Abstract
Advancing the field of fish ecology requires a shift in focus from describing patterns in species occurrences to understanding the mechanisms that regulate distributions and abundances across broad scales. For stream fish ecology, this includes understanding environmental mechanisms that regulate stream fish demographic properties at the scale of stream networks or riverscapes. Despite the fact that Banded Sculpin Cottus carolinae occupy a diversity of habitats and stream sizes across the southeastern United States, relatively little is known about the demography of this species. We assessed annual demographic properties (reproduction, growth, and survival) of C. carolinae collected monthly from four sites distributed longitudinally along the Roaring River riverscape in Tennessee to simultaneously describe life history attributes of the species and address riverscape-scale variation in population dynamics. Cottus carolinae lived for a maximum of four years, local populations were dominated by age-0 and age-1 individuals, reproduction began after one year, spawning occurred during December and January, and mean ova number was 398. A life history tradeoff between growth (robustness) and survival was evident at one site where water temperature and flow were least variable, otherwise life history attributes were consistent across the riverscape despite longitudinal changes in abiotic variables. Our work illustrates the potential for muted population responses to a strong hydrologic gradient in stream size and highlights the stability inherent with fish life history adaptations to natural environmental regimes across broad spatial scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fishes are embedded within ecosystems through ecological mechanisms that regulate species distributions and abundances (Matthews 1998). Regulatory mechanisms include geographic filters (e.g., spatial variability in habitat templates and geographic barriers to dispersal), interactions with other organisms (e.g., competition and predation), and dynamic abiotic regimes (e.g., hydrologic disturbances) that drive life history adaptations and ultimately control population demographics (Winemiller and Rose 1992; Gido and Jackson 2010; Ross 2013). For example, in guppies (Poelicia reticulata), variation in life history attributes (growth, survival, reproduction) are linked to spatial variation in food availability (Reznick and Yang 1993), occurrence of predators (Reznick et al. 1996), and forest canopy cover (Grether et al. 2001). For stream fishes, species occurrences and abundances depend largely on mechanistic linkages between hydrological factors (e.g., flow magnitude) and the functional traits of fishes that control population growth (Poff and Allan 1995; Olden and Kennard 2010; Perkin et al. 2017). Although multiple theoretical frameworks exist for describing longitudinal gradients in hydrologic factors (e.g., stream ordering, Strahler 1957) and the associated consequences for species occurrences along these gradients (e.g., River Continuum Concept; Vannote et al. 1980), few studies have tracked broad-scale spatial variability in fish populations across riverscapes to inform fish ecology and management (Fausch 2010).
Stream fish population regulation is linked to demographic properties such as growth, survival and reproduction that are in turn altered by environmental variability along a stream order gradient (Werner and Gilliam 1984). For example, flow magnitude and habitat diversity increase downstream as stream order increases (Horwitz 1978; Schlosser 1982), but discharge variability and canopy cover increase in an upstream direction (Vannote et al. 1980; Schlosser 1987; Dodds et al. 2004). These abiotic gradients are linked to fish demographic properties including greater population growth (Schlosser 1990), extinction rate (Taylor and Warren 2001), and recolonization rate (Schlosser 1990) among upstream segments. However, other demographic properties such as life span (Schlosser 1990) and carrying capacity (Vannote et al. 1980) are maximized among downstream segments. Each of these examples document only one or two demographic properties among a multitude of species, and broad scale tests of environmental controllers of demographic properties within a single species are rare. Advancing the field of fish ecology by assessing riverscape-scale variation in fish population dynamics will require documenting patterns in demographic responses along longitudinal gradients ranging from small headwater streams to large rivers. Because such gradients typically range beyond the spatial distribution of a single species (e.g., Troia and Gido 2014), cosmopolitan species that inhabit broad ranges of habitat are critical to testing riverscape-scale hypotheses (Adams and Schmetterling 2007).
Fishes in the family Cottidae inhabit a wide range of stream orders and represent an opportunity to test riverscape-scale hypotheses regarding environmental controllers of fish populations. For example, previous studies of Cottus spp. document longitudinal attenuations in survival and density (Anderson 1985; Keeler et al. 2007), yet Anderson (1985) also suggested growth rate and fecundity of individuals increased longitudinally. Banded Sculpin Cottus carolinae (Gill 1861) inhabits streams of most dimensions, ranging from small headwater streams to medium-sized rivers (Etnier and Starnes 1993). Across this range of stream sizes, C. carolinae utilize habitats similarly regardless of stream scale (Gebhard et al. 2017) and exhibit leptokurtic dispersal in which most individuals are stationary and few are mobile (Wells et al. 2017). Collectively, these recent observations suggest environmental controllers of C. carolinae demography operate similarly across local habitat templates and are likely to be independent of other locations within a stream network given the restricted movement of the species. However, little else is known about the ecology of C. carolinae, including basic life history attributes such as longevity, age at maturation, spawning seasonality, sex ratios, ova size, and clutch size. Existing studies reporting on portions of C. carolinae life history are often paired with uncertain or contradicting accounts. For example, C. carolinae reportedly nest over rubble, gravel, and sand in crevices on the undersurfaces of logs and rocks (Wallus and Grannemann 1979), although Williams and Robins (1970) suggested singly laid eggs or broadcast spawning. Spawning occurs between January and February in Alabama (Williams and Robins 1970; Wallus and Grannemann 1979) and from January to March in Kentucky (Craddock 1965) when water temperatures range between 9 and 13 °C (Wallus and Grannemann 1979); however, spawning is documented between March and early April in Tennessee (Hunt 1989) and southern Illinois (Smith 1979). These contradictory accounts illustrate a level of uncertainty surrounding C. carolinae reproductive ecology and suggest additional research might simultaneously advance knowledge of C. carolinae basic biology and assess riverscape-scale variation in fish demographic properties.
