Abstract
Estuarine systems play a critical role as nursery areas for some fish species. Nursery function, nevertheless, is likely to vary among estuaries, mostly due to differences in habitat quality. High quality nursery habitats are those in which growth and survival of early stages of fish are enhanced. The nutritional condition of larval fishes has vital implications for their mortality and growth, and thereby their recruitment. This study aimed to compare the nutritional condition of Gichristella aestuaria larvae, using individual RNA-DNA ratios and growth rates in the upper reaches of six estuaries in South Africa to find the environmental factors that better determine the nutritional condition of fish larvae. Physico-chemical factors as well as calorific value of zooplankton were used to correlate to fish body condition. Results showed that the larvae of G. aestuaria from the freshwater rich Gamtoos and the Sundays estuaries were in better nutritional condition than the larvae from other estuaries, while larvae from the Swartkops Estuary, a highly eutrophic system, presented the worst nutritional condition of all studied larvae. Salinity and the abundance of zooplankton were the major factors determining the nutritional condition of G. aestuaria larvae in these warm temperate estuaries. In addition, our results suggest that the match-mismatch hypothesis might also be important in estuarine systems. This study represents one of the few studies worldwide that applied a multispecies growth model for fish larvae in warm temperate estuaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine systems play a critical role in the dynamics of coastal environments and are particularly important for some fish species that are widely tolerant of the high environmental variability that characterizes estuaries (Whitfield 1998; Beck et al. 2001; Able et al. 2006; Vasconcelos et al. 2010). The ecological dynamics of these fish species in southern Africa has been a longstanding focus of research (Whitfield 2010). As a result, southern African estuaries act as essential nursery habitats for several coastal fish species (Whitfield 1999; Strydom et al. 2003; Harrison 2005; Harrison and Whitfield 2012; Strydom 2014). Nursery function, nevertheless, is likely to vary among estuaries, mostly due to differences in habitat quality (Gibson 1994; Vasconcelos et al. 2011).
High quality nursery habitats are those in which growth and survival of early stages of fish are enhanced (Gibson 1994; Heck et al 2003; Vasconcelos et al. 2010). However, the effects of the major agents determining habitat quality are not well studied in estuarine systems. For example, the availability of food is a critical link to the growth and survival of larval fishes (O’Connell and Raymond 1970; Buckley and Durbin 2006; Baumann et al. 2007). The quality or nutritional value of the available prey also affects the condition of fish larvae (Cruz-Rivera and Hay 2000; Vos et al. 2000) and is often expressed in terms of energetic value or caloric content of the prey (Davis 1993). Other important factors that might influence the quality of a nursery are abiotic traits, particularly temperature, salinity, oxygen and turbidity (Beck et al. 2001; Summers et al. 1995).
The most common approaches used to evaluate nursery quality compare the habitat-specific growth and/or mortality rates of selected fish species (Able et al. 1999; Beck et al. 2001). The study of the condition of early stages of fish has important implications for the prediction of their mortality and growth, and therefore their recruitment success (Houde 2008). Condition indices based on biochemical parameters are generally a very effective method of evaluating the condition status of aquatic animals (Ferron and Leggett 1993; Suthers 1992; Catalán et al. 2007). Amidst the biochemical methods available, the RNA-DNA ratio has been extensively used for the assessment of the nutritional condition of fish larvae in the ocean especially because of its high sensitivity, and because it allows for individual quantifications and responds to changes in feeding levels in a short period (Buckley 1984; Ferron and Leggett 1993; Clemmesen 1994; Catalán et al. 2007; Costalago et al. 2014).
Whilst the concentration of DNA is considered constant in somatic tissues, and is directly related to the numbers of cells of an individual, the amount of RNA is linked to the protein synthesis, and thereby to growth (Catalán et al. 2007). Thus, RNA/DNA serves as a proxy of the cell’s protein synthetic capacity and of individual growth (Buckley et al. 1999, 2008). Consequently, nutritional condition of fish larvae, assessed through the analysis of nucleic acids, can be used as an indicator of habitat quality (Amara et al. 2009). In addition, some of the most recent studies of larval fish condition (Chícharo et al. 2012; Grote et al. 2012; Paulsen et al. 2014) relate the RNA-DNA ratio with individual growth rates and environmental temperature through a multispecies larval fish growth model (Buckley et al. 2008). This approach allows for comparisons of larval fish RNA-DNA ratio among habitat types at different temperatures.
