Abstract
The majority of allelopathic studies on invasive plants have focused primarily on their leaf-mediated allelopathy, with relatively little attention paid to their root-mediated allelopathy, especially co-allelopathy mediated by both leaves and roots. It is conceivable that the diversified composition of acid rain may influence the allelopathy of invasive plants. This study aimed to evaluate the leaf and root-mediated co-allelopathy of the invasive plant Solidago canadensis L. under acid rain with different nitrogen-sulfur ratios (N/S) on Lactuca sativa L. via a hydroponic incubation. The root-mediated allelopathy of S. canadensis was found to be more pronounced than the leaf-mediated allelopathy of S. canadensis with nitric acid at pH 4.5, but the leaf-mediated allelopathy of S. canadensis was observed to be more pronounced than the root-mediated allelopathy of S. canadensis with sulfuric-rich acid at pH 4.5. The leaf and root-mediated co-allelopathy of S. canadensis was more pronounced than that of either part alone with sulfuric acid at pH 5.6 and nitric acid at pH 4.5, but not with nitric-rich acid at pH 4.5 and sulfuric-rich acid at pH 4.5. Sulfuric acid and sulfuric-rich acid with stronger acidity intensified the leaf-mediated allelopathy of S. canadensis. Nitric acid and nitric-rich acid attenuated the leaf-mediated allelopathy of S. canadensis, and most types of acid rain (especially nitric acid and nitric-rich acid) also attenuated the root-mediated allelopathy of S. canadensis and the leaf and root-mediated co-allelopathy of S. canadensis. Sulfuric acid and sulfuric-rich acid produced a more pronounced effect than nitric acid and nitric-rich acid. Hence, the N/S ratio of acid rain influenced the allelopathy of S. canadensis under acid rain with multiple N/S ratios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive plants have the potential to significantly alter the community composition of native ecosystems (Wang et al. 2019; Nsikani et al. 2021; Sampaio et al. 2021; Dimitrakopoulos et al. 2022). Elucidating the mechanisms that facilitate the successful establishment of invasive species is a significant and compelling task for ecologists (Glisson et al. 2022; Keet and Richardson 2022; Xu et al. 2023; Yu et al. 2023a).
A multitude of invasive plants have been observed to gain a competitive advantage by releasing the allelopathic chemicals that interfere with or even inhibit the establishment and growth of their competitors (Girotto et al. 2021; Sucur et al. 2021; Xu et al. 2023; Yu et al. 2023b). The allelopathic chemicals released by invasive plants can cause significant allelopathy on the seed germination and seedling growth (abbreviated as Sge-sgr) of their competitors, especially native plants (Cheng et al. 2021a; Mozdzen et al. 2021; Xu et al. 2023; Yu et al. 2023b). The allelopathic chemicals of invasive plants are primarily released from their leaves (including leaf debris) and roots (through root secretions) (Chon and Nelson 2011; Ferreira et al. 2020; Sun et al. 2021; Yu et al. 2022; Wang et al. 2024). Nevertheless, research on the allelopathy of invasive plants has predominantly focused on the leaf-mediated allelopathy, with relatively little attention paid to the root-mediated allelopathy, especially co-allelopathy mediated by both leaves and roots (Yu et al. 2022).
China is among the top three regions in the world most affected by acid rain (Liu et al. 2017; Wang et al. 2017b; Du et al. 2020; Zhong et al. 2022). China is currently undergoing a transformation in the composition of acid rain, shifting from sulfuric-rich acid rain to mixed sulfuric and nitric acid rain with an increased ratio of nitrogen to sulfur (N/S) in rainfall in China. This change has been attributed to the alterations in the adjustment of energy policy, particularly the strict control of coal use (Liu et al. 2017; Du et al. 2020; Zhong et al. 2022; Zhong et al. 2023). However, the alterations in the type of acid rain may alter the competitive advantage of invasive plants by changing their allelopathy (Wang et al. 2012a; Wang et al. 2012b; Wang et al. 2016; Cheng et al. 2021a; Zhong et al. 2023). Consequently, it is of great importance to elucidate the allelopathy of invasive plants with a particular focus on the leaf and root-mediated co-allelopathy of invasive plants, under the influence of multiple types of acid rain. This will facilitate an understanding of the mechanisms that drive the successful invasion of invasive plants under different acid rain regimes. However, there has been minimal advancement in this field.
This study aimed to evaluate the leaf (using the leaf aqueous extracts, abbreviated as SCL) and root (using the root aqueous extracts, abbreviated as SCR)-mediated co-allelopathy of the invasive plant Solidago canadensis L. under acid rain with multiple N/S ratios on Sge-sgr of Lactuca sativa L. via a hydroponic incubation. As an Asteraceae perennial herb, S. canadensis originated in North America and was introduced as an ornamental plant in China during the 1930s. The successful spread of this plant is mainly attributed to its allelopathy (Zandi et al. 2020; Cheng et al. 2021a; Yu et al. 2022; Zhou et al. 2022). Lactuca sativa is a commonly encountered horticultural plant in the environments (e.g., agricultural land and wasteland), where S. canadensis also thrives. Due to the high sensitivity of Sge-sgr to the allelopathic chemicals, L. sativa has been extensively studied in the field of allelopathic research (Kyaw and Kato-Noguchi 2020; Rob et al. 2020; da Cruz-Silva et al. 2021; Medic et al. 2021). The regions of China where S. canadensis is present have been subjected to severe acid rain with multiple N/S ratios (Wang et al. 2016; Liu et al. 2018; Liu et al. 2020; Cheng et al. 2021a).
This study investigated three questions: (i) Which of the two parts, leaf or root, is the primary source of the allelopathy of S. canadensis? (ii) Does the leaf and root-mediated co-allelopathy of S. canadensis result in synergistic effects on L. sativa that are greater than those observed when either part is considered separately? (iii) Does acid rain, and if so, which type, intensify the leaf and root-mediated co-allelopathy of S. canadensis?
Materials and methods
Preparation of SCL and SCR
The leaf and root samples of S. canadensis were collected from Zhenjiang (32.20–32.22°N, 119.51–119.53°E), China in late September 2021. The collected leaf and root samples of S. canadensis were meticulously cleaned and air-dried at 25 °C until the weight remained constant. The air-dried leaf and root samples of S. canadensis were leached in sterile distilled water at a concentration of 10 g L−1 (Lu et al. 2020; Yu et al. 2023b; Zhong et al. 2023; Li et al. 2024) for ≈48 h at 25 °C to obtain the SCL and SCR of S. canadensis. The SCL and SCR of S. canadensis were applied to L. sativa seeds, either alone (representing the condition with the leaf-mediated allelopathy or the root-mediated allelopathy of S. canadensis) or combined SCL and SCR (abbreviated as SCRL) at a 1:1 v/v mixture (representing the condition with the leaf and root-mediated co-allelopathy of S. canadensis), in comparison to distilled water as the control (0 g L−1; representing the condition without the allelopathy of S. canadensis). The SCL and SCR were stored at 4 °C for a maximum of 7 d to prevent contamination by bacteria and the production of algae.
