Abstract
Alterations in river flow can impact fish growth, an important metric for assessing fish health. Here, we use a known sentinel benthic fish species, Slimy Sculpin (Cottus cognatus, Richardson), to assess fish growth below a 15 MW dam and in a naturally flowing river in Ontario, Canada. We investigated fish growth under a restricted hydropeaking regime where the rate of discharge change was limited to 25% of the previous hour’s flow, and an unrestricted hydropeaking regime. Both biological (otolith back-calculated annual growth) and physical (hydrological) indices over a 9-year time period were collected. Slimy Sculpin growth was not significantly different between the two hydropeaking regimes in the regulated river, or the time periods encompassing the hydropeaking regimes in the naturally flowing river. There was a longitudinal gradient whereby Slimy Sculpin growth decreased with increasing distance from the dam. Slimy Sculpin growth in the naturally flowing river was significantly lower relative to the regulated river. Literature-based hydrologic indices explained little of the variation in annual growth. Indirect effects of altered flow such as changes to invertebrate abundance and downstream water temperatures may explain the differences in growth between rivers and the low correlation between annual growth and hydrologic indices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroelectric dams are an important source of energy in Canada, providing over 60% of the country’s electricity (Statistics Canada, 2013). Dams contribute significantly to the proportion of both total and renewable electricity produced, but can have major impacts on downstream physical conditions and biological communities (Sabater, 2008). Hydroelectric dams alter river discharge patterns by reducing the variability of flows, changing the timing of flow events, and dampening seasonal trends (Poff et al., 2007). Hydroelectric peaking operations, in particular, induce large daily fluctuations in discharge to meet daily patterns in electricity demand (Morrison & Smokorowski, 2000). While peaking gives electricity producers flexibility in responding to market forces, large daily fluctuations are a phenomenon that have no parallel in natural freshwater systems (Poff et al., 1997). Aquatic communities are adapted to the gradient of physical conditions along the length of a river (Vannote et al., 1980), and can adjust to the many discontinuities imposed by river regulation (Ward & Stanford, 1983). Thus, hydroelectric operations in general (Baxter, 1977), and peaking operations in particular, can have lasting impacts on downstream organisms (Cushman, 1985; Moog, 1993).
A variety of impacts from altered flow regimes have been identified for fish, including mortality as a result of stranding (Hvidsten, 1985; Young et al., 2011), changes in abundance (Freeman et al., 2001), an increase in invasive species (Marchetti & Moyle, 2001; Brown & Ford, 2002), changes in growth (Korman & Campana, 2009; Bond et al., 2015), and changes in fish movement patterns (Dare et al., 2002; Murchie & Smokorowski, 2004). The magnitude and direction of impacts on fish populations vary by study, and the identification of general trends in the response of biotic communities to flow alteration has proved elusive (Poff & Zimmerman, 2010). The inapplicability of simple overarching and straightforward flow rules (Arthington et al., 2006) make it difficult to design and implement effective environmental flow regulations. Thus, an increase in long-term experimental studies, which focus on hydrological alterations and their impacts on biological communities, is needed to identify mechanistic relationships and facilitate ecologically-based river management (Monk et al., 2007; Mims & Olden, 2013).
Fish growth is a useful individual-based metric for characterizing the effects of river regulation on downstream fish communities. The release of reservoir water from hydroelectric dams can indirectly influence fish growth as a result of altered water temperatures (Olden & Naiman, 2010), increasing invertebrate drift (Lagarrigue et al., 2002; Lauters et al., 1996) and increased abundance of benthic invertebrate communities (Jones, 2013). However, few studies assess the impact of river regulation on fish growth. Korman & Campana (2009) determined that reduced hourly flow fluctuations on weekends corresponded with increased daily growth in young-of-the-year Rainbow Trout (Oncorhynchus mykiss, Walbaum) in a hydropeaking river (Korman & Campana, 2009), while Finch et al. (2015) found that growth of juvenile Humpback Chub (Gila cypha, Miller) had higher growth rates under hydropeaking flows relative to steady flows. Bond et al. (2015) found higher Slimy Sculpin (Cottus cognatus, Richardson) growth rates in two regulated rivers relative to eight naturally flowing nearby rivers. Given that responses to altered flow are likely species-specific, and most studies to date have been carried out over short time periods, we suggest that long-term temporal studies assessing fish growth under multiple hydropeaking regimes would provide further valuable insight into the impacts of hydropeaking on fish.
To better understand the potential impacts of flow regimes altered by hydroelectric operations on fish, we determined annual growth rates for Slimy Sculpin collected along a longitudinal river gradient from a pair of boreal rivers in Ontario (ON), Canada, one with a 15 MW peaking dam, the other with a natural flow regime. We assessed the impact of river regulation on fish growth under two different hydroelectric operating regimes and investigated the suitability of hydrologic indices for explaining differences in fish growth.
Slimy Sculpin (hereafter referred to as “sculpin”) are a small bodied benthic fish, common throughout Canada and have been proposed as a sentinel species (Edwards & Cunjak, 2007) because of their small home range (Gray et al., 2004) and site fidelity (Cunjak et al., 2005). As a result, sculpin have been shown to reflect local conditions along a river continuum (Edwards & Cunjak, 2007) and can be used to indicate environmental degradation (Galloway et al., 2003). In addition, the behavioural traits of sculpin make them an interesting species with which to assess the impacts of altered flow regimes. For example, the closely related Mottled Sculpin (C. bairdii, Girard) have been shown to maintain position with no change in oxygen consumption at velocity ranges of up to 8 body lengths/second (Facey & Grossman, 1992) by staying close to the substrate, a strategy facilitated by their large pectoral fins (Facey & Grossman, 1990). Thus, sculpin may not be as susceptible as salmonids to the increased energetic demands associated with fluctuating flow regimes (Scruton et al., 2008; Cocherell et al., 2011), and may be able to take advantage of increased food availability in the regulated system.