Many South African estuarine systems are being increasingly altered by global and regional environmental disturbances, such as declining freshwater inflows, fishing, agricultural and industrial practices, pollution or climate change (Whitfield 1992, 2010). Thus, it is imperative to understand the consequences of these changes for feeding young fishes, particularly changes that affect food productivity (Strydom and Whitfield 2002). Mesohaline zones (salinity ranging 5–18) occurring in the middle and upper reaches of temperate South African estuaries have been suggested as important feeding areas for larval fishes based on zooplankton and larval fish density maxima in these areas (Strydom 2014). Freshwater rich estuaries have prominent mesohaline zones but the implications of richer zooplankton stocks on body condition of fish larvae has not been explored in South Africa. It was therefore necessary to explore the relationship between the value of zooplankton (density and calorific value) and its effect on instantaneous body condition of the larvae in the estuary at that time. Given that estuarine productivity and freshwater supply are positively correlated (Strydom and Whitfield 2002), the abovementioned relationship could only be explored by selecting estuaries ranging in freshwater supply and therefore zooplankton productivity.
The estuarine round-herring, Gilchristella aestuaria (Family Clupeidae) was selected as a candidate species to examine the quality of these ecosystems as nursery habitats through the assessment of the nutritional condition of the larvae. This species is an estuary resident species and the most abundant fish in southern African estuaries. It is an important forage fish and plays a key ecological role as mid-trophic species (Harrison 2005). The species occurs all along the coast of southern Africa (Smith 1965) and completes its entire life cycle in estuaries (Haigh and Whitfield 1993), feeding primarily on zooplankton (Coetzee 1982; Strydom et al. 2014). Gilchristella aestuaria spawn in the middle reaches of estuaries, with peak larval abundance typically in mesohaline zones of estuaries (Strydom 2014).
The present study thus aimed to compare the nutritional condition and growth rates of G. aestuaria larvae in six well studied estuarine systems in South Africa (Harrison 2005; Whitfield 2010; Strydom 2014) using the RNA/DNA method in order to ascertain which environmental factors best correlate with the nutritional condition of larvae. In addition, this study provides RNA-DNA ratio-based growth rates that allow for comparison of fish larvae from environments with different temperature regimes. This is one of the few studies worldwide that has applied the multispecies growth model to fish larvae at temperatures over 20 °C (Buckley et al. 2008).
Materials and methods
Study sites and field sampling
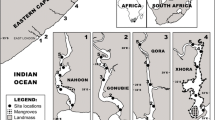
Samples were collected from known G. aestuaria breeding areas in the upper reaches of six permanently open estuaries with varying freshwater supply along the warm temperate coast of South Africa (Fig. 1). The Kromme and Kariega estuaries are classified as freshwater poor estuaries; the Swartkops receives a moderate supply of freshwater and the Gamtoos, Sundays and Great Fish estuaries are classified as freshwater rich (Strydom and Whitfield 2002). Further in-depth information about the physico-chemical and geomorphological characteristics of these systems can be found in Strydom (2014). Samples were collected after dark on six consecutive nights in November 2013. Physico-chemical parameters (temperature, salinity, turbidity and dissolved oxygen) were measured at each station in the upper reaches where larvae were collected. Measurements were recorded from surface to bottom every 0.5 m of depth with a YSI 6600 multi-parameter probe (Strydom and Whitfield 2002). Averaged values from the stations where fish larvae were found were used for analyses. To provide an estimation of the average depth of the estuaries at the sampling stations where larvae were collected, the last (closest to the bottom) measurements of the probe were taken at 2, 1, 2.5, 2, 2 and 1.5 m in the Kromme, Gamtoos, Swartkops, Sundays, Kariega and Great Fish Estuaries, respectively.
Fish larvae and zooplankton were collected at a similar time in each estuary, to avoid diel variations in RNA contents (Bergeron 1997), from the subsurface waters (<4 m) by means of two modified WP2 plankton nets (570 mm mouth diameter and 0.2 mm mesh aperture) fitted with Kahlsico 005 WA 130 flowmeters. Both nets were lowered simultaneously with two short booms (1.5 m length) fixed on either side of the bow of a single-hulled boat and towed horizontally. Sampling speed was approximately 2 knots and each tow lasted 5–10 min (Strydom et al. 2003). Samples (zooplankton and fish larvae) from one net were preserved in 5 % buffered formalin for quantitative analyses. Zooplankton from the other net were kept on ice until arrival at the laboratory, where it was frozen at −20 °C until calorimetric analysis was performed. Gilchristella aestuaria larvae were preserved individually in vials containing RNAlater (Sigma-Aldrich) for nucleic acids analysis. Once in the laboratory, all fish larvae were stored at −80 °C.