Preparation of acid rain solution
Acid rain solution was prepared to simulate acid rain by mixing 0.5 M HNO3 and 0.5 M H2SO4 with two acidity levels (i.e., pH 5.6 and pH 4.5; representing the condition with acid rain). In general, the pH of normal rainfall without acid rain and the pH of natural rainfall in Zhenjiang are ≈5.6 and ≈4.5, respectively; and NO3‒ and SO42‒ were the main acidic components of natural rainfall in Zhenjiang (Wang et al. 2016; Liu et al. 2017; Liu et al. 2018; Zhong et al. 2022). The modeled acid rain comprised four types with different N/S ratios: (1) nitric acid (N/S ratio = 1:0); (2) sulfuric acid (N/S ratio = 0:1); (3) nitric-rich acid (N/S ratio = 3:1); and (4) sulfuric-rich acid (N/S ratio = 1:3) (Wang et al. 2017b; Zhong et al. 2023). Distilled water (pH 7.0; representing the absence of acid rain) was used as the control.
Experimental design of hydroponic incubation
The seeds of L. sativa (cultivar name: cv. Chunfeng) were surface-sterilized by inimmersion in 1% NaClO for ≈15 min and subsequently washed with deionized water for ≈30 min. A total of 30 seeds of L. sativa with uniform in size were placed in a Petri dish (9 cm) with two layers of filter paper per treatment. The Petri dishes were maintained at a temperature of ≈25 °C with a 12-hour light cycle from April 1st to April 8th, 2022. The light intensity was ≈27.5 µmol m–2 s–1. During the incubation period, 1 mL of deionized water, the SCL and SCR, or acid rain solution was added to each Petri dish daily. The SCL and SCR, and acid rain solution in the combined treatments were mixed in an equivalent ratio. The number of seeds that had undergone normal germination was confirmed daily basis throughout the course of the hydroponic incubation. Germination was defined as the emergence of the radicle.
The hydroponic incubation of L. sativa involves three factors (i.e., the source of aqueous extracts, the acidity of acid rain, and the type of acid rain). Each factor is categorized into two, three, or four levels, respectively. The source of aqueous extracts is categorized into three levels (i.e., SCL, SCR, and SCRL). The acidity of acid rain is categorized into two levels (i.e., pH 4.5 and pH 5.6). The type of acid rain is categorized into four levels (i.e., nitric acid, sulfuric acid, nitric-rich acid, and sulfuric-rich acid). A total of three Petri dishes were utilized for each treatment, with each dish containing 90 seeds of L. sativa per treatment.
Determination of Sge-sgr variables
Following a seven-day period of hydroponic incubation, ten seedlings of L. sativa were collected from each Petri dish, thus a total 30 seedlings of L. sativa per treatment, which were used for the determination of Sge-sgr variables. This was done in order to evaluate the growth status of L. sativa.
The stress intensity of the treatments on Sge-sgr variables was calculated using the stress intensity index.
The methodology employed for the measurement of Sge-sgr variables and the stress intensity index is detailed in Supplementary Table S1.
Statistical analysis
The deviations from normality and homogeneity of the variances were estimated by employing the Shapiro–Wilks’s test and Bartlett’s test, respectively. A multiple comparison with the Tukey’s test was employed to assess differences in the values of L. sativa variables and the stress intensity index among the various treatments. A three-way ANOVA was employed to assess the effects of the source of aqueous extracts, the acidity of acid rain, and the type of acid rain on the values of L. sativa variables and the stress intensity index. The Partial Eta Squared (η2) values were also evaluated to estimate the effect size of each factor for use in two-way ANOVA. Statistically significant differences were indicated by a threshold of p ≤ 0.05. Statistical analyses were conducted using IBM SPSS Statistics (26.0).
Results
Effects of aqueous extracts of S. canadensis on L. sativa seed germination variables under acid rain with multiple N/S ratios
The sources of aqueous extracts did not significantly affect all seed germination variables compared to the control (Fig. 1a–e; p > 0.05). The acidity and type of acid rain also did not significantly affect the germination percentage or the germination potential (Fig. 1a, b; p > 0.05).
The values (means and SE; n = 30) of seed germination variables of L. sativa (a, germination percentage; b, germination potential; c, germination index; d, germination rate index; e, germination vigor index). Bars with different letters indicate statistically significant differences (p < 0.05). CK control, SCL leaf aqueous extracts of S. canadensis, SCR root aqueous extracts of S. canadensis, SCRL combined leaf and root aqueous extracts, N nitric acid, S sulfuric acid, MixN nitric-rich acid, MixS sulfuric-rich acid, 5.6 acid rain at pH 5.6, 4.5 acid rain at pH 4.5
SCL treated with sulfuric acid and sulfuric-rich acid at pH 4.5 significantly reduced the germination index compared to SCL (Fig. 1c; p < 0.05). SCR treated with sulfuric acid at pH 4.5 significantly reduced the germination index compared to SCR (Fig. 1c; p < 0.05). SCRL treated with nitric acid at pH 4.5 significantly reduced the germination index compared to SCRL (Fig. 1c; p < 0.05). SCL treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 4.5 significantly reduced the germination index compared to SCL treated with those types of acid rain at pH 5.6 (Fig. 1c; p < 0.05). SCR treated with sulfuric acid at pH 4.5 significantly reduced the germination index compared to SCR treated with sulfuric acid at pH 5.6 (Fig. 1c; p < 0.05). SCRL treated with nitric acid at pH 4.5 significantly reduced the germination index compared to SCRL treated with nitric acid at pH 5.6 (Fig. 1c; p < 0.05). The germination index under SCL treated with nitric acid and nitric-rich acid was significantly higher than that under SCL treated with sulfuric acid and sulfuric-rich acid regardless of the acidity (Fig. 1c; p < 0.05). The germination index under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 5.6 (Fig. 1c; p < 0.05). The germination index under SCR treated with nitric acid at pH 4.5 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 1c; p < 0.05). The germination index under SCRL treated with nitric-rich acid at pH 4.5 was significantly higher than that under SCRL treated with nitric acid at pH 4.5 (Fig. 1c; p < 0.05).
SCL treated with sulfuric acid and sulfuric-rich acid at pH 4.5 significantly reduced the germination rate index compared to SCL (Fig. 1d; p < 0.05). Both SCR and SCRL treated with sulfuric acid at pH 4.5 significantly reduced the germination rate index compared to SCR and SCRL, respectively (Fig. 1d; p < 0.05). SCL treated with sulfuric acid at pH 4.5 significantly reduced the germination rate index compared to SCL treated with sulfuric acid at pH 5.6 (Fig. 1d; p < 0.05). SCRL treated with nitric acid at pH 4.5 significantly reduced the germination rate index compared to SCRL treated with nitric acid at pH 5.6 (Fig. 1d; p < 0.05). The germination rate index under SCL treated with nitric acid was significantly higher than that under SCL treated with sulfuric-rich acid regardless of the acidity (Fig. 1d; p < 0.05). The germination rate index under SCL treated with nitric acid and nitric-rich acid at pH 4.5 was also significantly higher than that under SCL treated with sulfuric acid at pH 4.5 (Fig. 1d; p < 0.05). The germination rate index under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 5.6 (Fig. 1d; p < 0.05). The germination rate index under SCR treated with nitric acid at pH 4.5 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 1d; p < 0.05). The germination rate index under SCRL treated with nitric-rich acid at pH 4.5 was significantly higher than that under SCRL treated with nitric acid at pH 4.5 (Fig. 1d; p < 0.05).