We collected basic life history information for C. carolinae across multiple streams of increasing size to assess riverscape-scale patterns in demographic processes including growth, reproduction, and survival. Specific objectives were to (1) describe general life history traits including longevity, age at maturation, spawning seasonality, sex ratio, ova size, and clutch size, and (2) test for spatial variability in three fundamental demographic properties including growth, reproduction, and survivorship. Based on finer scale works with other Cottus fishes (Anderson 1985; Keeler et al. 2007), we hypothesized that life history attributes would vary across a riverscape in which growth and fecundity increased in a downstream direction but at a cost (i.e., tradeoff) to survival. This work ultimately addresses a key research need regarding improved understanding of broad-scale demography of fish populations to inform fish ecology, conservation, and management (Fausch 2010).
Materials and methods
Study area
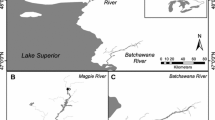
This study was conducted in the Roaring River basin of Tennessee within the Eastern Highland Rim ecoregion (Fig. 1). Study sites were selected along a longitudinal stream order gradient and represented a transition from a small headwater stream to a medium-size river. Streams included Little Creek (2nd order) on Tennessee Technological University Shipley Farm (N 36° 11′ 40.5″ W 85° 32′ 38.4″), West Blackburn Fork (3rd order) near the State Highway 290 crossing (N 36° 13′ 20.9″ W 85° 34′ 26.0″), Blackburn Fork (4th order) 12.01 km downstream of a series of waterfalls that separate the upper and lower basins (N 36° 17′ 46.9″ W 85° 33′ 40.4″), and the Roaring River (5th order) at the Tennessee Wildlife Resources Agency Boils Wildlife Management Area (N 36° 21′ 03.5″ W 85° 33′ 53.9″). Streams were dominated by gravel and cobble substrates, mixes of riffle and pool habitats, and intact riparian corridors at all sites. Major land uses in the basin in descending order of coverage include forest, rangeland, agriculture, and urbanization (see Crumby et al. 1990).
Site heterogeneity
Site heterogeneity was assessed using a combination of transect measurements and continuous data loggers. Ten evenly spaced transects established at each site were used to measure active channel width (m), canopy cover, and substrate composition. Active channel width was defined according to Davies-Colley (1997) and included the width of stream with no terrestrial vegetation on substrates despite the fact that substrates might be exposed or out of the water. We measured canopy cover at the center of each transect using a concave densitometer, facing upstream, and enumerating the proportion of open canopy. We measured substrate composition at 10 evenly spaced points along each transect (100 points of substrate per site) using Bovee (1982) substrate classifications (i.e., silt, sand, gravel, cobble, boulder, and bedrock). At each site, continuous water pressure (kPa) and temperature (°C) were monitored hourly using HOBO pressure transducers (Model U20 L-01, Onset Computer Corporation, Bourne, MA). A metered stage was secured in close proximity to each pressure transducer to monitor changes in water level (m). Discharge measurements (m3 s−1) were taken approximately bi-monthly at each site using the U.S. Geological Survey cross-sectional discharge approach of measuring depth and velocity at 60% depth at 20 points across the channel (Turnipseed and Sauer 2010).
Fish collections
Cottus carolinae were collected monthly from each site starting in May 2015 and ending in April 2016. Fish were collected at Little Creek and West Blackburn Fork using a backpack electrofishing unit (100–150 V, direct current) with two dip netters (3.2 mm mesh) moving in an upstream direction and sampling all available habitat. At the larger Blackburn Fork and Roaring River sites, electrofishing samples were collected in concert with a stretched 4.5 m by 1.8 m seine with 3.2 mm mesh. Per the methods of Potoka et al. (2016), this process involved a two-person crew setting a seine in place and a third person starting 3 m upstream of the seine and shocking in a downstream direction into the seine. A minimum of 10 and maximum of 30 seine sets during each visit were employed at Blackburn Fork and Roaring River. All sites were sampled for fish within a two-day period once a month. Targeted sample size for measurements of total length (TL, mm) was 30 fish per site, and from these fish the first 10 were retained for laboratory analyses. On rare occasions, sites did not produce 10 individuals and periodically more than 10 individuals were kept from a site to ensure all size classes were analyzed. Retained individuals were stored in 10% formalin (buffered with sodium bicarbonate to a pH of ~7) for a minimum of 48 h and processed in the laboratory within one week of field collections.
Laboratory protocols
In the laboratory, we collected morphological and life history attribute data, including total weight (g), standard length (SL, mm), total length (TL, mm), eviscerated weight (EW, entire digestive tract and gonads removed, g), gonad weight (g), and sex. Sagittal otoliths were removed from each individual and stored dry until further analysis. Gonads were removed and preserved in 10% neutrally buffered formalin. Reproductive stage of females was evaluated and classified as immature-resting (oocytes transparent with a visible nucleus and no yolk deposition), developing (oocytes translucent with a white to cream appearance), or vitellogenic (oocytes yellow in coloration; Heins and Baker 1987; Heins and Rabito 1988; Marconato and Bisazza 1988). Clutch size was calculated by teasing apart the left ovary of each reproductively active female in a petri dish, counting vitellogenic oocytes, and multiplying by two (Wilde and Durham 2008; Perkin et al. 2012). Oocyte size was estimated by choosing 100 oocytes at random to measure diameter (mm) using a trinocular stereomicroscope (Meiji Techno America, San Jose, CA, USA) fitted with a calibrated digital camera (i-Solutions, Vancouver, BC, Canada). Reproductive investment and seasonality were measured using the gonadosomatic index (GSI):
where GW is gonad weight and EW is the eviscerated weight of the fish (Copp et al. 2002; Martínez-Gómez et al. 2012; Kacem et al. 2015). Longevity was estimated by visually aging otoliths after immersion in glycerin for 48–120 h (Jearld 1983). The aging process was conducted by placing whole otoliths on a glass slide under a dissecting microscope with a drop of glycerin and viewing them in reflected light (Jearld 1983). Age estimation followed Quist et al. (2012) including a second blind otolith reading on all otoliths and a third reading when necessary to reach age consensus.