We formulated several hypotheses for the potential responses of sculpin growth to the altered flow regime created by a hydropeaking dam with a minimum environmental flow, based on the changes to food availability in hydropeaked systems (Lauters et al., 1996; Lagarrigue et al., 2002; Jones, 2013), the potential for sculpin to avoid increased energetic demands (Facey & Grossman, 1992) and a previous study on sculpin growth (Bond et al., 2015). First, we hypothesized that sculpin annual growth would be higher in the regulated river than the natural river, and higher under the unrestricted ramping regime relative to the restricted ramping regime. Second, a gradient of growth effects with distance from the dam would be seen in the regulated river, with higher annual growth at sites closest to the dam, but no gradient with distance from the upstream valley segment would be seen in the naturally flowing reference river. Finally, we investigated a set of hydrologic indices proposed in the literature to identify the aspect(s) of the natural and hydropeaked flow regimes that may lead to differences in sculpin growth. Given that water releases from dams can increase lentic and lotic invertebrate abundance downstream (Jones, 2013), we hypothesized that more frequent or higher discharge peaking events would indirectly increase the sculpin food supply and, therefore, hydrologic indices which capture the magnitudes and frequencies of peaking events would be positively related to sculpin growth.
Methods
Study sites
Samples were obtained from a pair of boreal shield rivers in northern Ontario selected to assess the impact of varying hydroelectric ramping regimes on downstream systems relative to a proximate river with similar physical conditions and a natural flow regime (e.g. Smokorowski et al., 2010). The Batchawana River near Sault Ste. Marie ON (47°0′N; 84°30′W) has a natural flow regime and was chosen as the comparative control to the regulated Magpie River near Wawa ON (48°4′N; 84°44′W), which has a 15 MW hydropeaking facility (Fig. 1). The river reach sampled in the Batchawana River was selected on the basis of proximity and similar drainage area, geology, river geomorphology, fish species composition, historical mean annual discharge, and the availability of long-term flow gauge data (Marty et al., 2009; Smokorowski et al., 2010). We chose a section to sample within the control river which was not immediately downstream from a lake to simulate the placement of a dam within a river reach and to avoid the confounding factor of increased water temperatures often observed downstream from lake outlets (Wotton, 1995).
The Steephill Falls dam and generating station (SHF) are located together on the Magpie River. The SHF facility is located at a natural falls, has been operational since 1989, draws water from a depth of 10 m and has a maximum passable flow of 44 m3 s−1. From 1989 through 2004, regulations for the “restrictive regime” required a minimum flow of 7.5 m3 s−1, and ramping restrictions of ≤1 m3 s−1 h−1 from October 10th through November 15th, 2 m3 s−1 h−1 from November 16th through the spring freshet, and no greater than a 25% increase or decrease from the previous hour’s flow from the spring freshet to October 9th (Smokorowski et al., 2010). The ramping rate restrictions were removed in October 2004 (“unrestricted ramping regime”), although the minimum flow requirement of 7.5 m3 s−1, measured at SHF was maintained. The unrestricted ramping regime enabled the hydro operator to increase from the minimum required flows to the maximum passable flow through the turbines and back down again on a daily or sub-daily basis, while under the restricted ramping regime daily discharge changes of this magnitude were not possible (Smokorowski et al., 2010).
Sampling
Sculpin were collected by backpack electrofishing during July and August of 2002 through 2012 (resulting in growth estimates for the years 2002 through 2010) at randomly selected transects below SHF in the Magpie River. Randomly selected transects of 100 m in analogous river valley segments were sampled in the Batchawana River to ensure that similar habitats between rivers were sampled (Smokorowski et al., 2010). An additional site upstream of the SHF reservoir in the Magpie River was sampled as an in-river control. Sites were fished to a depth of 60 cm, which was a safe depth for backpack electrofishing in these rivers and which represents a significant proportion of the total area in both rivers. The longitudinal distance along which the river was sampled was 12 km on the Batchawana River, and 19 km on the Magpie River. Backpack electrofishing was standardized to a rate of 4 m2 s−1. The majority of fish were preserved in formalin (24–30 h) followed by ethanol, while a subset of fish were frozen to avoid preservation biases (Kelly et al., 2006; Storm-Suke et al., 2007) for future stable isotope analysis. Species identification was confirmed in the lab following keys in Scott & Crossman (1973) and McAllister (1964), sex was determined by examination of the gonads, and length (mm) and weight (g) measurements were obtained. Sculpin density and biomass were calculated for each 100 m transect sampled as number of sculpin per m2, and the total weight (g) of sculpin per m2, respectively.
Sagittal otoliths from sculpin were removed and stored dry in plastic snap cap vials. A preliminary study showed no difference in otolith diameter (µm), radius (µm) or weight (mg) between dried, frozen, or ethanol preserved otoliths (Student’s t test, P < 0.05, data not shown). Ageing was conducted with a dissecting microscope (Nikon SMZ 1000, Nikon Instruments Inc.) using whole otoliths placed in deionized water under reflected light. All otoliths were read twice by the same reader and where discrepancies existed, otoliths were polished or thin sectioned. If a discrepancy between ages persisted, the sample was removed from the analysis. Corroboration of ages included consultation with other sculpin age readers and examination of the length frequency diagram by age-class. Otolith increments consisting of an annual cycle were measured at 90° from the rostral radius along a line from the nucleus towards the dorsal edge using NIS Elements software (Nikon Instruments Inc.). Only full annuli were measured, such that the partial growth during year of capture was not included in the analysis. Only the first and second full year’s growth could be included in the statistical analysis due to low sample numbers of 3+, 4+ and 5+ aged fish. Growth (ΔL) was measured as the individual absolute increase in length for one annual cycle where length at age was determined by back-calculation using the scale proportional hypothesis as outlined in (Francis, 1990):
where L i is the length of the fish at age i, L c is the length of the fish at capture, S i is the radius of the otolith at age i, S c is the radius of the otolith at capture, and a and b are the intercept and slope, respectively, of the regression of otolith radius on fish length. The regression of otolith radius on fish length was conducted separately by site within each river, given the potential for differences in growth rates and otolith formation rates amongst sites.