SCL treated with nitric-rich acid at pH 5.6 significantly increased the germination vigor index compared to SCL (Fig. 1e; p < 0.05). SCL treated with nitric acid at pH 4.5 significantly increased the germination vigor index but SCL treated with sulfuric acid at pH 4.5 significantly reduced the germination vigor index compared to SCL (Fig. 1e; p < 0.05). SCR treated with nitric acid at pH 5.6 significantly increased the germination vigor index compared to SCR (Fig. 1e; p < 0.05). SCL treated with nitric-rich acid at pH 4.5 significantly reduced the germination vigor index compared to SCL treated with nitric-rich acid at pH 5.6 (Fig. 1e; p < 0.05). Both SCR and SCRL treated with nitric acid at 4.5 significantly reduced the germination vigor index compared to SCR and SCRL treated with nitric acid at pH 5.6, respectively (Fig. 1e; p < 0.05). The germination vigor index under both SCL and SCRL treated with nitric acid at pH 5.6 was significantly higher than that under SCL and SCRL treated with sulfuric acid at pH 5.6, respectively (Fig. 1e; p < 0.05). The germination vigor index under SCL treated with nitric-rich acid at pH 5.6 was also significantly higher than that under SCL treated with sulfuric acid and sulfuric-rich acid at pH 5.6 (Fig. 1e; p < 0.05). The germination vigor index under SCL treated with nitric acid at pH 4.5 was significantly higher than that under SCL treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 4.5 (Fig. 1e; p < 0.05). The germination vigor index under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 5.6 (Fig. 1e; p < 0.05). The germination vigor index under SCR treated with nitric acid at pH 4.5 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 1e; p < 0.05).
Effects of aqueous extracts of S. canadensis on L. sativa seedling growth variables under acid rain with multiple N/S ratios
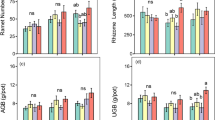
All sources of aqueous extracts did not significantly affect seedling height compared to the control (Fig. 2a; p > 0.05). All sources of aqueous extracts treated with all types of acid rain at pH 5.6 significantly increased seedling height compared to only aqueous extracts (Fig. 2a; p < 0.05). SCL treated with sulfuric acid and sulfuric-rich acid at pH 4.5 significantly reduced and increased seedling height compared to SCL, respectively (Fig. 2a; p < 0.05). SCR treated with sulfuric acid at pH 4.5 significantly increased seedling height compared to SCR (Fig. 2a; p < 0.05). SCRL treated with nitric acid and sulfuric acid at 4.5 significantly reduced seedling height compared to SCRL (Fig. 2a; p < 0.05). Seedling height under all sources of aqueous extracts treated with all types of acid rain at pH 5.6 was significantly higher than that under all sources of aqueous extracts treated with all types of acid rain at pH 4.5 (except under SCL treated with sulfuric-rich acid) (Fig. 2a; p < 0.05). Seedling height under SCL treated with nitric acid and nitric-rich acid was significantly higher than that under SCL treated with sulfuric acid and sulfuric-rich acid regardless of the acidity (Fig. 2a; p < 0.05). Seedling height under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid at pH 5.6 (Fig. 2a; p < 0.05). Seedling height under SCR treated with nitric acid and nitric-rich acid at pH 4.5 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 2a; p < 0.05). Seedling height under SCRL treated with sulfuric acid at pH 5.6 was significantly lower than that under SCRL treated with nitric acid, nitric-rich acid, and sulfuric-rich acid at pH 4.5 (Fig. 2a; p < 0.05). Seedling height under SCRL treated with nitric-rich acid at pH 4.5 was significantly higher than that under SCR treated with nitric acid and sulfuric acid at pH 4.5 (Fig. 2a; p < 0.05).
The values (means and SE; n = 30) of seedling growth variables of L. sativa (a, seedling height; b, radicle length; c, leaf length; d, leaf width; e, fresh weight; f, dry weight). Bars with different letters indicate statistically significant differences (p < 0.05). Abbreviations have the same meanings as defined in Fig. 1
All sources of aqueous extracts significantly increased radicle length compared to the control (Fig. 2b; p < 0.05). All sources of aqueous extracts treated with all types of acid rain regardless of the acidity significantly increased radicle length compared to only aqueous extracts (Fig. 2b; p < 0.05). Radicle length under SCL treated with nitric acid at pH 5.6 was significantly higher than that under SCL treated with nitric acid at pH 4.5, but it’s the opposite for SCR (Fig. 2b; p < 0.05). Radicle length under SCL treated with nitric acid at pH 4.5 was significantly higher than that under SCL treated with sulfuric acid, nitric-rich acid and sulfuric-rich acid at pH 4.5 (Fig. 2b; p < 0.05). Radicle length under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid, nitric-rich acid and sulfuric-rich acid at pH 5.6 (Fig. 2b; p < 0.05). Radicle length under SCRL treated with nitric acid and sulfuric acid at pH 4.5 was significantly higher than that under SCRL treated with sulfuric-rich acid at pH 4.5 (Fig. 2b; p < 0.05).
All sources of aqueous extracts did not significantly affect leaf length compared to the control (Fig. 2c; p > 0.05). SCL treated with nitric acid and nitric-rich acid at pH 5.6 significantly increased leaf length compared to SCL (Fig. 2c; p < 0.05). SCL treated with nitric acid and sulfuric acid at pH 4.5 significantly increased and decreased leaf length compared to SCL, respectively (Fig. 2c; p < 0.05). SCR treated with nitric acid, nitric-rich acid, and sulfuric-rich acid at pH 5.6 significantly increased leaf length compared to SCR (Fig. 2c; p < 0.05). SCR treated with nitric acid and nitric-rich acid at pH 4.5 significantly increased leaf length, but SCR treated with sulfuric acid at pH 4.5 significantly decreased leaf length, compared to SCR (Fig. 2c; p < 0.05). SCRL treated with nitric acid, nitric-rich acid, and sulfuric-rich acid regardless of the acidity significantly increased leaf length compared to SCRL (Fig. 2c; p < 0.05). Leaf length under SCL treated with nitric acid at pH 5.6 was significantly higher than that under SCL treated with nitric acid at pH 4.5, but it’s the opposite for sulfuric acid, nitric-rich acid, and sulfuric-rich acid (Fig. 2c; p < 0.05). Leaf length under SCR treated with sulfuric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 2c; p < 0.05). Leaf length under SCRL treated with nitric-rich acid at pH 5.6 was significantly lower than that under SCRL treated with nitric-rich acid at pH 4.5 (Fig. 2c; p < 0.05). Leaf length under all sources of aqueous extracts treated with different types of acid rain regardless of the acidity generally exhibited a decreasing order as follows: nitric acid > nitric-rich acid > sulfuric-rich acid > sulfuric acid (Fig. 2c; p < 0.05).
All sources of aqueous extracts did not significantly affect leaf width compared to the control (Fig. 2d; p > 0.05). SCL and SCR treated with nitric acid regardless of the acidity significantly increased leaf width compared to SCL and SCR, respectively (Fig. 2d; p < 0.05). Leaf width under SCL treated with nitric acid at pH 5.6 was significantly higher than that under SCL treated with nitric acid at pH 4.5 (Fig. 2d; p < 0.05). Leaf width under SCL treated with nitric acid at pH 4.5 was significantly higher than that under SCL treated with sulfuric acid, nitric-rich acid, and sulfuric-rich acid at pH 4.5 (Fig. 2d; p < 0.05). Leaf width under SCR treated with sulfuric acid at pH 4.5 was significantly lower than that under SCR treated with nitric acid, nitric-rich acid, and sulfuric-rich acid at pH 4.5 (Fig. 2d; p < 0.05). Leaf width under SCRL treated with nitric acid at pH 5.6 was significantly higher than that under SCRL treated with sulfuric acid at pH 5.6 (Fig. 2d; p < 0.05).