Data analysis
Environmental variables including sensor depth, daylight hours, stream discharge, and water temperature were recorded for an annual cycle at each site. Pressure transducer depth (sensor depth) was estimated using pressure data from HOBO loggers combined with daily atmospheric pressure from the Upper Cumberland Regional Airport near Sparta, TN (USAF WBAN ID: 723,274 99,999; NOAA 2016). Daily duration of daylight was downloaded from the United States Naval Observatory (Cookeville, TN: W 085° 30′00″, N 36° 10′00″; UNSO 2016). Time series data were used to illustrate temporal change in sensor depth, daylight hours, and water temperature among sites. Finally, probability exceedance curves were constructed for discharge (m3 s−1) based on field measurements described above and temperature (°C) recorded by pressure transducers to illustrate longitudinal differences among sites.
Reproductive biology and life history attributes of C. carolinae were analyzed using descriptive statistics and regression. Longevity was measured as the oldest individual captured among all four sites across the duration of the study (Hunt 1989). Size and age at maturation were estimated using the length at which the probability of being reproductively mature exceeded 0.5 for fishes collected during December and January (females) and November through January (males), and then relating the corresponding length to age in years based on otolith aging. We defined reproductively mature individuals as those with GSI values greater than half the maximum average GSI value observed across all months included in this analysis, which corresponded to GSI ≥4 for females and GSI ≥0.3 for males. We then fit a logistic regression model using length as the independent variable and reproductive maturity (0 = immature, 1 = mature) as the response variable using the ‘glm’ function from the ‘stats’ package in Program R. Spawning seasonality was determined using the months during which GSI values were elevated (i.e., > 50% of maximum average value) for males and females. Sex ratio was calculated as the number of males/females returned to the laboratory. To determine if the sex ratio was 1:1 across sites, a Chi-Square test in Program R function ‘MASS’ was conducted. Mean ova size was calculated across all reproductive mature females with vitellogenic oocytes (Perkin et al. 2009, 2012). Mean clutch size was measured as the number of vitellogenic oocytes present in ovaries of reproductively mature females. Distribution of 100 randomly measured oocyte diameters (including latent, developing, and mature) from mature females was used to evaluate spawning bouts based on probability density functions (PDF) from package ‘MASS’ (Venables and Ripley 2002). Monthly size structures for age 0 (cohort from 2015), age 1 (cohort from 2014), and age 2 (cohort from 2013) were analyzed by combining all sites for each month to create PDFs using package ‘MASS’ (Venables and Ripley 2002). We elected to use PDFs rather than frequency histograms because PDFs provide summarized distributions that facilitate comparison of continuous response variables (e.g., oocyte diameter, fish size) among individuals or sites without the need to identify appropriate data bins. However, we did include histograms of oocyte sizes (bin = 0.25 mm) and fish total lengths (bin = 5 mm) to facilitate interpretation of PDF plots.
Site-specific differences in demographic processes were analyzed using mixed modelling and Leslie matrix modelling. Growth (i.e., robustness) was quantified as the relationship between EW (dependent variable) and TL (independent variable) and was summarized using a generalized additive mixed model (GAMM). A GAMM approach was necessary because the relationship was non-linear with non-standardized variances and because individuals were collected from the same sites over time (i.e., non-independent). We included an adjustment for autocorrelation (i.e., AR1) because fish of similar size are more likely to have similar weights, used site and sex as fixed factors, included a fixed smoothing term for TL, used repeated measurements of individuals at each site as a random variable to account for pseudoreplication, and modeled the relationship using a quasipoisson error distribution to account for overdispersion (Zuur et al. 2007). Reproduction timing was quantified as the relationship between GSI (dependent variable) and time (month; independent variable) and summarized using a similar GAMM approach in which autocorrelation and pseudoreplication adjustments were included, sex and site were fixed factors, a smoothing term was fit to month, and a quaisipoisson error distribution employed. All GAMMs were conducted using the ‘gamm4’ function from the ‘mgcv’ package in Program R (Wood and Scheipl 2014). Survival was based on the abundance of individuals assigned to each age class at each site and analyzed using Heincke’s method of estimating annual mortality (Heincke 1913). This model assumes constant recruitment and that only individuals that have recruited to catchable size are modeled (Heincke 1913). Because age-0 individuals were too small for capture in standardized sampling gear (i.e., 3.2 mm mesh on dip nets and seines), and because age 3+ individuals were rare, annual survival of only age-1 and age-2 fish were modeled. We first calculated annual survivorship for age-1 and age-2 fish following the methods of Miranda and Bettoli (2007), and then used the “leave one out” approach described by Vaughan and Saila (1976) in a Leslie matrix to estimate age-0 survival. Specifically, we populated the Leslie matrix using fecundity estimates based on ovary dissections as described above and held constant across sites (Wilde and Durham 2008), age-1 and age-2 survival estimates based on the Heincke method, and then iteratively solved for an age-0 survival that produced a population growth rate equal to 1 (Vaughan and Saila 1976). Leslie matrix calculations were conducted using the ‘Matrix’ package from Program R (Bates and Maechler 2015).
Results
Site heterogeneity
Environmental variables measured at each site were consistent with increasing stream size from upstream to downstream. Mean active channel width, canopy openness, and percent gravel substrate increased in the downstream direction (Table 1). Exceedance plots illustrated increased flow magnitude along the longitudinal gradient (Fig. 2a), while water temperature did not follow a longitudinal pattern and instead converged among all sites at 12.9 °C (Fig. 2b). Flow and temperature regimes followed consistent timing but differing magnitudes (as illustrated in exceedance plots) among sites. Flow regime magnitude measured as sensor depth illustrated consistent timing but increased magnitudes in flow pulses along the river continuum (Fig. 3). The site with the coldest recorded water temperature was Blackburn Fork (1.1 °C), followed by West Blackburn Fork (3.8 °C), and then Roaring River and Little Creek with the same low (3.9 °C). Maximum temperatures recorded by site did not follow a longitudinal gradient and included Blackburn Fork (30.1 °C), Little Creek (29.3 °C), Roaring River (25.2 °C), and West Blackburn Fork (22.6 °C). Daylight duration was greatest (i.e., 14.62 h) during June and least (i.e., 9.70 h) during December.