River discharge data were measured with data loggers (Keller 173-L pressure transducer (2002-October 2004), and Solinst Leveloggers (October 2004 through 2010)) every half hour in 2002 and every 15 min thereafter from 2003 through 2010 (Metcalfe, unpublished data). Data loggers were located upstream from the SHF reservoir and at four locations downstream from SHF in the Magpie River, as well as at two locations in the Batchawana River. In addition we accessed flow data from two Water Survey of Canada gauges, 02BD007 (Magpie River) and 02BF001 (Batchawana River).
Data analysis
Statistical analyses were completed in R (The R Project for Statistical Computing www.r-project.org). Normal quantile–quantile plots were used to assess the normality of the data while Bartlett’s test was used to assess homogeneity of variance (Bartlett, 1937). Individual fish were treated as replicates within each river for between river comparisons, and within each site for among-site comparisons. Differences in first and second year annual growth and length at ages 1 and 2 between male and female sculpin were tested within each river using a Welch’s t test. No significant differences were found (p > 0.05) and male and female sculpin were pooled in subsequent analyses. A Welch’s t test (Welch, 1947) was used to test for differences in ΔL between ramping regimes in the regulated river and between the regulated and natural rivers for first and second year growth. A difference in ΔL between the time periods encompassed by the two ramping regimes was also tested with Welch’s t test in the natural river to control for potential weather and climate related impacts. Linear regression was used to assess differences in ΔL with distance from the dam in the regulated river and distance downstream in the natural river. Distance downstream in the naturally flowing river was measured from the beginning of the river valley segment within which the randomly selected sampling transects were located. Pearson’s partial correlation coefficient was used to assess whether sculpin density and biomass were significantly correlated with sculpin growth when distance downstream was controlled for.
A linear model was used to investigate more directly the impact of discharge on ΔL. Numerous hydrologic indices have been applied in the literature to characterize river flow patterns and have been used to explain differences in macroinvertebrate assemblages (Monk et al., 2007; Konrad et al., 2008; Kennen et al., 2010), fish diversity and abundance (Yang et al., 2008) and fish recruitment (Nicola et al., 2009). Here, hydrologic indices were chosen to represent each of the fundamental components of the flow regime: magnitude, timing, duration, frequency and rate of change (Richter et al., 1996; Poff et al., 1997). Nine hydrologic indices were chosen in total, corresponding to each of the nine flow regime components outlined by Olden & Poff (2003). Each of the chosen indices is known to have a large absolute loading in a statistically significant principle components analysis for streams in the US (Olden & Poff, 2003). Slight modifications from the calculations in Olden & Poff (2003) were sometimes necessary to suit study and data availability (Table 1). For example, flow indices were calculated for the growing season (June 1st to September 30th) of each year in the study, rather than for the entire period of record. As well, the absolute rate of change of discharge was calculated in this study instead of a separate calculation for the rate of increase and the rate of decrease. Discharge data used to calculate the nine hydrologic indices were taken from the data logger 2.5 km below SHF in the Magpie River and from the Water Survey of Canada station 02BF001 in the Batchawana River. Multi-collinearities were identified and removed by running the model using a correlation matrix based on the Pearson product-moment correlation coefficient (R Core Team, 2014). The Akaike information criterion (AIC) (Hirogotu 1974) was used to refine the linear model, balancing goodness of fit and prediction power (R package MASS; Venables & Ripley, 2002). The relative importance of each of the variables included in the final model was approximated following Lindeman et al. (1980) using a ranking based on standardized regression coefficients (R package relaimpo; Grömping, 2006).
Results
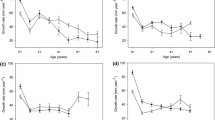
Sculpin (n = 1163) collected from 2002 through 2012 ranged in length from 12 to 126 mm and resulted in first and second year ΔL estimates for the years 2002 through 2010. However, degradation of some otoliths from fish preserved in formalin followed by ethanol resulted in no ΔL estimates for second year growth for 2006 and only one estimate for 2008 in the Magpie River. Average sculpin ΔL ± standard deviation in the Magpie River (regulated) for first and second growth years was 47.9 ± 8.0 and 18.6 ± 7.3 mm/year, respectively, during the restricted ramping period, and 47.5 ± 8.1 and 19.1 ± 7.0 mm/year during the unrestricted ramping period. In the Batchawana River (natural) sculpin ΔL for first and second growth years was 41.5 ± 4.2, and 13.6 ± 5.1 mm/year, respectively, during the period of restricted ramping in the regulated river, and 42.1 ± 4.9 and 15.7 ± 5.2 mm/year during the period of unrestricted ramping in the regulated river (Fig. 2).
Annual first (A) and second (B) year growth (mm year−1) with error bars indicating ± standard deviation for the naturally flowing Batchawana River (circles) and the regulated Magpie River (triangles). Closed symbols indicate growth for the period of restricted ramping, while open symbols indicate growth for the unrestricted ramping period in the regulated river. The same time periods were analysed in the naturally flowing river and are similarly represented with open and closed symbols
There was no difference in ΔL between ramping regimes for first year growth (Welch’s t = −0.16, df = 165.2, P > 0.05) or second year growth (Welch’s t = −1.68, df = 21.8, P > 0.05) in the regulated river (Fig. 2). There was also no difference in ΔL between the time periods encompassed by the ramping regimes for first year growth (Welch’s t = −1.19, df = 223.7, P > 0.05) or second year growth (Welch’s t = −1.84, df = 37.2, P > 0.05) in the naturally flowing river (Fig. 2). As such, the remainder of comparisons were computed using growth data from all years combined. There was a significant difference between ΔL in the regulated and natural river for first year growth (Welch’s t = 10.3, df = 507.8, P < 0.05) and second year growth (Welch’s t = 3.04, df = 113.4, P < 0.05), with growth in the regulated river being higher (Fig. 2). Variance in annual growth was significantly different between rivers for first year ΔL (Bartlett’s test, K-squared1 = 1.96, P < 0.05) and second year ΔL (Bartlett’s test, K-squared1 = 1.96, P < 0.05) and was higher in the regulated river (Fig. 2).