All sources of aqueous extracts did not significantly affect fresh weight compared to the control (Fig. 2e; p > 0.05). SCR and SCRL treated with nitric acid at pH 5.6 significantly increased fresh weight compared to SCR and SCRL, respectively (Fig. 2e; p < 0.05). Fresh weight under SCRL treated with nitric acid at pH 5.6 was significantly higher than that under SCRL treated with nitric acid at pH 4.5 (Fig. 2e; p < 0.05). Fresh weight under SCL treated with sulfuric acid was significantly lower than that under SCR treated with nitric acid and nitric-rich acid regardless of the acidity (Fig. 2e; p < 0.05). Fresh weight under SCR treated with nitric acid at pH 5.6 was significantly higher than that under SCR treated with sulfuric acid and sulfuric-rich acid at pH 5.6 (Fig. 2e; p < 0.05). Fresh weight under SCR treated with nitric-rich acid at pH 4.5 was significantly higher than that under SCR treated with sulfuric acid at pH 4.5 (Fig. 2e; p < 0.05). Fresh weight under SCRL treated with nitric acid at pH 5.6 was significantly higher than that under SCRL treated with sulfuric acid at pH 5.6 (Fig. 2e; p < 0.05). Fresh weight under SCRL treated with nitric acid and nitric-rich acid at pH 4.5 was significantly higher than that under SCRL treated with sulfuric acid at pH 4.5 (Fig. 2e; p < 0.05).
No significant differences in dry weight were detected among all treatments (Fig. 2f; p > 0.05).
Differences in the value of the stress intensity index under aqueous extracts of S. canadensis treated with and without acid rain with multiple N/S ratios on L. sativa variables
No significant differences in the value of the stress intensity index were detected among all sources of aqueous extracts (Fig. 3; p > 0.05). The value of the stress intensity index under all sources of aqueous extracts treated with nitric acid regardless of the acidity was significantly lower than that under only aqueous extracts (Fig. 3; p < 0.05). The value of the stress intensity index under SCL and SCR treated with nitric-rich acid at pH 5.6 was significantly lower than that under SCL and SCR, respectively (Fig. 3; p < 0.05). The value of the stress intensity index under SCR and SCRL treated with nitric-rich acid at pH 4.5 was significantly lower than that under SCR and SCRL, respectively (Fig. 3; p < 0.05). The value of the stress intensity index under SCL and SCR treated with sulfuric acid at pH 4.5 was significantly higher than that under SCL and SCR, respectively (Fig. 3; p < 0.05). The value of the stress intensity index under SCL treated with sulfuric-rich acid at pH 4.5 was significantly higher than that under SCL (Fig. 3; p < 0.05). The value of the stress intensity index under SCR treated with sulfuric-rich acid at pH 5.6 was significantly lower than that under SCR (Fig. 3; p < 0.05). The value of the stress intensity index under SCRL treated with sulfuric-rich acid at pH 4.5 was significantly lower than that under SCRL (Fig. 3; p < 0.05). The value of the stress intensity index under all sources of aqueous extracts treated with different types of acid rain regardless of the acidity generally exhibited a decreasing order as follows: sulfuric acid > sulfuric-rich acid > nitric-rich acid > nitric acid (Fig. 3; p < 0.05). The value of the stress intensity index under SCL treated with nitric acid at pH 4.5 was significantly lower than that under SCR and SCRL treated with nitric acid at pH 4.5, but it’s the opposite for sulfuric-rich acid (Fig. 3; p < 0.05). The value of the stress intensity index under SCL treated with nitric-rich acid at pH 4.5 was significantly higherer than that under SCRL treated with nitric-rich acid at pH 4.5 (Fig. 3; p < 0.05).
The values (means and SE; n = 30) of the stress intensity index of different treatments on L. sativa variables. Bars with different letters indicate statistically significant differences (p < 0.05). Abbreviations have the same meanings as defined in Fig. 1
The effects of the source of aqueous extracts, the acidity of acid rain, and the type of acid rain on the values of L. sativa variables and the stress intensity index
Based on the results of three-way ANOVA analysis, the source of aqueous extracts significantly affected radicle length, leaf dimensions, fresh weight, and stress intensity index (p < 0.05; Supplementary Table S2). The acidity of acid rain significantly affected all Sge-sgr variables (except dry weight) (p < 0.05; Supplementary Table S2). The type of acid rain significantly affected the germination index, germination rate index, germination vigor index, seedling height, radicle length, leaf dimensions, fresh weight, and stress intensity index (p < 0.05; Supplementary Table S2). The interaction of the source of aqueous extracts and the acidity of acid rain significantly affected the germination index, germination rate index, radicle length, leaf length, and stress intensity index (p < 0.05; Supplementary Table S2). The interaction of the source of aqueous extracts and the type of acid rain significantly affected all Sge-sgr variables (except seedling biomass) (p < 0.05; Supplementary Table S2). The interaction of the source of the acidity of acid rain the type of acid rain significantly affected the germination index, germination rate index, seedling height, radicle length, leaf length, leaf width, and stress intensity index (p < 0.05; Supplementary Table S2). The interaction of the source of aqueous extracts, the acidity of acid rain, and the type of acid rain significantly affected all Sge-sgr variables (except germination percentage, germination potential, and dry weight) (p < 0.05; Supplementary Table S2).
Discussion
Allelopathy is a crucial factor in the success of invasive plants (Ferreira et al. 2020; Kalisz et al. 2021; Ullah et al. 2021; Kumar and Garkoti 2022), including S. canadensis (Mozdzen et al. 2020; Zandi et al. 2020; Yu et al. 2022; Zhou et al. 2022). The three sources of aqueous extracts of S. canadensis did not exhibit any significant allelopathy on L. sativa, and only significantly improved the radicle length of L. sativa compared to the control (Fig. 2b). This may be attributed to the neutralizing effect of the positive and negative effects of the aqueous extracts of S. canadensis on L. sativa. The negative effects of the aqueous extracts of invasive plants on their competitors may be mainly due to the allelopathic chemicals that interfere with the physiological processes of the recipient plants. These processes include reduced nutrient uptake, reduced cell division, suppressed cellular functions, and inhibited material synthesis (Amoo et al. 2008; Svensson et al. 2013; Jmii et al. 2020; Zhong et al. 2023). The positive effects of the aqueous extracts of invasive plants on their competitors may be due to the hormonal effects via the reactive oxygen molecules generated (Duke et al. 2006; Agathokleous 2018; Erofeeva 2022; Sebastiano et al. 2022), and/or the facilitated priming effects triggered by the nutrient elements (especially nitrogenous substances) contained in the aqueous extracts of invasive plants (Wang et al. 2020a; Wei et al. 2020a; Wei et al. 2020b).