Probability of exceedance for a discharge (m3 s−1) and b temperature ( ̊C) at four sites in the Roaring River basin, Tennessee (see Fig. 1 for locations)
Hourly temperature, hourly sensor depth, and daylight duration for each site in order of increasing stream order, including a Little Creek, b West Blackburn Fork, c Blackburn Fork, and d Roaring River. Timing of discharge readings used to constructing the exceedance curves in Fig. 2 are denoted by vertical bars near the x-axis
Basic life history
A total of 511 C. carolinae were examined in this study. Among the individuals examined, 42.0% were age 0 (n = 214), 48.4% were age 1 (n = 247), 8.8% were age 2 (n = 45), 0.06% were age 3 (n = 3), and 0.02% were age 4 (n = 1). Age-0 fishes were spawned during 2015 and were <50 mm TL during May 2015 when sampling began (Fig. 4). The 2015 cohort was distinctly smaller in size than the 2014 cohort, but grew most rapidly during May–October. The 2014 cohort experienced loss of larger individuals during June–September, and was most truncated during January and February. The 2013 cohort was collected during May, November, and December of 2015, and March of 2016. The estimated size at maturity was 95 mm TL for females (Fig. 5a) and 105 mm TL for males (Fig. 5b), and corresponded with age-1 based on otolith aging. Female spawning activity occurred during December and January when GSI values averaged 7.8 (Fig. 5c), and male spawning activity occurred during October–January when GSI values averaged 0.60 (Fig. 5d). The sex ratio was 0.87:1.00, including 234 females (46%) and 268 males (54%) across all sites, and was not significantly different from 1:1 (x2 = 2.30, df = 1, P = 0.13). The mean (± standard deviation) oocyte diameter across 9 mature females was 1.33 mm (±0.29) with a range of 0.26–2.37 mm. Clutch size varied among ages, including a mean of 377 ova for age 1, 390 ova for age 2, and 538 ova for age 3, with an overall mean of 398 (range 266–538) ova. Probability density functions for oocyte diameters showed polymodal distributions with high probabilities of oocytes exceeding 1.0 mm for mature females collected during December 2015, but reduced probabilities of larger oocytes during January 2016 (Fig. 6), suggesting spawning activity before or during January.
Monthly total length (mm) probability density distributions for Cottus carolinae year classes 2013 (dash-dot-dot), 2014 (solid), and 2015 (dashed) collected from the Roaring River basin during a May, b June, c July, d August, e September, f October, g November, and h December of 2015 and i January, j February, k March, and l April of 2016. Bars illustrate raw data frequency histograms (bin size = 5 mm)
Relationship between size and probability of maturity for a female and b male Cottus carolinae in the Roaring River basin of Tennessee. Logistic regression was used to estimate the size at which probability of maturity exceeded 0.5 (95 mm TL for females, 105 mm TL for males). Relationship between time (month) and gonadosomatic index for c female and d male C. carolinae collected from four sites (see Fig. 1 for locations). A generalized additive mixed model was fit across sites and months and is illustrated by the fitted values (dark gray line) and 95% confidence intervals (light gray shaded area)
Oocyte probability density distributions for nine reproductively mature female Cottus carolinae collected from the Roaring River basin, Tennessee during December 2015 and January 2016. TL represents total length (mm) and GSI represents gonadosomatic index. Probability density distributions were conducted using 100 randomly selected oocytes from the left ovary of each reproductively mature female, and raw data are shown as frequency histograms (bin size = 0.25 mm)
Riverscape-scale variation in demographic properties
Reproduction timing was consistent among sites across the riverscape, but growth and survival were not. The GAMM used to model GSI as a function of time included a significant term for sex (t = −11.81, p < 0.001) and month (estimated df = 7.69, F = 44.06, p < 0.001, adjusted R 2 = 0.65). Sex-specific relationships between GSI and month were then tested using Little Creek as the baseline. The GAMM fit to only females included a significant term for month (estimated df = 8.02, F = 52.41, p < 0.001, adjusted R 2 = 0.58) characterized by increased GSI values during December and January, and this pattern did not differ between Little Creek and West Blackburn Fork (t = 0.16, p = 0.88), Blackburn Fork (t = −0.86, p = 0.40), or Roaring River (t = 0.37, p = 0.71). The GAMM fit to only males included a significant term for month (estimated df = 5.64, F = 30.18, p < 0.001, adjusted R 2 = 0.58) characterized by increased GSI values during October–January, and this pattern did not differ between Little Creek and West Blackburn Fork (t = −1.89, p = 0.06), Blackburn Fork (t = −0.77, p = 0.45), or Roaring River (t = 1.64, p = 0.11). The GAMM fitted to weight as a function of length illustrated length-weight relationships were not consistent across sites when Little Creek was used as the baseline. Growth was consistent between Little Creek and Blackburn Fork (t = −1.62, p = 0.11) and Little Creek and Roaring River (t = 1.03, p = 0.30), but not between Little Creek and West Blackburn Fork (t = 96.71, p < 0.001). We then fit a GAMM using only data from West Blackburn Fork (estimated df = 5.23, F = 1532, p < 0.001, adjusted R 2 = 0.98) and a second GAMM using data combined from Little Creek, Blackburn Fork, and Roaring River (estimated df = 6.41, F = 2975, p < 0.001, adjusted R 2 = 0.97). The two independently fit GAMMs illustrated greater growth (i.e., weight at length) at West Blackburn compared to all other sites (Fig. 7). Age-specific survival differed among sites, but there was no evidence of a longitudinal pattern. Estimated age-0 survival was consistently low across sites (0.00395–0.00450), age-1 survival was highest at Little Creek (0.32) but lowest at West Blackburn Fork (0.09), and age-2 survival was zero at two sites, 0.06 at Little Creek, and 0.15 at Roaring River (Table 2).