Both first and second year ΔL in the regulated river were significantly related to distance from the dam (P < 0.05, r 2 = 0.12 and 0.19 for first and second year growth, respectively) (Fig. 3), with ΔL decreasing with distance downstream. A between-site ANOVA followed by Tukey’s post hoc test showed that both first and second year sculpin growth in the regulated river decreased to rates not significantly different from the upstream control site at a distance of 4 km downstream from the dam (ANOVA, F = 10.68, P < 0.05). First year ΔL in the natural river was also significantly related to distance (P < 0.05, r 2 = 0.03), but in contrast to the regulated river increased with distance downstream. There was no significant relationship between growth and distance for second year ΔL in the natural river. There was also no significant correlation between growth and density or biomass in either river when distance downstream was controlled for (Pearson’s partial correlation coefficient, P > 0.05).
First and second year growth for Slimy Sculpin in the naturally flowing Batchawana River (A) and the regulated Magpie River (B) along a longitudinal gradient. Distance is measured from the dam in the regulated river and from the beginning of the river valley segment in the naturally flowing river. Regression lines are included where the relationship between growth and distance is significant
Growth season values for the nine flow regime components included in the multiple regression are listed in Table 2. Consistent dam operations, and thus consistent values for the flow regime components, were expected within each flow regime. However, there were two anomalous years in the unrestricted ramping rate period on the regulated river: 2008 was an uncharacteristically wet year, leading to higher discharge, while 2010 was an uncharacteristically dry year, leading to lower discharge. In both those years, ramping was reduced relative to other years throughout the study period.
When explanatory power of the model relating sculpin growth to the tested hydrological indices was significant, it was low and the pattern of hydrologic indices contributing to the explanatory power was not consistent among sites. The variation in sculpin ΔL explained by the flow regime components was significant for first year ΔL in the regulated river (r 2 = 0.12), but not for second year ΔL. Upstream in the regulated river the model was significant for both growth years (r 2 = 0.25 and 0.33, respectively) (Table 3). The model was also significant for both first and second year ΔL in the natural river (r 2 = 0.11 and 0.11, respectively), but explained only a small proportion of the variance in ΔL. The hydrologic index responsible for the highest proportion of variation explained by the model differed between upstream (FL1) and downstream (DH15) in the regulated river and between the regulated and natural river (MH1) (Table 2). Rate of change was positively related to ΔL in the regulated river, but negatively related to ΔL in the natural river. When the anomalous years 2008 and 2010 were removed from the data set used to estimate the model, there was no longer a significant relationship between hydrologic indices and first year ΔL in the regulated river. An ANOVA and Tukey’s HSD showed that first year ΔL in 2008 was significantly higher than in years 2002 and 2010, and first year ΔL in 2009 was significantly higher than in 2010 (F 7, 220 = 4.07, P < 0.05) (Fig. 4). There were no significant differences in second year ΔL between years in the regulated river (F 5, 44 = 1.71, P > 0.05), however, only one second year growth sample was available for 2008.
Discussion
Sculpin annual growth rates did not differ between the periods encompassed by the two different ramping rate regimes in either the regulated Magpie River or the naturally flowing Batchawana River. Growth rates differed between the rivers, with higher average annual growth rates observed in the regulated river. A decrease in growth rates for both growth years was detected with increasing distance from the dam in the regulated river, while an increase in growth rates for first year growth was observed in the naturally flowing river. Sculpin density and biomass did not account for any variation in growth beyond that which was explained by distance downstream. Tested hydrologic indices accounted for only a small proportion of the explained variation in sculpin growth in either river, and significant hydrological indices were not consistent among sites. Thus, while data obtained for sculpin indicate there are impacts associated with changing river flow regimes (natural vs. regulated), sculpin in general do not indicate strong growth-flow correlations under either natural or altered flow conditions.
Flow patterns resulting from a hydropeaking regime could be expected to increase energetic costs, and thus lower growth as a result of increased movement during peak flow events (Scruton et al., 2008; Cocherell et al., 2011; Murchie & Smokorowski, 2004), exposure to higher velocities (Korman & Campana, 2009) and/or changes in foraging behaviour (Cocherell et al., 2011). In contrast, our higher sculpin growth in the hydropeaking river relative to the naturally flowing river could be explained by potential benefits provided by the dam and its operations, such as increased nutrient delivery (Hildebrand, 1980), increased benthic invertebrate abundance (Jones, 2013; Patterson & Smokorowski, 2011), changes to the thermal regime (Olden & Naiman, 2010) which shift water temperatures closer to the optimum for sculpin, and maintenance of a minimum flow requirement (Weisberg & Burton, 1993). In addition, sculpin display life-history traits and behaviour that may enable them to cope with increased peaking without reducing the resources allocated to growth. Below we explore the validity of these possibilities within the context of our study systems.
In the regulated river sampled in this study, the metalimnetic dam draw (Smokorowski et al., 2010) results in cooler water temperatures throughout the summer (by an average of 2.2°C) and slightly warmer temperatures throughout the winter (by an average of 1°C). While the preferred water temperature for sculpin is 10°C (Otto & Rice, 1977), temperatures in both rivers routinely exceed this point in the summer and can also exceed the upper incipient (21°C; Otto & Rice, 1977) and ultimate incipient lethal (26°C; Otto & Rice, 1977) levels for sculpin. However, the regulated river remains cooler in the growing season relative to the natural river (1.7°C cooler on average during restricted ramping and 2.4°C cooler on average during unrestricted ramping 2.5 km below the dam). High temperatures in the natural river would cause an increase in standard metabolic rate and a reduction or cessation of feeding (e.g. Elliott, 1994), resulting in less assimilated energy being allocated towards growth. The cooling effect of the metalimnetic draw in the regulated river would maintain a thermal environment conducive to sculpin feeding throughout more of the growing season, while sculpin would maintain a lower standard metabolic rate. As such, the more favourable water column temperatures in the regulated river may have reduced fish energetic costs and increased feeding opportunities, contributing to higher growth.