The allelopathic chemicals of invasive plants originate from both leaf and root (Chon and Nelson 2011; Ferreira et al. 2020; Medina-Villar et al. 2020; Yu et al. 2022). The stress intensity of SCL was found to be lower than that of SCR treated with nitric acid at pH 4.5, whereas the stress intensity of SCL was found to be higher than that of SCR treated with sulfuric-rich acid at pH 4.5 (Fig. 3). Consequently, the allelopathy of SCR was more pronounced than that of SCL treated with nitric acid at pH 4.5, whereas the allelopathy of SCL was more pronounced than that of SCR treated with sulfuric-rich acid at pH 4.5. It can therefore be concluded that the root-mediated allelopathy may be largely attributed to the allelopathy of S. canadensis treated with nitric acid at pH 4.5, whereas the leaf-mediated allelopathy may have a stronger contribution to the allelopathy of S. canadensis treated with sulfuric-rich acid at pH 4.5. This indicates that the dissimilarities in the allelopathy between the leaf and root of S. canadensis may be influenced by the type of acid rain, specifically the N/S ratio of acid rain. One potential explanation for this phenomenon is the varying solubility and metabolic activity of the allelopathic chemicals present in the leaf and root under different types of acid rain (Wang et al. 2016; Zhong et al. 2023). Moreover, it is possible that the quantity and quality of the allelopathic chemicals contained in the leaf and root may diverge due to the morphological and physiological differences between the two organs. Consequently, it can be expected that marked differences in the allelopathy may be observed between leaf and root of invasive plants.
It has been demonstrated that the allelopathic chemicals present in the leaf and root of S. canadensis can naturally interact with one another under normal conditions (Yu et al. 2022). Following the mixing of the allelopathic chemicals present in leaf and root of S. canadensis, these chemicals can undergo alteration (Yu et al. 2022). The stress intensity of SCRL was found to be higher than that of SCR but it was approximately equal to that of SCL treated with sulfuric acid at pH 5.6, and the stress intensity of SCRL was found to be higher than that of SCL but approximately equal to that of SCR treated with nitric acid at pH 4.5. Conversely, the stress intensity of SCRL was found to be lower than that of SCL whereas it is approximately equal to that of SCR treated with sulfuric-rich acid at pH 4.5, and the stress intensity of SCRL was also found to be lower than that of SCL treated with nitric-rich acid at pH 4.5 (Fig. 3). Thus, SCRL caused stronger co-allelopathy than either part alone treated with sulfuric acid at pH 5.6 and nitric acid at pH 4.5, but conversely with nitric-rich acid at pH 4.5 and sulfuric-rich acid at pH 4.5. Accordingly, the leaf and root of S. canadensis exhibited a synergistic co-allelopathy on L. sativa, which was more pronounced than that observed when either part was used alone with sulfuric acid at pH 5.6 and nitric acid at pH 4.5, but contrary to that with nitric-rich acid at pH 4.5 and sulfuric-rich acid at pH 4.5. In other words, the leaf and root-mediated co-allelopathy of S. canadensis treated with sulfuric acid at pH 5.6 and nitric acid at pH 4.5 can be mainly attributed to its root, but the leaf and root-mediated co-allelopathy of S. canadensis treated with nitric-rich acid at pH 4.5 and sulfuric-rich acid at pH 4.5 can be mainly attributed to its leaf. Thus, the leaf and root-mediated co-allelopathy of S. canadensis may also be influenced by the type of acid rain, specifically the N/S ratio of acid rain. It can be reasonably assumed that the various types of acid rain may have exerted a significant influence on the synergistic effects of the mixed allelopathic chemicals present in leaf and root (Wang et al. 2016).
Acid rain can affect the secondary metabolic processes, which in turn can influence the production of secondary metabolites by plants (Shumejko et al. 1996; Debnath et al. 2020; Zhang et al. 2020). It has been observed that acid rain has recently increased and is expected to gradually increase in China in the near future (Liu et al. 2017; Du et al. 2020; Zhong et al. 2022; Zhong et al. 2023). Thus, the allelopathy of invasive plants may undergo a transformation, potentially becoming more pronounced in the context of elevated acid rain. Previous studies have also demonstrated that acid rain, particularly when of a higher acidity, can increase the allelopathy of invasive plants (Wang et al. 2012a; Wang et al. 2012b; Wang et al. 2016; Cheng et al. 2021a; Zhong et al. 2023). Normally, abiotic stresses can increase the allelopathy of plants via the enhanced secondary metabolic processes, as proposed by the Stress Hypothesis of Allelopathy (Reigosa et al. 2002). A number of studies have also corroborated this hypothesis (Selmar and Kleinwächter 2013; Hashoum et al. 2021; Zhou et al. 2022; Zhong et al. 2023). The stress intensity of SCL treated with sulfuric acid at pH 4.5 and sulfuric-rich acid) at pH 4.5 was found to be greater than that of SCL (Fig. 3). This result is consistent with the Stress Hypothesis of Allelopathy (Reigosa et al. 2002), which posits that sulfuric acid and sulfuric-rich acid with higher acidity enhance the leaf-mediated allelopathy of S. canadensis. This finding may be attributed to the increased acidity, which has been demonstrated to have toxic effects on plant growth. This is evidenced by the increased energy cost and the suppressed nutrient use efficiency for L. sativa performance. Another contributing factor may also be, the accelerated extraction and release processes of acid-soluble substances from SCL (Wang et al. 2012a; Wang et al. 2012b; Cheng et al. 2021a; Zhong et al. 2023), such as phenolics (especially polyphenols), which were identified as one of the main components of the allelopathic chemicals of invasive plants (Djurdjević et al. 2011; Djurdjević et al. 2012; Hussain et al. 2020; Stefanowicz et al. 2021). Consequently, the allelopathy of S. canadensis as well as sulfuric acid and sulfuric-rich acid with higher acidity, were found to have an antagonistic influence on the performance of L. sativa.
It is noteworthy that the stress intensity of SCL with nitric acid regardless of the acidity and nitric-rich acid at pH 5.6 was found to be lower than that with SCL; the stress intensity of SCR with all types of acid rain (except sulfuric acid regardless of the acidity and sulfuric-rich acid at pH 4.5) was found to be lower than that with SCR but in contrast to sulfuric acid at pH 4.5; the stress intensity of SCRL with nitric acid regardless of the acidity, nitric-rich acid at pH 4.5, and sulfuric-rich acid at pH 4.5 was found to be lower than that with SCRL of S. canadensis (Fig. 3). It can be concluded that nitric acid and nitric-rich acid have the effect of reducing the leaf-mediated allelopathy of S. canadensis, and most types of acid rain (especially nitric acid and nitric-rich acid) also have the effect of reducing the root-mediated allelopathy and the leaf and root-mediated co-allelopathy of S. canadensis. This finding may be attributed to the increased nitrogen availability to L. sativa, which has been demonstrated to mitigate the adverse effects mediated by the allelopathy of S. canadensis (Shen et al. 2008; Wang et al. 2017a; Wang et al. 2020b). Previous research has also demonstrated that the application of exogenous nitrogen can enhance plant resistance to adverse environments largely due to the most essential role of nitrogen in plant growth (Hassan et al. 2005; Hassan et al. 2008; Cheng et al. 2020; Cheng et al. 2021b). The reduced allelopathy of S. canadensis with acid rain, especially nitric acid and nitric-rich acid, may also be attributed to the increased application of exogenous nitrogen, which reduces the concentration of phenolics (especially polyphenols) (Guillén-Román et al. 2018; Min and Shi 2018; Sun et al. 2020), but phenolics (especially polyphenols) are one of the major components of the allelopathic chemicals produced by invasive plants (Djurdjević et al. 2011; Djurdjević et al. 2012; Hussain et al. 2020; Stefanowicz et al. 2021). Thus, the effects of acid rain on the allelopathy of S. canadensis may also be contingent upon the specific type of acid rain, specifically the N/S ratio of acid rain.