Length-weight relationship across all dates and sites with a fitted generalized additive mixed model (GAMM; black solid line) and 95% confidence intervals (dark gray shading) for West Blackburn Fork and a fitted GAMM (dark gray solid line) with a 95% confidence interval (light gray shading) for all remaining sites (i.e., Little Creek, Blackburn Fork, and Roaring River). Allometric growth equations fit to raw data for each site are given
Discussion
Cottus carolinae in the Roaring River basin live for a maximum of four years, begin reproduction at age-1, and spawn during December and January when ova averaging 1.33 mm in size are produced in clutches of 266–538. We found the Roaring River population was characterized by a 1:1 sex ratio and was dominated by age-0 and age-1 individuals. Age-0 survival was low compared to age-1 and age-2, suggesting high mortality at early life stages. Average longevity was two years and maximum life span was four years, as evidenced by dominance by only two cohorts across most of our sampling, low vital rates for age-2 and age-3 individuals, and collection of only one age-4 individual during this study. We initially predicted three demographic properties would vary across the riverscape, including increases in growth and fecundity in a downstream direction but at a cost to survival. Instead, we found that reproductive investment and timing were consistent across the riverscape, but growth and survival differed at only one site where greater growth came at a cost to age-1 survival. These findings collectively show that C. carolinae is a short-lived, early maturing species with relatively low fecundity and low juvenile survival, and therefore fits the opportunistic life history strategy described by Winemiller and Rose (1992). Life history theory predicts that opportunistic life history strategists should be most abundant in dynamic and unpredictable environments (Mims and Olden 2012). Dynamic stream systems such as the Roaring River basin exist throughout the southeastern United States, including the range of C. carolinae, and are dominated by fishes employing an opportunistic life history strategy (Mims et al. 2010). These similarities highlight a deep evolutionary history between C. carolinae and dynamic and unpredictable stream habitats.
Cottus carolinae life history attributes in the Roaring River basin were consistent with some previous Cottus biology studies, but contrasted others. It appears female C. carolinae in the Roaring River basin spawn during a short reproductive season corresponding with winter high flows. Similarly, congener species like Mottled Sculpin Cottus bairdii (Girard 1850) and Slimy Sculpin Cottus cognatus (Richardson 1836) are reported to spawn during narrow spawning seasons (Downhower and Brown 1980; Becker 1983). Wallus and Grannemann (1979) extracted fertilized C. carolinae eggs from a stream in Alabama and successfully hatched 225 larval individuals after 15–19 days of incubation. Based on their observations, Wallus and Grannemann (1979) predicted clutch-sizes to average 100–300 ova, a value similar to our finding as well as the mean clutch size of 477 ova (range 222–1258) reported by Craddock (1965). However, the longevity of four years in the Roaring River basin contrasts previous suggestions of six year classes among Tennessee populations (Etnier and Starnes 1993). Furthermore, the observed December to January spawning season in the Roaring River extends the known initiation of spawning by one month compared to studies documenting the beginning of spawning in January (Craddock 1965; Williams and Robins 1970; Wallus and Grannemann 1979), and represents a truncation in spawning season length compared to reports of spawning ending in March or April (Craddock 1965). Discrepancies in reproductive life history information might be related to multi-scale spatial differences in the environmental factors that control life history adaptations, including photoperiod and water temperature as suggested for Cyprinidae and Percidae fishes (Hubbs 1985; Gotelli and Pyron 1991; Perkin et al. 2012). At the scale of the Roaring River basin, environmental variables did not affect spawning magnitude or timing based on GSI values. This finding is surprising given the presumed effect of discharge on fish reproduction (Munz and Higgins 2013) and the fact that discharge varied over two orders of magnitude across the Roaring River riverscape. Life history adaptations in highly-studied guppy populations suggest abiotic mechanisms such as environmental gradients in canopy cover, as well as biotic mechanisms such as the presence of predators can result in variation in life history attributes across space and time (Reznick and Yang 1993; Reznick et al. 1996; Grether et al. 2001). Detailed studies of C. carolinae population and life history attributes are currently limited, but our data are useful for comparisons with future studies conducted across the range of the species and allow for assessing transferability of life history predictions among riverscapes (Gebhard et al. 2017). It is clear from our analysis that some level of life history plasticity should be expected within and among populations.
There was evidence for a demographic trade-off between growth and survival for C. carolinae in West Blackburn Fork. Survival of age-1 fish was lower, but growth (or ‘robustness’) was significantly higher at West Blackburn Fork compared to Little Creek. Anderson (1985) found total length of C. bairdii and C. cognatus increased along a longitudinal stream gradient and suggested increased predation by piscivores (Rock Bass Ambloplites rupestris) (Rafinesque 1817) that do not occur in headwater streams disproportionally targeted smaller sculpin among downstream sites. Predatory fishes including A. rupestis are absent from headwaters (Little Creek) and increase in prevalence among larger stream orders (West Blackburn Fork, Blackburn Fork, and Roaring River) in the Roaring River basin (Hooper 1977; Amy Gebhard, personal observation). The occurrence of predators might result in disproportional survival of larger (heavier) sculpin among sites where predators are abundant, and might explain the overall reduction of age-1 survival among larger streams. This trade-off represents a ‘live fast, die young’ framework in which C. carolinae obtain larger sizes or higher condition, but suffer greater mortality in doing so (Metcalfe and Monaghan 2003). However, predation alone does not explain consistent robustness among sites outside of West Blackburn Fork. At these sites, mechanisms such as counter-gradient variation in growth rates might allow for consistent robustness if fishes add weight more rapidly where growth conditions are truncated, and this hypothesis might be tested in future experimentation (e.g., Schultz et al. 1996). Robustness is determined by energy availability, and condition is maximized near thermal optima when prey is abundant (Kappenman et al. 2009). Across sites in the Roaring River riverscape, West Blackburn Fork maintained the most consistent thermal regime, and analysis of stomach contents removed from fish collected during this study suggests prey availability was most consistent at West Blackburn Fork (William Curtis, Tennessee Technological University, unpublished manuscript). The availability and diversity of prey at West Blackburn Fork might allow individuals to be more robust even though predation pressure is high. Regardless of the mechanism(s), our findings suggest demographic patterns among C. carolinae populations do not follow a strong longitudinal pattern even though at least one local trade-off was apparent. Instead, at the riverscape scale, local ecosystem processes operated within broader reach, segment, and watershed processes to determine local population demographics (Fausch et al. 2002). Given that C. carolinae fit an opportunistic life history strategy, life history theory predicts that environmental factors should control population growth more strongly than biotic processes (Winemiller and Rose 1992). Population dynamics of short-lived, opportunistic fishes are most sensitive to age-1 survival and reproduction (Vélez-Espino et al. 2006) and understanding the manner in which abiotic processes regulate these demographic properties is critical to advancing fish ecology. Our findings suggest that although local demographic properties may be regulated by local abiotic processes, hydrologic connectivity (sensu Pringle 2003) in the form of consistent timing and seasonality of flow across the basin might act to synchronize local populations. Ultimately, this results in demographic consistency and therefore muted life history divergence across inconsistent local sites distributed along strong environmental gradients.