Zooplankton analyses
Quantification and identification of major groups of zooplankton were carried out under a Leica M80 stereo microscope at × 100 magnification. Zooplankton samples were analyzed in three aliquots representing each about 10 % of the total volume and individuals identified to the lowest possible taxonomical level until counting at least 400 copepods and mysids were counted. For the calorimetric analysis, frozen zooplankton samples were thawed and sieved to separate organisms larger than 355 μm, which are not feasible prey for G. aestuaria larvae because of their size (Strydom et al. 2014). Copepods were also separated from the samples. Three replicates of zooplankton and copepod samples from each estuary were dried in an oven at 60 °C over 24 h and homogenized using mortar and pestle. Dry weight of replicates ranged from 55 to 131 mg. Calorimetry analyses of dried replicates were performed with a Parr 1425 semi-micro bomb calorimeter. Zooplankton and copepod energy contents are reported as cal/g dry weight.
Nucleic acids extraction
Once thawed, the standard length of 80 larvae from each estuary was measured to the nearest 0.01 mm with a Mitutoyo digital calliper. Individual samples were freeze-dried and then homogenized using the protocol of Caldarone et al. (2001) adapted by Costalago et al. (2014). 1.50 ml of sarcosil Tris-EDTA buffer pH 8.0 (containing 0.1 % of N-lauroylsarcosine sodium salt in 0.05 M Tris, 0.01 M EDTA, and 0.1 M NaCl) and three glass beads (3 mm diameter) were added to each RNase-DNase free vial containing the larvae. After 5–6 min for rehydration of the larvae, vials were vortexed in a balanced box for 10 min, sonicated for 1 min at 4 °C and then vortexed for an additional 20–30 min. After total homogenization, samples were centrifuged in a cooled centrifuge at 4 °C for 20 min at 4000 rpm (12.3 g) in an Eppendorf 5804R centrifuge. Supernatant was collected and stored at −80 °C for nucleic acids quantification.
Nucleic acids quantification
RNA and DNA quantification was completed using the procedure described by Berdalet et al. (2005) and adapted by Costalago et al. (Costalago et al. 2014). SYBR Green II fluorochrome (Lonza) and standards DNA from herring sperm and RNA from baker’s yeast (Sigma-Aldrich) were used. Standards were diluted at six different concentrations, ranging from 0.38 to 6.06 μg/ml, to obtain the standard curve. Enzymes RNase and RNase-free DNase were acquired from Qiagen. Duplicate (50 μL) samples, standards and blank (containing 0.01 % sarcosil Tris-EDTA) were pipetted into black 96-well plates (Greiner Bio-One). Fifty microliter of SYBR Green II were added to each well and the fluorescence (Ex492nm:Em520nm) were measured using a BioTek PowerWave XS microplate reader linked to the Gen5 2.0 Data Analysis software. In a second plate, 7.5 μL RNase per well was added; and 5 μL DNase per well in the third plate. These plates were incubated for 30 min at 37 °C until enzymes degraded nucleic acids completely, then 50 μL of SYBR Green II per well added and fluorescence measured. The amounts of DNA and RNA were calculated, respectively, as the difference between the total nucleic acids in the first plate (without enzymes) and the amount of nucleic acids in the second plate (which contained RNase) and in the third plate (which contained DNase). The RNA-DNA ratios were then standardized (sRD) using this information and the reference slope ratio of 3.9, according to Caldarone et al. (2006).
Growth rate calculation
To minimize possible bias due to the differences in temperature and mean larval size among estuaries, the larval instantaneous growth rates were calculated according to Buckley et al. (2008). The best-fit multispecies growth model that was chosen for further calculation was:
where Gi is the instantaneous growth rate, sRD the standardized RNA-DNA ratio and T the temperature at the given site. Results have to be interpreted in the way that a value of 0 would mean no growth at all and a value of 1 would be a doubling of the weight of the larva per day.