It is of greater significance that the stress intensity after treatment with the three sources of aqueous extracts of S. canadensis treated with sulfuric acid was found to be greater than that with the remaining types of acid rain, and the stress intensity generally decreased in the following order: sulfuric acid > sulfuric-rich acid > nitric-rich acid > nitric acid (Fig. 3). It can be concluded that sulfuric acid and sulfuric-rich acid produced a more pronounced effect on L. sativa than nitric acid and nitric-rich acid in general. Hence, the influence of acid rain on L. sativa increases with the proportion of sulfur and decreases with the proportion of nitrogen. Consequently, the N/S ratio of acid rain is a key issue that profoundly affects its ecological function. This is primarily attributable to the disparities in ion exchange capacity with hydroxyl between nitrate and sulfate ions (Lindberg et al. 1990; Christ et al. 1995; Chen et al. 2013; Huang et al. 2019). In contrast, nitric acid and nitric-rich acid have been observed to exert nutrient enrichment effects, whereas sulfuric acid and sulfuric-rich acid have not (Hassan et al. 2005; Hassan et al. 2008; Villar-Salvador et al. 2013; van den Elzen et al. 2018).
Conclusions
The root-mediated allelopathy of S. canadensis was found to be more pronounced than the leaf-mediated allelopathy of S. canadensis treated with nitric acid at pH 4.5, but the leaf-mediated allelopathy of S. canadensis was observed to be greater than the root-mediated allelopathy of S. canadensis treated with sulfuric-rich acid at pH 4.5. The leaf and root-mediated co-allelopathy of S. canadensis was more pronounced than that of either part alone treated with sulfuric acid at pH 5.6 and nitric acid at pH 4.5, but in contrast with nitric-rich acid at pH 4.5 and sulfuric-rich acid at pH 4.5. Sulfuric acid and sulfuric-rich acid with higher acidity resulted in an intensification of the leaf-mediated allelopathy of S. canadensis. The application of nitric acid and nitric-rich acid resulted in a reduction in the leaf-mediated allelopathy of S. canadensis, and most types of acid rain (especially nitric acid and nitric-rich acid) also exhibited a reduction in the root-mediated allelopathy and the leaf and root-mediated co-allelopathy of S. canadensis. Sulfuric acid and sulfuric-rich acid exhibited a more pronounced effect on L. sativa than nitric acid and nitric-rich acid. Thus, the allelopathy of S. canadensis under acid rain with multiple N/S ratios was found to be influenced by the N/S ratio of acid rain. The findings of this study provide a substantial practical foundation for environmental management of plant species, including the effective early warning prevention and control of invasive plants, especially under different types of acid rain.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (e.g., non-commercial academic research).
References
Agathokleous E (2018) Environmental hormesis, a fundamental nonmonotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol Environ Saf 148:1042–1053
Amoo SO, Jo AU, Van Staden J (2008) Allelopathic potential of Tetrapleura tetraptera leaf extracts on early seedling growth of five agricultural crops. South Afr J Bot 74:149–152
Chen J, Wang WH, Liu TW, Wu FH, Zheng HL (2013) Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol Biochem 64:41–51
Cheng HY, Wang S, Wei M, Yu YL, Wang CY (2021a) Effect of leaf water extracts of four Asteraceae alien invasive plants on germination performance of Lactuca sativa L. under acid deposition. Plant Ecol 222:433–443
Cheng HY, Wei M, Wang S, Wu BD, Wang CY (2020) Atmospheric N deposition alleviates the unfavorable effects of drought on wheat growth. Braz J Bot 43:229–238
Cheng HY, Wu BD, Wang S, Wei M, Wang C (2021b) Nitrogen application and osmotic stress antagonistically affect wheat seed germination and seedling growth. Int J Phytoremediat 23:1289–1300
Chon SU, Nelson CJ (2011) Allelopathy in Compositae plants. In: Lichtfouse E, Hamelin M, Navarrete M, Debaeke P (eds) Sustainable Agriculture Volume 2. Springer Netherlands, Dordrecht, pp 727–739
Christ M, Zhang YM, Likens GE, Driscoll CT (1995) Nitrogen retention capacity of a northern hardwood forest soil under ammonium sulfate additions. Ecol Appl 5:802–812
da Cruz-Silva CTA, Cantrell CL, Nobrega LHP, Ali A, Duke SO (2021) Bioassay-guided isolation of phytotoxins from three Salvia species. Allelopath J 54:13–24
Debnath B, Li M, Liu S, Pan TF, Ma CL, Qiu DL (2020) Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotoxicol Environ Saf 200:110720
Dimitrakopoulos PG, Koukoulas S, Michelaki C, Galanidis A (2022) Anthropogenic and environmental determinants of alien plant species spatial distribution on an island scale. Sci Total Environ 805:150314
Djurdjević L, Gajić G, Kostić O, Jarić S, Pavlović M, Mitrović M, Pavlović P (2012) Seasonal dynamics of allelopathically significant phenolic compounds in globally successful invader Conyza canadensis L. plants and associated sandy soil. Flora 207:812–820
Djurdjević L, Mitrović M, Gajić G, Jarić S, Kostić O, Oberan L, Pavlović P (2011) An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L. Flora 206:921–927
Du JJ, Qv MX, Zhang YY, Cui MH, Zhang HZ (2020) Simulated sulfuric and nitric acid rain inhibits leaf breakdown in streams: A microcosm study with artificial reconstituted fresh water. Ecotoxicol Environ Saf 196:110535
Duke SO, Cedergreen N, Velini ED, Belz RG (2006) Hormesis: is it an important factor in herbicide use and allelopathy? Outlooks Pest Manag 17:29–33
Erofeeva EA (2022) Environmental hormesis: From cell to ecosystem. Curr Opin Environ Sci Health 29:100378
Ferreira PJ, Zonetti PDC, Paiola Albrecht AJ, Rosset IG, Moreira Silva AF, Albrecht LP, Vieira AH, Paulert R (2020) Conyza sumatrensis allelopathy effect on Bidens pilosa (Asteraceae) seed germination. Botanical Sci 98:348–354
Girotto L, Franco AC, Nunez CV, Oliveira SCC, Scheffer de Souza MC, Fachin-Espinar MT, Ferreira CS (2021) Phytotoxicity and allelopathic potential of extracts from rhizomes and leaves of Arundo donax, an invasive grass in neotropical savannas. Not Botanicae Horti Agrobot Cluj Napoca 49:12440
Glisson WJ, Muthukrishnan R, Wagner CK, Larkin DJ (2022) Invasive Nitellopsis obtusa (starry stonewort) has distinct late-season phenology compared to native and other invasive macrophytes in Minnesota, USA. Aquat Bot 176:103452