This study strengthens our knowledge of C. carolinae in particular and fish ecology in general. We found that winter-time seasonal fluctuations in photoperiod, water temperature, and discharge magnitude occurred during a period of synchronized spawning by C. carolinae across an entire riverscape. Reproductive output measured as GSI was similar across stream orders despite strong differences in habitat area and volume. However, robustness and annual mortality were neither consistent across all sites nor oriented along a longitudinal gradient. Instead, growth and survival were consistent at three sites and potentially operating as a trade-off at the fourth site. These results support recent findings that C. carolinae populations exhibit similar ecological patterns across sites distributed among differing basins (Gebhard et al. 2017). Future studies should continue to shed light on C. carolinae ecology, morphology, and demographic processes due to their ecological importance (e.g., glochidia host: Zale and Neves 1982; Yeager and Saylor 1995). Additionally, the plasticity of C. carolinae was recently linked to speciation, driven by isolation and environmental selection within different habitats (e.g., Grotto Sculpin Cottus specus; Adams et al. 2013; Day et al. 2016) and further investigation of C. carolinae across its broad distribution could elucidate more facets of the species. From a general fish ecology perspective, our results suggest strong environmental gradients do not always solicit strong population responses, even among opportunistic life history strategists that are expected to respond most readily to environmental variability (Winemiller and Rose 1992). Stability of C. carolinae demographics across sites with varying levels of environmental variability is likely an artifact of life history adaptations to a variable environment (Lytle and Poff 2004). Similar adaptations by native fish species are reported elsewhere (e.g., McManamay and Frimpong 2015) and represent a mechanism through which populations maintain stability in unpredictable environments (Gido et al. 2000). Robust and plastic demographic properties are therefore critical for fishes that persist along a wide range of environmental conditions (Jackson et al. 2001), and this study provides quantitative evidence for the existence of demographic consistency across a riverscape (Fausch 2010).
References
Adams SB, Schmetterling DA (2007) Freshwater sculpins: phylogenetics to ecology. Trans Am Fish Soc 136:1736–1741
Adams GL, Burr BM, Starkey DE (2013) Cottus Specus, a new troglomorphic species of sculpin (Cottidae) from southeastern Missouri. Zootaxa 3609:484–494. doi:10.11646/zootaxa.3609.5.4
Anderson CS (1985) The structure of sculpin populations along a stream size gradient. Environ Biol Fish 13:93–102
Bates D, Maechler M (2015) Matrix: sparse and dense matrix classes and methods. R package version 1.2–1. http://CRAN.R-project.org/package=Matrix. Accessed 1 Oct 2016
Becker GC (1983) Fishes of Wisconsin. University of Wisconsin Press, Madison
Bovee KD (1982) A guide to stream habitat analysis using the instream flow incremental methodology. U.S. Fish and Wildlife Service biological services program report FWS/OBS-82/26. U.S. fish & wildlife service, Fort Collins
Copp GH, Kovac V, Blacker F (2002) Differential reproductive allocation in sympatric stream-dwelling sticklebacks Gasterosteus aculeatus and Pungitius pungitius. Folia Zool-Praha 51:337–351
Craddock JE (1965) Some aspects of the life history of the banded Sculpin, Cottus carolinae, in doe run, Meade County Kentucky (final report). Louisville University of Kentucky
Crumby WD, Webb MA, Bulow FJ, Cathey HJ (1990) Changes in biotic integrity of a river in north-central Tennessee. Trans Am Fish Soc 119:885–893
Davies-Colley RJ (1997) Stream channels are narrower in pasture than in forest. N Z J Mar Freshw 31:599–608. doi:10.1080/00288330.1997.9516792
Day J, Gerken JE, Adams GL (2016) Population ecology and seasonal demography of the endangered grotto sculpin (Cottus specus). Ecol Freshw Fish 25:27–37. doi:10.1111/eff.12184
Dodds WK, Gido K, Whiles MR, Fritz KM, Matthews WJ (2004) Life on the edge: the ecology of Great Plains prairie streams. Bioscience 54:205–216. doi:10.1641/0006-3568(2004)054[0205:LOTETE]2.0.CO;2
Downhower JF, Brown L (1980) Mate preferences of female mottled sculpins, Cottus bairdi. Anim Behav 28:728–734. doi:10.1016/S0003-3472(80)80132-1
Etnier DA, Starnes WC (1993) The fishes of Tennessee. University of Tennessee Press, Knoxville
Fausch KD (2010) Preface: a renaissance in stream fish ecology. In: Gido KB, Jackson DA (eds) community ecology of stream fishes: concepts, approaches, and techniques. American fisheries society, symposium 73, Bethesda, p 199–206
Fausch KD, Torgersen CE, Baxter CV, Li HW (2002) Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. Bioscience 52:483–498. doi:10.1641/00063568(2002)052[0483:LTRBTG]2.0.CO;2
Gebhard AE, Paine RT, Hix LA, Johnson TC, Wells WG, Ferrell HN, Engle AN, Perkin JS (2017) Testing cross-system transferability of fish habitat associations using Cottus carolinae (banded Sculpin). Southeast Nat 16:70–86
Gido KB, Jackson DA (2010) Community ecology of stream fishes. American Fisheries Society, Bethesda
Gido KB, Matthews WJ, Wolfinbarger WC (2000) Long-term changes in a reservoir fish assemblage: stability in an unpredictable environment. Ecol Appl 10:1517–1529. doi:10.2307/2641301