Food availability and foraging success, which are key determinants of fish growth, are impacted by river impoundment in general, as well as by the different ramping regimes in our regulated river. Although we did not measure invertebrate abundance directly in this study, several publications have reported on this topic in detail for our two study rivers for the years encompassed by our study (Jones, 2013; Tuor et al., 2014; Bond et al., 2015; Patterson & Smokorowski, 2011). In our hydropeaking river, there was a higher abundance of invertebrates in the benthos (Jones, 2013; Patterson & Smokorowski, 2011) relative to nearby naturally flowing rivers. In addition, stomach contents of small bodied fish (including sculpin) had a higher abundance of invertebrates in the regulated river relative to the naturally flowing river (Tuor et al., 2014). A higher abundance of invertebrates in the benthos, an important food source for sculpin (Petrosky & Waters, 1975), coupled with a higher abundance of invertebrates in stomach contents suggests a higher foraging success in the regulated river, resulting in the higher observed growth. Indeed, Bond et al. (2015) found a positive correlation between sculpin intrinsic growth rates and invertebrate density for a set of rivers including both the Magpie and Batchawana Rivers.
However, Tuor et al. (2014) also found a higher abundance of invertebrates in the stomach contents of small bodied fish during the unlimited ramping rate regime in the regulated river relative to the restricted ramping regime, and a change in the species composition of stomach contents to a higher proportion of primary consumers relative to predatory macroinvertebrates. The lack of difference in growth between the two ramping regimes, despite the increase in stomach contents, suggests that increased energy intake during unlimited ramping was offset by increased energy outputs, or nutritional quality was decreased by the shift in taxonomic composition in stomach contents, or a combination of both. The possibility of increased energetic costs suggests a response threshold exists for sculpin where positive forage-related gains from increased variable flows are ultimately outweighed by the associated energetic costs of standard metabolic rate. Thus, the absence of a significant difference in sculpin growth between the two ramping regimes does not mean that the two hydropeaking regimes were equivalent in their impacts on sculpin.
Invertebrate abundance in the regulated river was highest at the site closest to the dam, but decreased to levels found in natural rivers 5–8 km below the dam (Jones, 2013). This coincides well with our finding that sculpin growth was highest near the dam, with growth decreasing to levels not significantly different from upstream, 4 km below the dam. In the naturally flowing Batchawana River, benthic invertebrate abundance increases with distance downstream (Jones, 2013), as do our first year annual growth rate measurements for sculpin. These results may be expected based on current stream ecological theory. Cooler water temperatures, increased particulate drift and increased abundance of filter feeding invertebrates below dams (Ward & Stanford, 1983; the serial discontinuity concept) would produce conditions beneficial for sculpin growth. As river water temperatures are attenuated and benthic invertebrate abundances decrease to levels similar to naturally flowing rivers with distance downstream, sculpin growth would be expected to, likewise, decrease with distance downstream. Conversely, in the middle reaches of a naturally flowing river reach without a significant input of particulate organic matter, the filter feeding invertebrate community may have a lower abundance relative to river reaches below a dam. As the river shifts from heterotrophy to autotrophy with distance downstream and the benthic community of collectors and grazers increases in abundance (Vannote et al., 1980, the river continuum concept), food availability for sculpin may increase, driving an increase in sculpin growth with distance downstream.
While invertebrate abundance can increase immediately below dams (Spence & Hynes, 1971; Jones, 2013), with consequences for productivity at higher trophic levels, the same phenomenon can occur in riverine habitats below naturally occurring lakes (Richardson & Mackay, 1991; Jones, 2010). The river reach sampled in the Batchawana River as a control for our regulated Magpie River was not immediately downstream from a lake. Yet, given that our study dam has a cool water draw, the ideal environmental control may not be obtainable. The outlet water from natural lakes is composed of lake surface water, which is often warmer than downstream river temperatures, increasing water temperatures immediately downstream (Wotton, 1995). As discussed above, the alteration of downstream water temperatures is an important factor for coldwater species such as sculpin. Higher water temperatures from the outlet of a natural lake would increase sculpin metabolic costs relative to a lake-less river reach, potentially offsetting benefits from an increased food supply. Therefore we chose to compare our study river with a lake-less river reach to determine the impacts of a hydroelectric dam built within a river reach as opposed to at the outlet of a lake.
During both experimental periods, the requirement of a minimum discharge (7.5 m3 s−1) in the regulated river remained in place, which has demonstrated benefits to fish (Travnichek et al., 1995; Weisberg & Burton, 1993). The requirement often resulted in a mid-summer discharge in the regulated river above that which was experienced in the naturally flowing river (see discharge parameter ML1, Table 2). In general, discharge parameters positively correlated with the frequency and duration of high discharge events were important components of the explained variation in annual growth in both rivers, suggesting that higher discharge provides benefits realized in higher sculpin growth rates. For example, Weisberg & Burton (1993) noted an increase in invertebrate abundance and increased foraging success, once a minimum flow requirement was instituted below the Conowingo Dam in Maryland, US. Therefore, continuation of the minimum discharge requirement, as well as higher mean flows (Table 2), may in part explain the higher annual sculpin growth rates in the regulated river relative to the natural river.
Variance in growth rates was higher in the regulated river relative to the natural river. Variability in discharge (Rand et al., 2006) and resource availability (Nislow et al., 2004) can lead to variability in energy allocation, resulting in differential growth. Invertebrate abundance and community composition differs along both lateral and longitudinal gradients in both the rivers in this study with depth and velocity being important factors contributing to the gradients in the regulated river (Jones, 2013). The longitudinal gradient in invertebrate abundance in the regulated river likely contributes to the longitudinal gradient in growth in the regulated river, as discussed above, driving the higher variation in growth rates seen in the Magpie River.