Guillén-Román CJ, Guevara-González RG, Rocha-Guzmán NE, Mercado-Luna A, Pérez-Pérez MCI (2018) Effect of nitrogen privation on the phenolics contents, antioxidant and antibacterial activities in Moringa oleifera leaves. Ind Crops Products 114:45–51
Hashoum H, Gavinet J, Gauquelin T, Baldy V, Dupouyet S, Fernandez C, Bousquet-Melou A (2021) Chemical interaction between Quercus pubescens and its companion species is not emphasized under drought stress. Eur J For Res 140:333–343
Hassan MJ, Shafi M, Zhang GP, Zhu ZJ, Qaisar M (2008) The growth and some physiological responses of rice to Cd toxicity as affected by nitrogen form. Plant Growth Regul 54:125–132
Hassan MJ, Wang F, Ali S, Zhang GP (2005) Toxic effect of cadmium on rice as affected by nitrogen fertilizer form. Plant Soil 277:359–365
Huang J, Wang HY, Zhong YD, Huang JG, Fu XF, Wang LH, Teng WC (2019) Growth and physiological response of an endangered tree, Horsfieldia hainanensis merr., to simulated sulfuric and nitric acid rain in southern China. Plant Physiol Biochem 144:118–126
Hussain MI, El-Sheikh MA, Reigosa MJ (2020) Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants Basel 9:1228
Jmii G, Khadhri A, Haouala R (2020) Thapsia garganica allelopathic potentialities explored for lettuce growth enhancement and associated weed control. Sci Hortic 262:109068
Kalisz S, Kivlin SN, Bialic-Murphy L (2021) Allelopathy is pervasive in invasive plants. Biol Invasions 23:367–371
Keet JH, Richardson DM (2022) A rapid survey of naturalized and invasive eucalypt species in southwestern Limpopo, South Africa. South Afr J Bot 144:339–346
Kumar M, Garkoti SC (2022) Allelopathy effects of invasive alien Ageratina adenophora on native shrub species of chir pine forest in the central Himalaya, India. J For Res 27:53–62
Kyaw EH, Kato-Noguchi H (2020) Allelopathic potential of Acacia pennata (L.) Willd. leaf extracts against the seedling growth of six test plants. Not Botanicae Horti Agrobot Cluj Napoca 48:1534–1542
Li Y, Li C, Zhong SS, Xu ZL, Yu YL, Wang CY, Du DL (2024) Does salt stress intensify the allelopathic effect of four Asteraceae invasive plants? Pol J Ecol 71:59–74
Lindberg SE, Bredemeier M, Schaefer DA, Qi L (1990) Atmospheric concentrations and deposition of nitrogen and major ions in conifer forests in the United States and Federal Republic of Germany. Atmos Environ Part A Gen Top 24:2207–2220
Liu X, Fu ZH, Zhang B, Zhai L, Meng MJ, Lin J, Zhuang JY, Wang GG, Zhang JC (2018) Effects of sulfuric, nitric, and mixed acid rain on Chinese fir sapling growth in Southern China. Ecotoxicol Environ Saf 160:154–161
Liu X, Zhang B, Zhao WR, Wang L, Xie DJ, Huo WT, Wu YW, Zhang JC (2017) Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci Total Environ 601-602:669–678
Liu XY, You SH, Liu HJ, Yuan BJ, Wang HY, James EK, Wang F, Cao WD, Liu ZK (2020) Diversity and geographic distribution of microsymbionts associated with invasive mimosa species in Southern China. Front Microbiol 11:563389
Lu YJ, Wang YF, Wu BD, Wang S, Wei M, Du DL, Wang CY (2020) Allelopathy of three Compositae invasive alien species on indigenous Lactuca sativa L. enhanced under Cu and Pb pollution. Sci Hortic 267:109323
Medic A, Zamljen T, Slatnar A, Hudina M, Veberic R (2021) Is juglone the only naphthoquinone in Juglans regia L. with allelopathic effects? Agriculture 11:784
Medina-Villar S, Uscola M, Esther Perez-Corona M, Jacobs DF (2020) Environmental stress under climate change reduces plant performance, yet increases allelopathic potential of an invasive shrub. Biol Invasions 22:2859–2881
Min J, Shi WM (2018) Nitrogen discharge pathways in vegetable production as non-point sources of pollution and measures to control it. Sci Total Environ 613-614:123–130
Mozdzen K, Barabasz-Krasny B, Zandi P, Kliszcz A, Pula J (2020) Effect of aqueous extracts from Solidago canadensis L. leaves on germination and early growth stages of three cultivars of Raphanus sativus L. var. Radicula Pers. Plants 9:9111549
Mozdzen K, Tatoj A, Barabasz-Krasny B, Soltys-Lelek A, Gruszka W, Zandi P (2021) The allelopathic potential of rosa blanda aiton on selected wild-growing native and cultivated plants in Europe. Plants 10:10091806
Nsikani MM, Gaertner M, Latombe G, Esler KJ (2021) Soil nitrogen availability favours the growth but not germination of secondary invaders after clearing invasive Acacia saligna. South Afr J Bot 143:198–204
Reigosa MJ, Pedrol N, Sánchez-Moreiras A, González L (2002) Stress and Allelopathy. In: Reigosa MJ, Pedrol N (eds) Allelopathy from Molecules to Ecosystems. Science Publisher Inc., Enfield, p. 231–256
Rob MM, Iwasaki A, Suenaga K, Ozaki K, Teruya T, Kato-Noguchi H (2020) Potential use of Schumannianthus dichotomus waste: the phytotoxic activity of the waste and its identified compounds. J Environ Sci Health Part B 55:1099–1105
Sampaio JAGE, Reis CRG, Cunha-Lignon M, Nardoto GB, Salemi LF (2021) Plant invasion affects vegetation structure and sediment nitrogen stocks in subtropical mangroves. Mar Environ Res 172:105506
Sebastiano M, Messina S, Marasco V, Costantini D (2022) Hormesis in ecotoxicological studies: A critical evolutionary perspective. Curr Opin Toxicol 29:25–30
Selmar D, Kleinwächter M (2013) Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol 54:817–826
Shen LH, Guo QX, Xiong J, Li GQ, Lin WX (2008) Solidago canadensis L. allelopathy and resource competitiveness under different nitrogen supply. Chin J Eco Agric 16:900–904
Shumejko P, Ossipov V, Neuvonen S (1996) The effect of simulated acid rain on the biochemical composition of Scots pine (Pinus sylvestris L.) needles. Environ Pollut 92:315–321
Stefanowicz AM, Kapusta P, Stanek M, Frac M, Oszust K, Woch MW, Zubek S (2021) Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci Total Environ 767:145439
Sucur J, Konstantinovic B, Crnkovic M, Bursic V, Samardzic N, Malencic D, Prvulovic D, Popov M, Vukovic G (2021) Chemical composition of Ambrosia trifida L. and its allelopathic influence on crops. Plants 10:10102222
Sun WH, Yang GF, Cong LL, Sun J, Ma LC (2021) Allelopathic potency and an active substance from Hairy Vetch. Legume Res 44:46–50
Sun YM, Guo JJ, Li YR, Luo GW, Li L, Yuan HY, Mur LAJ, Guo SW (2020) Negative effects of the simulated nitrogen deposition on plant phenolic metabolism: A meta-analysis. Sci Total Environ 719:137442
Svensson JR, Nylund GM, Cervin G, Toth GB, Pavia H (2013) Novel chemical weapon of an exotic macroalga inhibits recruitment of native competitors in the invaded range. J Ecol 101:140–148