Gotelli NJ, Pyron M (1991) Life history variation in north American freshwater minnows: effects of latitude and phylogeny. Oikos 62:30–40. doi:10.2307/3545443
Grether GF, Millie DF, Bryant MJ, Reznick DN, Mayea W (2001) Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology 82:1546–1559
Heincke F (1913) Investigations on the plaice. General report 1: the plaice fishery and protective measures. Rapp P-V Reurz cons perm Int Explor Mer 16
Heins DC, Baker JA (1987) Analysis of factors associated with intraspecific variation in Propagule size of a stream-dwelling fish. In: Matthews WJ, Heins DH (eds) Community & evolutionary ecology of north American stream fishes. University of Oklahoma Press, Norman, pp 223–231
Heins DC, Rabito FG Jr (1988) Reproductive traits in populations of the weed shiner, Notropis texanus, from the Gulf coastal plain. Southwest Nat 33:147–156. doi:10.2307/3671889
Hooper RC (1977) Fish communities of Blackburn fork as studied by diversity indices and factor analysis in relation to stream order. Thesis, Tennessee Technological University
Horwitz RJ (1978) Temporal variability patterns and the distributional patterns of stream fishes. Ecol Monogr 48:307–321. doi:10.2307/2937233
Hubbs C (1985) Darter reproductive seasons. Copeia 1985:56–68. doi:10.2307/1444790
Hunt TD (1989) Microhabitat selection and some aspects of the life history of the banded Sculpin, Cottus carolinae (gill). Thesis, Tennessee Technological University
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can J fish Aquat Sci 58:157–170
Jearld A (1983) Age determination. In: Nielsen LA, Johnson DL (eds) Fisheries Techniques, 1st edn. American Fisheries Society, Bethesda, pp 301–324
Kacem H, Boudaya L, Neifar L (2015) Age, growth and longevity of the grey triggerfish, Balistes capriscus Gmelin, 1789 (Teleostei, Balistidae) in the Gulf of Gabès, southern Tunisia, Mediterranean Sea. J Mar Biol Assoc UK 95:1061–1067. doi:10.1017/S0025316414002148
Kappenman KM, Fraser WC, Toner M, Dean J, Webb MA (2009) Effect of temperature on growth, condition, and survival of juvenile shovelnose sturgeon. Trans Am Fish Soc 138:927–937. doi:10.1577/T07-265.1
Keeler RA, Breton A, Peterson DP, Cunjak RA (2007) Apparent survival and detection estimates for PIT-tagged slimy sculpin in five small New Brunswick streams. Trans Am Fish Soc 136:281–292. doi:10.1577/T05-131.1
Lytle DA, Poff NL (2004) Adaptation to natural flow regimes. Trends Ecol Evol 19:94–100. doi:10.1016/j.tree.2003.10.002
Marconato A, Bisazza A (1988) Mate choice, egg cannibalism and reproductive success in the river bullhead, Cottus gobio L. J Fish Biol 33:905–916. doi:10.1111/j.1095-8649.1988.tb05539.x
Martínez-Gómez C, Fernández B, Benedicto J, Valdés J, Campillo JA, León VM, Vethaak AD (2012) Health status of red mullets from polluted areas of the Spanish Mediterranean coast, with special reference to Portmán (SE Spain). Mar Environ Res 77:50–59. doi:10.1016/j.marenvres.2012.02.002
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman and Hall, New York
McManamay RA, Frimpong EA (2015) Hydrologic filtering of fish life history strategies across the United States: implications for stream flow alteration. Ecol Appl 25:243–263. doi:10.6084/m9.figshare.c.3296702.v1
Metcalfe NB, Monaghan P (2003) Growth versus lifespan: perspectives from evolutionary ecology. Exp Gerontol 38:935–940. doi:10.1016/S0531-5565(03)00159-1
Mims MC, Olden JD (2012) Life history theory predicts fish assemblage response to hydrologic regimes. Ecology 93:35–45. doi:10.1890/11-0370.1
Mims MC, Olden JD, Shattuck ZR, Poff NL (2010) Life history trait diversity of native freshwater fishes in North America. Ecol Freshw Fish 19:390–400. doi:10.1111/j.1600-0633.2010.00422.x
Miranda LE, Bettoli PW (2007) Mortality. In: Guy CS, Brown ML (eds) Analysis and interpretation of freshwater fisheries data. American Fisheries Society, Bethesda, pp 229–277
Munz JT, Higgins CL (2013) The influence of discharge, photoperiod, and temperature on the reproductive ecology of cyprinids in the Paluxy River, Texas. Aquat Ecol 47:67–74. doi:10.1007/s10452-012-9425-9
National Oceanic and Atmospheric Administration (NOAA) (2016) Climate 726 data online. Available here: http://www.ncdc.noaa.gov/cdo-web/. Accessed 2 Aug 2016
Olden JD, Kennard MJ (2010) Intercontinental comparison of fish life history strategies along a gradient of hydrologic variability. In: Gido KB, Jackson DA (eds) Community ecology of stream fishes: concepts, approaches, and techniques. American fisheries society, symposium 73, Bethesda, pp 83–107
Perkin JS, Williams CS, Bonner TH (2009) Aspects of chub shiner Notropis potteri life history with comments on native distribution and conservation status. Am Midl Nat 162:276–288. doi:10.1674/0003-0031-162.2.276
Perkin JS, Shattuck ZR, Bonner TH (2012) Life history aspects of a relict Ironcolor shiner Notropis chalybaeus population in a novel spring environment. Am Midl Nat 167:111–126. doi:10.1674/0003-0031-167.1.111
Perkin JS, Knorp NE, Boersig TC, Gebhard AE, Hix LA, Johnson TC (2017) Life history theory predicts long-term fish assemblage response to stream impoundment. Can J Fish Aquat Sci 74:228–239
Poff NL, Allan JD (1995) Functional organization of stream fish assemblages in relation to hydrological variability. Ecology 76:606–627. doi:10.2307/1941217
Potoka KM, Shea CP, Bettoli PW (2016) Multispecies occupancy modeling as a tool for evaluating the status and distribution of darter in the Elk River, Tennessee. Trans Am Fish Soc 145:1110–1121. doi:10.1111/eff.12198