Higher annual growth rates of sculpin in the regulated river and decreasing growth rates with distance from the dam coincide with trends between rivers reported for sculpin by Bond et al. (2015), but not the specific growth-flow relationships reported by Korman & Campana (2009) for Rainbow Trout. While Korman & Campana (2009) reported an increase in daily growth with reduced ramping, sculpin growth was not higher under the restricted ramping regime in the regulated river. The closely related Mottled Sculpin are most often found underneath the cover of rocks or clinging to substrate (Facey & Grossman 1992), with the tactical use of substrate necessitated by their poor swimming ability (Facey & Grossman, 1990). Field observations in this study suggest that sculpin behave similarly to Mottled Sculpin, with the apparent advantage of reducing energetic expenditure even under high, ramping induced discharge rates and rates of change. Thus, sculpin likely avoid the negative consequences of increased discharge by taking refuge in the substrate, while juvenile Rainbow Trout which maintain position during peaking events do not (Korman & Campana, 2009). However, it cannot be presumed that the higher growth rates in the regulated river relative to the naturally flowing river are a beneficial response to river regulation or will necessarily lead to higher relative abundance in the regulated river, as higher sculpin growth rates in the Magpie River led to younger age at maturity and higher instantaneous mortality rates (Bond et al., 2015). To understand the impacts of hydropeaking on fish growth more fully, investigations specific to particular fish body morphologies, age-class and behavioural strategies are needed (e.g. Scruton et al., 2003; Murchie & Smokorowski, 2004).
Weak correlations between hydrologic indices and annual sculpin growth rates, despite significant differences in growth rates between rivers, highlight the importance of the indirect effects of altering flow regimes on fish species. Indeed, when two anomalous years during which peaking operations were suspended are removed from our analysis, there was no significant relationship between first or second year sculpin growth and hydrologic indices in the regulated river. However, changes in flow regimes do not have ubiquitous effects for all species, as not all fish species respond equally to flow, and indirect impacts associated with flow alteration may compensate for negative impacts. Therefore it is imperative that both biotic (Bunn & Arthington, 2002) and abiotic (Cushman, 1985) factors be included in study designs to properly understand the impacts of altered hydrologic regimes, and how such alterations interact with species specific life history traits. This paper provides valuable insight into the effects of two different hydroelectric peaking regimes on the growth of a little studied but widely distributed forage fish species with benthic behaviour. Our detailed hydrologic analysis highlights the importance of direct and indirect effects of flow alteration and lends support to current ecological theories, shedding light on how the ecological impacts of hydroelectric dams are manifested by a benthic fish species.
References
Arthington, A. H., S. E. Bunn, N. L. Poff & R. J. Naiman, 2006. The challenge of providing environmental flow rules to sustain river ecosystems. Ecological Applications 16: 1311–1318.
Bartlett, M. S., 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London Series A 160: 268–282.
Baxter, R. M., 1977. Environmental effects of dams and impoundments. Annual Review of Ecology and Systematics 8: 255–283.
Bond, M. J., N. E. Jones & T. J. Haxton, 2015. Growth and life history patterns of a small-bodied stream fish, Cottus cognatus, in hydropeaking and natural rivers of northern Ontario. River Research and Applications. doi:10.1002/rra.2886.
Brown, L. R. & T. Ford, 2002. Effects of flow on the fish communities of a regulated California river: Implications for managing native fishes. River Research and Applications 18: 331–342.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Cocherell, S. A., D. E. Cocherell, G. J. Jones, J. B. Miranda, L. C. Thompson, J. J. Cech Jr & A. P. Klimley, 2011. Rainbow trout Oncorhynchus mykiss energetic responses to pulsed flows in the American River, California, assessed by electromyogram telemetry. Environmental Biology of Fishes 90: 29–41.
Cunjak, R. A., J.-M. Roussel, M. A. Gray, J. P. Dietrich, D. F. Cartwright, K. R. Munkittrick & T. D. Jardine, 2005. Using stable isotope analysis with telemetry or mark-recapture data to identify fish movement and foraging. Oecologia 144: 636–646.
Cushman, R. M., 1985. Review of ecological effects of rapidly varying flows downstream from hydroelectric facilities. North American Journal of Fisheries Management 5: 330–339.
Dare, M. R., W. A. Hubert & K. G. Gerow, 2002. Changes in habitat availability and habitat use and movements by two trout species in response to declining discharge in a regulated river during winter. North American Journal of Fisheries Management 22: 917–928.
Edwards, P. A. & R. A. Cunjak, 2007. Influence of water temperature and streambed stability on the abundance and distribution of sculpin (Cottus cognatus). Environmental Biology of Fishes 80: 9–22.
Elliott, J. M., 1994. Quantitative Ecology and the Brown Trout. Oxford University Press, Oxford.
Facey, D. E. & G. D. Grossman, 1990. The metabolic cost of maintaining position for four North American stream fishes: effects of season and velocity. Physiological Zoology 63: 757–776.
Facey, D. E. & G. D. Grossman, 1992. The relationship between water velocity, energetic costs, and microhabitat use in four North American stream fishes. Hydrobiologia 239: 1–6.
Finch, C., W. E. Pine III & K. E. Limburg, 2015. Do hydropeaking flows alter juvenile fish growth rates? A test with juvenile Humpback Chub in the Colorado River. River Research and Applications 31: 156–164.
Francis, R. I. C. C., 1990. Back-calculation of fish length: a critical review. Journal of Fish Biology 36: 883–902.
Freeman, M. C., Z. H. Bowen, K. D. Bovee & E. R. Irwin, 2001. Flow and habitat effects on juvenile fish abundance in natural and altered flow regimes. Ecological Applications 11: 179–190.
Galloway, B. J., K. R. Munkittrick, S. Currie, M. A. Gray, R. A. Curry & C. S. Wood, 2003. Examination of the responses of sculpin (Cottus cognatus) and white sucker (Catostomus commersoni) collected on the Saint John River (Canada) downstream of pulp mill, paper mill, and sewage discharges. Environmental Toxicology and Chemistry 22: 2898–2907.