Ullah MS, Sun JF, Rutherford S, Ullah I, Javed Q, Rasool G, Ajmal M, Du DL (2021) Evaluation of the allelopathic effects of leachate from an invasive species (Wedelia triobata) on its own growth and performance and those of a native congener (W. chinensis). Biol Invasions 23:3135–3149
van den Elzen E, van den Berg LJL, van der Weijden B, Fritz C, Sheppard LJ, Lamers LPM (2018) Effects of airborne ammonium and nitrate pollution strongly differ in peat bogs, but symbiotic nitrogen fixation remains unaffected. Sci Total Environ 610-611:732–740
Villar-Salvador P, Peñuelas JL, Nicolás-Peragón JL, Benito LF, Domínguez-Lerena S (2013) Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? N For 44:733–751
Wang CY, Liu J, Xiao HG, Zhou JW, Du DL (2017a) Nitrogen deposition influences the allelopathic effect of an invasive plant on the reproduction of a native plant: Solidago canadensis versus Pterocypsela laciniata. Pol J Ecol 65:87–96
Wang CY, Wu BD, Jiang K, Zhou JW, Liu J, Lv YN (2019) Canada goldenrod invasion cause significant shifts in the taxonomic diversity and community stability of plant communities in heterogeneous landscapes in urban ecosystems in East China. Ecol Eng 127:504–509
Wang CY, Xiao HG, Zhao LL, Liu J, Wang L, Zhang F, Shi YC, Du DL (2016) The allelopathic effects of invasive plant Solidago canadensis on seed germination and growth of Lactuca sativa enhanced by different types of acid deposition. Ecotoxicology 25:555–562
Wang CY, Zhou JW, Jiang K, Liu J, Du DL (2017b) Responses of soil N-fixing bacteria communities to invasive plant species under different types of simulated acid deposition. Sci Nat 104:43
Wang RL, Rehman SU, Liang XT, Song YY, Su YJ, Baerson SR, Zeng RS (2012a) Effects of simulated acid rain on the allelopathic potential of invasive weed Wedelia trilobata. Allelopath J 30:23–32
Wang RL, Staehelin C, Dayan FE, Song YY, Su YJ, Zeng RS (2012b) Simulated acid rain accelerates litter decomposition and enhances the allelopathic potential of the invasive plant Wedelia trilobata (Creeping Daisy). Weed Sci 60:462–467
Wang S, Cheng HY, Wei M, Wu BD, Wang CY (2020a) Litter decomposition process dramatically declines the allelopathy of Solidago canadensis L. on the seed germination and seedling growth of Lactuca sativa L. Int J Phytoremediat 22:1295–1303
Wang S, Wei M, Wu BD, Cheng HY, Wang CY (2020b) Combined nitrogen deposition and Cd stress antagonistically affect the allelopathy of invasive alien species Canada goldenrod on the cultivated crop lettuce. Sci Hortic 261:108955
Wang X, Liu YQ, Peng N, Yu HT, Ma Y, Zhang MX, Wang YY, Wang Y, Gao WW (2024) Allelopathy and identification of volatile components from the roots and aerial parts of Astragalus mongholicus Bunge. Plants 13:317
Wei M, Wang S, Cheng HY, Wu BD, Wang CY (2020a) The mixed silicon and cadmium synergistically impact the allelopathy of Solidago canadensis L. on native plant species Lactuca sativa L. Ecotoxicology 29:1095–1104
Wei M, Wang S, Wu BD, Cheng HY, Wang CY (2020b) Combined allelopathy of Canada goldenrod and horseweed on the seed germination and seedling growth performance of lettuce. Landsc Ecol Eng 16:299–306
Xu ZL, Zhong SS, Yu YL, Wang YY, Cheng HY, Du DL, Wang CY (2023) Rhus typhina L. triggered greater allelopathic effects than Koelreuteria paniculata Laxm under ammonium fertilization. Sci Hortic 309:111703
Yu YL, Cheng HY, Wang CY, Du DL (2023a) Heavy drought reduces the decomposition rate of the mixed litters of two composite invasive alien plants. J Plant Ecol 16:rtac047
Yu YL, Cheng HY, Xu ZL, Zhong SS, Wang CY, Guo EH (2022) Invasion intensity modulates the allelopathic impact of Solidago canadensis L. leaves and roots against Lactuca sativa L. during germination and early seedling stage. Int J Environ Res 16:48
Yu YL, Zhong SS, Xu ZL, Xu ZY, Wang CY, Du DL (2023b) Does the salt stress intensify the independent allelopathy and the co-allelopathy of Solidago canadensis L. and Conyza canadensis (L.) Cronq.? South Afr J Bot 153:37–45
Zandi P, Barabasz-Krasny B, Stachurska-Swakon A, Pula J, Mozdzen K (2020) Allelopathic effect of invasive Canadian goldenrod (Solidago canadensis L.) on early growth of red clover (Trifolium pratense L.). Not Botanicae Horti Agrobot Cluj Napoca 48:2060–2071
Zhang C, Yi X, Gao X, Wang M, Shao C, Lv Z, Chen J, Liu Z, Shen C (2020) Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol Environ Saf 192:110315
Zhong SS, Xu ZL, Li Y, Li C, Yu YL, Wang CY, Du DL (2023) What modulates the impacts of acid rain on the allelopathy of the two Asteraceae invasives? Ecotoxicology 32:114–126
Zhong SS, Xu ZL, Yu YL, Cheng HY, Wang S, Wei M, Du DL, Wang CY (2022) Acid deposition at higher acidity weakens the antagonistic responses during the co-decomposition of two Asteraceae invasive plants. Ecotoxicol Environ Saf 243:114012
Zhou JL, Xu ZL, Zhong SS, Yu YL, Xu ZY, Du DL, Wang CY (2022) Nitrogen influence to the independent invasion and the co-invasion of Solidago canadensis and Conyza canadensis via intensified allelopathy. Sustainability 14:11970
Acknowledgements
This study was supported by Special Research Project of School of Emergency Management, Jiangsu University (Grant No.: KY-C-01), Research project on the application of invasive plants in soil ecological restoration in Jiangsu (Grant No.: 20240110), Carbon Peak and Carbon Neutrality Technology Innovation Foundation of Jiangsu Province (Grant No.: BK20220030), National Natural Science Foundation of China (Grant No.: 32071521), and Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment (no grant number).
Author information
Authors and Affiliations
Contributions
Chuang Li: Data curation; Investigation; Methodology; Writing - review & editing. Yue Li: Data curation; Investigation; Methodology; Writing - review & editing. Zhelun Xu: Data curation; Investigation; Methodology; Writing - review & editing. Yingsheng Liu: Data curation; Investigation; Methodology; Writing - review & editing. Shanshan Zhong: Data curation; Investigation; Writing - review & editing. Congyan Wang: Conceptualization; Formal analysis; Funding acquisition; Project administration; Supervision; Writing - original draft. Daolin Du: Funding acquisition; Project administration; Supervision; Writing - review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Li, Y., Xu, Z. et al. The nitrogen-sulfur ratio of acid rain modulates the leaf- and root-mediated co-allelopathy of Solidago canadensis. Ecotoxicology 33, 893–904 (2024). https://doi.org/10.1007/s10646-024-02788-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-024-02788-2