Lv et al. 2014).
Previous studies showed that acid deposition enhanced the allelopathic potential of invasive species (Wedelia trilobata) and then facilitated its invasiveness (Wang et al. 2012a, b). This study achieved the same result. In particular, all types of acid deposition significantly enhanced the allelopathic effects of S. canadensis on root length, germination index, vigor index, and germination rate index of L. sativa. The phenomenon might be attributed to the following mechanisms. Firstly, in this study, acid deposition and allelopathic compounds all exhibited negative effects on seed germination and growth of L. sativa, thereby producing a synergistic effect by their interaction. Secondly, total phenolics (especially polyphenols) constitute some of the primary allelopathic compounds of S. canadensis (Zhang et al. 2011; Yuan et al. 2013). Low pH values induced by acid deposition could promote the activity of allelochemicals released by S. canadensis, which inhibited seed germination and growth of L. sativa presumably because environmental stress factors often enhance allelochemical production and thereby increase potential toxicity (Einhellig 1996; An 2005). Meanwhile, acid rain can also enhance the leaching of acid-soluble materials, such as polyphenols (Lee and Weber 1983). Thirdly, more recalcitrant material often formed after complexation between acid rain and polyphenols (Carreiro et al. 2000). Thus, the results of this study suggested that the allelopathic effects of invasive species on seed germination and growth of native species may be enhanced under acid deposition. Meanwhile, some types of acid deposition significantly enhanced the allelopathic effects of S. canadensis on seedling biomass, seedling height, and germination rate of L. sativa, respectively. Thus, the enhanced allelopathic effects of S. canadensis on the seed germination and growth of L. sativa under acid deposition varies with acid deposition types. This trend may be due to the difference in the exchange capacity with hydroxyl groups (OH−) between SO4 2− and NO3 − (Christ et al. 1995; Lv et al. 2014), as well as the fertilizing effects of nitric deposition but not sulfuric deposition (Jacobsen et al. 1990). Thus, the ratio of SO4 2− to NO3 − in acid deposition may perform an important function in the enhanced allelopathic effects of S. canadensis on seed germination and growth of L. sativa. The result agrees with the third hypothesis.
In general, the allelopathic effects of S. canadensis on seed germination and growth of native species may be enhanced under the increased and diversified acid deposition. Meanwhile, the ratio of SO4 2− to NO3 − in acid deposition is an important factor that profoundly affects the allelopathic effects of S. canadensis on seed germination and growth of L. sativa. In the future, the ratio of SO4 2− to NO3 − in acid deposition would change with changes in the natural ecosystem. The influence of the invasion process of invasive species via the variation in allelopathic effects on seed germination and growth of native species would also change.
References
Abhilasha D, Quintana N, Vivanco J, Joshi J (2008) Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J Ecol 96:993–1001
An M (2005) Mathematical modelling of dose-response relationship (hormesis) in allelopathy and its application. Nonlinearity Biol Toxicol Med 3:153–172
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Christ M, Zhang YM, Likens GE, Driscoll CT (1995) Nitrogen retention capacity of a northern hardwood forest soil under ammonium sulfate additions. Ecol Appl 5:802–812
Einhellig FA (1996) Interactions involving allelopathy in cropping systems. Agron J 88:886–893
Fan HB, Wang YH (2000) Effects of simulated acid rain on germination, foliar damage, chlorophyll contents and seedling growth of five hardwood species growing in China. For Ecol Manage 126:321–329
Hao ZP, Wang Q, Christie P, Li XL (2007) Allelopathic potential of watermelon tissues and root exudates. Sci Hortic 112:315–320
Jacobsen JS, Bethard T, Heller LI, Lassoise JP (1990) Response of Picea rubens seedlings to intermittent mist varying in acidity, and in concentrations of sulfur-, and nitrogen-containing pollutants. Physiologic Plant 78:595–601
Jefferson LV, Pennacchio M (2003) Allelopathic effects of foliage extracts from four Chenopodiaceae species on seed germination. J Arid Environ 55:275–285
Lee JJ, Weber DE (1983) Effects of sulfuric acid rain on decomposition rate and chemical element content of hardwood leaf litter. Can J Bot 61:872–879
Lin WX, Kim KU, Smin DH (2000) Rice allelopathic potential and its modes of action on barnyardgrass (Echinochloa crusgalli). Allelopathy J 7:215–224
Liu ZQ, Chen JL (2007) Research on present situation and development tendency of atmospheric environmental quality in China. Electr Power Environ Prot 23:23–27
Lu JZ, Weng ES, Wu XW, Weber E, Zhao B, Li B (2007) Potential distribution of Solidago canadensis in China. Acta Phytotaxonomica Sinica 45:670–674