Gray, M. A., R. A. Cunjak & K. R. Munkittrick, 2004. Site fidelity of sculpin (Cottus cognatus): insights from stable carbon and nitrogen analysis. Canadian Journal of Fisheries and Aquatic Science 61: 1717–1722.
Grömping, U., 2006. Relative importance for linear regression in R: the package relaimpo. Journal of Statistical Software 17: 1–27.
Hildebrand, S. G. (eds) 1980. Analysis of environmental issues related to small-scale hydroelectric development. III. Water Level Fluctuation. ORNL/TM-7453. Oak Ridge National Laboratory, Oak Ridge, TN.
Hirogotu, A., 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723.
Hvidsten, N. A., 1985. Mortality of pre-smolt Atlantic salmon, Salmo salar L., and brown trout, Salmo trutta L., caused by fluctuating water levels in the regulated River Nidelva, central Norway. Journal of Fish Biology 27: 711–718.
Jones, N. E., 2010. Incorporating lakes within the river discontinuum: longitudinal changes in ecological characteristics in stream-lake networks. Canadian Journal of Fisheries and Aquatic Science 67: 1350–1362.
Jones, N. E., 2013. Spatial patterns of benthic invertebrates in regulated and natural rivers. River Research and Applications 29: 343–351.
Kelly, B., J. B. Dempson & M. Power, 2006. The effects of preservation on fish tissue stable isotope signatures. Journal of Fish Biology 69: 1595–1611.
Kennen, J. G., K. Riva-Murray & K. M. Beaulieu, 2010. Determining hydrologic factors that influence stream macroinvertebrate assemblages in the northeastern US. Ecohydrology 3: 88–106.
Konrad, C. P., A. M. D. Brasher & J. T. May, 2008. Assessing streamflow characteristics as limiting factors on benthic invertebrate assemblages in streams across the western United States. Freshwater Biology 53: 1983–1998.
Korman, J. & S. E. Campana, 2009. Effects of hydropeaking on nearshore habitat use and growth of age-0 rainbow trout in a large regulated river. Transactions of the American Fisheries Society 138: 76–87.
Lagarrigue, T., R. Céréghino, P. Lim, P. Reyes-Marchant, R. Chappaz, P. Lavandier & A. Belaud, 2002. Diel and seasonal variations in brown trout (Salmo trutta) feeding patterns and relationship with invertebrate drift under natural and hydropeaking conditions in a mountain stream. Aquatic Living Resources 15: 129–137.
Lauters, F., P. Lavandier, P. Lim, C. Sabaton & A. Belaud, 1996. Influence of hydropeaking on invertebrates and their relationship with fish feeding habits in a Pyrenean river. Regulated Rivers: Research & Management 12: 563–573.
Lindeman, R. H., P. F. Merenda & R. Z. Gold, 1980. Introduction to Bivariate and Multivariate Analysis. Scott Foresman, Glenview, IL.
Marchetti, M. P. & P. B. Moyle, 2001. Effects of flow regime on fish assemblages in a regulated California stream. Ecological Applications 11: 530–539.
Marty, J., K. Smokorowski & M. Power, 2008. The influence of fluctuating ramping rates on the food web of boreal rivers. River Research and Applications 25: 962–974.
McAllister, D. E., 1964. Distinguishing characters for the sculpins Cottus bairdii and C. cognatus in Eastern Canada. Journal of the Fisheries Board of Canada 21: 1339–1342.
Mims, M. C. & J. D. Olden, 2013. Fish assemblages respond to altered flow regimes via ecological filtering of life history strategies. Freshwater Biology 58: 50–62.
Monk, W. A., P. J. Wood, D. M. Hannah & D. A. Wilson, 2007. Selection of river flow indices for the assessment of hydroecological change. River Research and Applications 23: 113–122.
Moog, O., 1993. Quantification of daily peak hydropower effects on aquatic fauna and management to minimize environmental impacts. Regulated Rivers: Research & Management 8: 5–14.
Morrison H. A. & K. E. Smokorowski, 2000. The applicability of various frameworks and models for assessing the effects of hydropeaking on the productivity of aquatic ecosystems. Canadian technical report of fisheries and aquatic sciences/Rapport technique canadien des sciences halieutiques et aquatiques. Imprint varies (2322), 67.
Murchie, K. J. & K. E. Smokorowski, 2004. Relative activity of brook trout and walleyes in response to flow in a regulated river. North American Journal of Fisheries Management 24: 1050–1057.
Nicola, G. G., A. Almodóvar & B. Elvira, 2009. Influence of hydrologic attributes on brown trout recruitment in low-latitude range margins. Oecologia 160: 515–524.
Nislow, K. H., A. J. Sepulveda & C. L. Folt, 2004. Mechanistic linkage of hydrologic regime to summer growth of age-0 Atlantic salmon. Transactions of the American Fisheries Society 133: 79–88.
Olden, J. D. & R. J. Naiman, 2010. Incorporating thermal regimes into environmental flows assessments: modifying dam operations to restore freshwater ecosystem integrity. Freshwater Biology 55: 86–107.
Olden, J. D. & N. L. Poff, 2003. Redundancy and the choice of hydrologic indices for characterizing streamflow regimes. River Research and Applications 19: 101–121.
Otto, R. G. & J. O. H. Rice, 1977. Responses of a freshwater sculpin (Cottus cognatus gracilis) to temperature. Transactions of the American Fisheries Society 106: 89–94.
Patterson, R. J. & K. E. Smokorowski, 2011. Assessing the benefit of flow constraints on the drifting invertebrate community of a regulated river. River Research and Applications 27: 99–112.
Petrosky, C. E. & T. F. Waters, 1975. Annual production by the sculpin population in a small Minnesota trout stream. Transactions of the American Fisheries Society 104: 237–244.
Poff, N. L., J. D. Allan, M. B. Bain, J. R. Karr, K. L. Prestegaard, B. D. Richter, R. E. Sparks & J. C. Stromberg, 1997. The natural flow regime. Bioscience 47: 769–784.