Pringle C (2003) What is hydrologic connectivity and why is it ecologically important? Hydrol Process 17:2685–2689. doi:10.1002/hyp.5145
Quist MC, Pegg MA, DeVries DR (2012) Age and growth. In: Murphy BR, Willis DW (eds) Fisheries techniques, 3rd edn. American Fisheries Society, Bethesda, pp 677–731
Reznick DR, Yang AP (1993) The influence of fluctuating resources on life history: patterns of allocation and plasticity in female guppies. Ecology 74:2011–2019
Reznick DN, Butler MJ IV, Rodd FH, Ross P (1996) Life-history evolution in guppies (Poeciliea reticulate) VI: differential mortality as a mechanism for natural selection. Evolution 50:1651–1660
Ross ST (2013) Ecology of north American freshwater fishes. University of California Press, Berkeley
Schlosser IJ (1982) Fish community structure and function along two habitat gradients in a headwater stream. Ecol Monogr 52:395–414. doi:10.2307/2937352
Schlosser IJ (1987) The role of predation in age-and size-related habitat use by stream fishes. Ecology 68:651–659. doi:10.2307/1938470
Schlosser IJ (1990) Environmental variation, life history attributes, and community structure in stream fishes: implications for environmental management and assessment. Environ Manag 14:621–628. doi:10.1007/BF02394713
Schultz ET, Reynolds KE, Conover DO (1996) Countergradient variation in growth among newly hatched Fundulus Heteroclitus: geographic differences revealed by common-environmental experiments. Funct Ecol 10:366–374
Smith PW (1979) The fishes of Illinois. University of Illinois Press, Urbana
Strahler AN (1957) Quantitative analysis of watershed geomorphology. Eos Trans Am Geophys Union 38:913–920. doi:10.1029/TR038i006p00913
Taylor CM, Warren ML (2001) Dynamics in species composition of stream fish assemblages: environmental variability and nested subsets. Ecology 82:2320–2330. doi:10.2307/2680234
Troia MJ, Gido KB (2014) Towards a mechanistic understanding of fish species niche divergence along a river continuum. Ecosphere 5:1–18. doi:10.1890/ES13-00399.1
Turnipseed DP, Sauer VB (2010) Discharge measurements at gaging stations. US geological survey (USFS) techniques and methods 3-A8. Washington, DC
United States Naval Observatory (UNSO) (2016) Duration of daylight/darkness table for one year. Available here: http://aa.usno.navy.mil/data/docs/Dur_OneYear.php. Accessed 25 Aug 2016
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137. doi:10.1139/f80-017
Vaughan DS, Saila SB (1976) A method for determining mortality rates using the Leslie matrix. Trans Am Fish Soc 105:380–383. doi:10.1577/1548-8659(1976)105<380:AMFDMR>2.0.CO;2
Vélez-Espino LA, Fox MG, McLaughlin RL (2006) Characterization of elasticity patterns of north American freshwater fishes. Can J Fish Aquat Sci 63:2050–2066. doi:10.1139/f06-093
Venables WN, Ripley BD (2002) Random and mixed effects. In: Venables WN, Ripley BD (eds) Modern applied statistics with S, 2nd edn. Springer, New York, pp 271–300
Wallus R, Grannemann KL (1979) Spawning behavior and early development of the banded Sculpin, Cottus carolinae (gill). In: Proceedings of a workshop on freshwater larval fishes. Tennessee Valley Authority, Knoxville, p 199–235
Wells WG, Johnson TC, Gebhard AE, Paine RT, Hix LA, Ferrell HN, Engle AN, Perkin JS (2017) March of the sculpin: measuring and predicting short-term movement of banded sculpin Cottus carolinae. Ecol Freshw Fish 26:280–291. doi:10.1111/eff.12274
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Wilde GR, Durham BW (2008) A life history model for peppered chub, a broadcast-spawning cyprinid. Trans Am Fish Soc 137:1657–1666. doi:10.1577/T07-075.1
Williams JD, Robins CR (1970) Variation in populations of the fish Cottus carolinae in the Alabama River system with description of a new subspecies from below the fall line. Am Midl Nat 83:368–381. doi:10.2307/2423950
Winemiller KO, Rose KA (1992) Patterns of life-history diversification in north American fishes: implications for population regulation. Can J Fish Aquat Sci 49:2196–2218. doi:10.1139/f92-242
Wood SN, Scheipl F (2014) gamm4: generalized additive mixed models using mgcv and lme4. Version 0.2–2. R package. From: http://CRAN.R-project.org/package=gamm4. Accessed 1 Oct 2016
Yeager BL, Saylor CF (1995) Fish hosts for four species of freshwater mussels (Pelecypoda: Unionidae) in the upper Tennessee River drainage. Am Midl Nat 133:1–6. doi:10.2307/2426342
Zale AV, Neves RJ (1982) Fish hosts of four species of lampsiline mussels (Mollusca: Unionidae) in big Moccasin Creek, Virginia. Can J Zool 60:2535–2542. doi:10.1139/z82-325
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer Press, New York
Acknowledgements
The funding of this work was provided by the Department of Biology at Tennessee Technological University. Fish collections were approved by the Tennessee Wildlife Resource Agency (TWRA; permit 1729 to JSP) and specimen handling protocols were approved by the Tennessee Tech Institutional Animal Care and Use Committee (permit TTU-IACUC-14-15-001 to JSP). Stream access was provided by Tennessee Technological University Shipley Farm, A. Ballinger, and TWRA. We thank J. Wellemeyer, E. Malone, W. Curtis, R. Paine, T. Johnson, C. Harty, S. Johnston, J. Ridgway, and Z. Tankersley for assistance with field sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gebhard, A.E., Perkin, J.S. Assessing riverscape-scale variation in fish life history using banded sculpin (Cottus carolinae). Environ Biol Fish 100, 1397–1410 (2017). https://doi.org/10.1007/s10641-017-0651-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0651-9