Lv YN, Wang CY, Jia YY, Wang WW, Ma X, Du JJ, Pu GZ, Tian XJ (2014) Effects of sulfuric, nitric and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests in China. Appl Soil Ecol 79:1–9
Neuvonen S, Suomela J (1990) The effects of simulated acid rain on pine needle and birch leaf litter decomposition. Appl Soil Ecol 2:857–872
Pabian SE, Ermer NM, Tzilkowski WM, Brittingham MC (2012) Effects of liming on forage availability and nutrient content in a forest impacted by acid rain. PLoS ONE 7:e39755
Powell KI, Chase JM, Knight TM (2013) Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 339:316–318
Schaberg PG, DeHayes DH, Hawley GJ (2001) Anthropogenic calcium depletion: a unique threat to forest ecosystem health? Ecosyst Health 7:214–228
Schmer MR, Xue Q, Hendrickson JR (2012) Salinity effects on perennial, warm-season (C4) grass germination adapted to the northern Great Plains. Can J Plant Sci 92:873–881
Si CC, Liu XY, Wang CY, Wang L, Dai ZC, Qi SS, Du DL (2013) Different degrees of plant invasion significantly affect the richness of the soil fungal community. PLoS ONE 8:e85490
Steinmaus SJ, Timonthy SP, Jodie SH (2000) Estimation of base temperature for nine weed species. J Exp Bot 51:275–286
Sun ZK, He WM (2010) Evidence for enhanced mutualism hypothesis: Solidago canadensis plants from regular soils perform better. PLoS ONE 5:e15418
Tu J, Wang HS, Zhang ZF, Jin X, Li WQ (2005) Trends in chemical composition of precipitation in Nanjing, China, during 1992–2003. Atmos Res 73:283–298
Wang WX, Xu PJ (2009) Research progress in precipitation chemistry in China. Prog Chem 21:266–281
Wang TJ, Jiang F, Li S, Liu Q (2007) Trends in air pollution during 1996–2003 and cross-border transport in city clusters over the Yangtze River Delta Region of China. Terr Atmos Oceanic Sci 5:995–1009
Wang CY, Guo P, Han GM, Feng XG, Zhang P, Tian XJ (2010) Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci Total Environ 408:2706–2713
Wang CY, Han GM, Jia Y, Feng XG, Guo P, Tian XJ (2011) Response of litter decomposition and related soil enzyme activities to different forms of nitrogen fertilization in a subtropical forest. Ecol Res 26:505–513
Wang RL, Staehelin C, Dayan FE, Song YY, Su YJ, Zeng RS (2012a) Simulated acid rain accelerates litter decomposition and enhances the allelopathic potential of the invasive plant Wedelia trilobata (creeping daisy). Weed Sci 60:462–467
Wang RL, Rehman SU, Liang XT, Song YY, Su YJ, Baerson SR, Zeng RS (2012b) Effects of simulated acid rain on the allelopathic potential of invasive weed Wedelia trilobata. Allelopathy J 30:23–32
Weber E (2001) Current and potential ranges of three exotic goldenrods (Solidago) in Europe. Conserv Biol 15:122–128
Xu RK, Ji GL (2001) Effects of H2SO4 and HNO3 on soil acidification and aluminum speciation in variable and constant charge soils. Water Air Soil Pollut 129:33–43
Yang RY, Mei LX, Tang JJ, Chen X (2007) Allelopathic effects of invasive Solidago canadensis L. on germination and root growth of native Chinese plants. Allelopathy J 19:241–248
Yang RY, Yu GD, Tang JJ, Chen X (2008) Effects of metal lead on growth and mycorrhizae of an invasive plant species (Solidago canadensis L.). J Environ Sci 20:739–744
Yuan YG, Wang B, Zhang SS, Tang JJ, Tu C, Hu SJ, Yong JWH, Chen X (2013) Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J Plant Ecol 6:253–263
Zhang YJ, Chang HR (2012) The impact of acid rain on China’s socioeconomic vulnerability. Nat Hazards 64:1671–1683
Zhang JE, Ouyang Y, Ling DJ (2007) Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67:2131–2137
Zhang SS, Zhu WJ, Wang B, Tang JJ, Chen X (2011) Secondary metabolites from the invasive Solidago canadensis L. accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl Soil Ecol 48:280–286
Zhao SY, Sun SG, Dai C, Gituru RW, Chen JM, Wang QF (2015) Genetic variation and structure in native and invasive Solidago canadensis populations. Weed Res 55:163–172
Acknowledgments
This study was supported by National Natural Science Foundation of China (31300343, 31570414), Natural Science Foundation of Jiangsu Province, China (BK20130500), Universities Natural Science Research Project of Jiangsu Province, China (13KJB610002), Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, Research Foundation for Advanced Talents, Jiangsu University (12JDG086), and the Student Scientific Research Project, Jiangsu University (Y13A229). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to the anonymous reviewers for the insightful and constructive comments that improved this manuscript greatly.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, C., Xiao, H., Zhao, L. et al. The allelopathic effects of invasive plant Solidago canadensis on seed germination and growth of Lactuca sativa enhanced by different types of acid deposition. Ecotoxicology 25, 555–562 (2016). https://doi.org/10.1007/s10646-016-1614-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1614-1