Poff, N. L., J. D. Olden, D. M. Merritt & D. M. Pepin, 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences 104: 5732–5737.
Poff, N. L. & J. K. H. Zimmerman, 2010. Ecological response to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55: 194–205.
R Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Rand, P. S., S. G. Hinch, J. Morrison, M. G. G. Foreman, M. J. MacNutt, J. S. Macdonald, M. C. Healey, A. P. Farell & D. A. Higgs, 2006. Effects of river discharge, temperature, and future climates on energetics and mortality of adult migrating Fraser River sockeye salmon. Transactions of the American Fisheries Society 135: 655–667.
Richardson, J. S. & R. J. Mackay, 1991. Lake outlets and the distribution of filter feeders: an assessment of hypotheses. Oikos 62: 370–380.
Richter, B. D., J. V. Baumgartner, J. Powell & D. P. Braun, 1996. A method for assessing hydrologic alteration within ecosystems. Conservation Biology 10: 1163–1174.
Sabater, S., 2008. Alterations of the global water cycle and their effects on river structure, function and services. Freshwater Reviews 1: 75–88.
Scott, W. B. & E. J. Crossman, 1973. Freshwater fishes of Canada. Fisheries Research Board of Canada, Ottawa.
Scruton, D. A., L. M. N. Ollerhead, K. D. Clarke, C. Pennell, K. Alfredsen, A. Harby & D. Kelley, 2003. The behavioural response of juvenile Atlantic salmon (Salmo salar) and brook trout (Salvelinus fontinalis) to experimental hydropeaking on a Newfoundland (Canada) river. River research and applications 19: 577–587.
Scruton, D. A., C. Pennell, L. M. N. Ollerhead, K. Alfredsen, M. Stickler, A. Harby, M. Robertson, K. D. Clarke & L. J. LeDrew, 2008. A synopsis of ‘hydropeaking’studies on the response of juvenile Atlantic salmon to experimental flow alteration. Hydrobiologia 609: 263–275.
Smokorowski, K. E., R. A. Metcalfe, S. D. Finucan, N. Jones, M. Marty, M. Power & R. Steele, 2010. Ecosystem level assessment of environmentally based flow restrictions for maintaining ecosystem integrity: a comparison of a modified peaking versus unaltered river. Ecohydrology. doi:10.1002/eco.167.
Spence, J. A. & H. B. N. Hynes, 1971. Differences in benthos upstream and downstream of an impoundment. Journal of the Fisheries Board of Canada 28: 35–43.
Statistics Canada. 2013. Table 127-0002. Electric power generation, by class of electric power producer. CANSIM (database) Available at http://www5.statcan.gc.ca/cansim/a33?RT=TABLE&themeID=4012&spMode=tables&lang=eng.
Storm-Suke, A., J. B. Dempson, F. Caron & M. Power, 2007. Effects of formalin and ethanol preservation on otolith δ18O stable isotope signatures. Rapid communications in mass spectrometry 21: 503–508.
Travnichek, V. H., M. B. Bain & M. J. Maceina, 1995. Recovery of a warm water fish assemblage after the initiation of a minimum-flow release downstream from a hydroelectric dam. Transactions of the American Fisheries Society 124: 836–844.
Tuor, K. M. F., K. E. Smokorowski & S. J. Cooke, 2014. The influence of fluctuating ramping rates on the diets of small-bodied fish species of boreal rivers. Environmental Biology of Fishes. doi:10.1007/s10641-014-0264-5.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Venables, W. N. & B. D. Ripley, 2002. Modern applied statistics with S, 4th ed. Springer, New York.
Ward, J. V. & J. A. Stanford, 1983. The serial discontinuity concept of lotic ecosystems. Dynamics of lotic ecosystems 10: 29–42.
Weisberg, S. B. & W. H. Burton, 1993. Enhancement of fish feeding and growth after an increase in minimum flow below the Conowingo Dam. North American Journal of Fisheries Management 13: 103–109.
Welch, B. L., 1947. The generalization of “Student’s” problem when several different population variances are involved. Biometrika 34: 28–35.
Wotton, R. S., 1995. Temperature and lake-outlet communities. Journal of Thermal Biology 20: 121–125.
Yang, Y. C. E., X. Cai & E. E. Herricks, 2008. Identification of hydrologic indicators related to fish diversity and abundance: a data mining approach for fish community analysis. Water Resources Research. doi:10.1029/2006WR005764.
Young, P. S., J. J. Cech & L. C. Thompson, 2011. Hydropower-related pulsed-flow impacts on stream fishes: a brief review, conceptual model, knowledge gaps, and research needs. Reviews in Fish Biology and Fisheries 21: 713–731.
Acknowledgments
The authors would like to thank Dr. Robert Metcalfe for collecting and processing the hydrologic and temperature data. Thanks to the many biologists and field technicians who conducted sampling throughout the project, particularly those who participated in 2011 and 2012 including Rick Elsner, Patrick Rivers, Lauren Siccoli, Evan Timusk, Marla Thibodeau, Bill Gardner and Kim Tuor. Funding for the project was provided by Fisheries and Oceans Canada - Center of expertise on Hydropower Impacts on Fish and Fish Habitat (DFO CHIF), Brookfield Renewable Power Ltd. in the form of direct support and contributions through the National Sciences and Engineering Research Council that funded a Collaborative Research and Development Grant to M. Power and an NSERC Industrial Postgraduate Scholarship sponsored by Natural Resources Solutions Inc. for B. Kelly. And special thanks to R. Steele for his enthusiastic support of the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Odd Terje Sandlund
Rights and permissions
About this article
Cite this article
Kelly, B., Smokorowski, K.E. & Power, M. Slimy Sculpin (Cottus cognatus) annual growth in contrasting regulated and unregulated riverine environments. Hydrobiologia 768, 239–253 (2016). https://doi.org/10.1007/s10750-015-2553-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2553-1