Statistical analysis
After testing whether data fit to normal distribution with a Shapiro-Wilks test, one-way analysis of variance was performed in order to identify significant differences among estuaries in the zooplankton caloric content and in the standard length of fish larvae, which were the only variables following a normal distribution. Differences in abiotic variables (temperature, salinity, turbidity and oxygen) among estuaries were analysed using a Kruskal-Wallis test and, when significant (** p < 0.05), Mann–Whitney U post-hoc was applied on pairs of estuaries, with a new value of significance of α = 0.003. A non-parametric test (Kruskal-Wallis) was applied in order to determine if there were statistically significant differences in the sRD among estuaries. Spearman’s rank-order correlation analysis was performed to identify and select relevant correlations between the mean sRD in each estuary and the studied variables (i.e., temperature, salinity, turbidity, oxygen concentration, density of zooplankton, density of copepods, caloric content of zooplankton and density of G. aestuaria larvae). All the statistical analyses were performed using the OpenStat free software.
Results
Physical-chemical variables
Mean temperature in the areas of highest density of larval G. aestuaria was significantly lower (p < 0.003) in the Swartkops Estuary (range 20.77–21.56 °C) than in the Sundays Estuary (21.88–24.36 °C) and showed high variability within the Gamtoos Estuary (Fig. 2a). Mean salinity was significantly higher in the Kromme Estuary (30.07–34.83) than in the other estuaries, and also was significantly higher in the Sundays Estuary (11.12–24.24) than in the Kariega (7.92–10.86) and Great Fish (1.47–7.03) estuaries. Variability of salinity in the water column in the Gamtoos Estuary was very high, as with temperature, but not within the other estuaries (Fig. 2b). Mean dissolved oxygen concentration (Fig. 2c) was significantly higher in the Kromme Estuary (7.47–8.25 mg/L) than in the Swartkops Estuary (3.49–8.83 mg/L). Turbidity (Fig. 2d) in the Kromme Estuary (0) was significantly lower than in all the other estuaries, it was also significantly lower in the Swartkops Estuary (1.6–3.9), and the highest significant turbidity was measured in the Great Fish Estuary (42.3–72.9). Other significant differences in the abiotic variables among estuaries were not found (Table 1).
Box-plots showing the ranges and median of the abiotic parameters: (a) Temperature, (b) Salinity, (c) Dissolved oxygen and (d) Turbidity, in the stations where Gilchristella aestuaria larvae were collected in each of the six estuaries. Estuaries sharing a common superscript (1–8) differ significantly (see Table 1)
Plankton analyses
Sundays (77878.90 ind/m3) and Kromme estuaries (36764.00 ind/m3) presented the highest densities of zooplankton (Table 1), and also of copepods (38367.99 ind/m3 and 5789.50 ind/m3, respectively) (Table 1). Gilchristella aestuaria larvae were more abundant in the Sundays Estuary (371.11 ind/m3), followed by the Gamtoos Estuary (33.59 ind/m3) and the Kromme Estuary (15.72 ind/m3); lower densities of G. aestuaria larvae were found in the Kariega Estuary (10.82 ind/m3), the Swartkops Estuary (4.50 ind/m3) and the Great Fish Estuary (1.56 ind/m3).
Significant differences (α = 0.05) were identified in the zooplankton caloric content among estuaries (Table 1), with the mean value in the Great Fish Estuary (5628.23 cal/g) being significantly higher than in the Gamtoos (4772.64 cal/g), Swartkops (3549.26 cal/g) and Kariega (1722.14 cal/g) estuaries, while in the Kariega Estuary zooplankton caloric content was significantly lower than in the other estuaries (Table 1).
Nucleic acids quantification and growth rates
Mean standard length of G. aestuaria larvae was significantly different (p < 0.05) among all estuaries except between the Kromme and the Gamtoos estuaries and between the Swartkops and the Kariega estuaries (Table 1).
The sRD was higher in the larvae of the Gamtoos (1.41) and the Sundays (1.44) estuaries than in the other estuaries, with intermediate values in the Kromme (1.29) and Kariega (1.11). The lowest values were recorded in the Swartkops Estuary (0.79) and the Great Fish Estuary (0.97) (Fig. 3, Table 1). There were significant differences in the sRD between the Gamtoos Estuary and the Swartkops and Kariega estuaries (Fig. 3).
The Spearman-ranks correlations did not show significant correlation between the sRD and any of the environmental variables (Table 2) except with larval fish density, for which a positive correlation was found (rs = 0.943, p < 0.05). Among the non-significant correlations, the highest one was the positive correlation between sRD and salinity (rs = 0.66) and between oxygen, and copepod and zooplankton densities (rs = 0.37) (Table 2).
Larvae from the Gamtoos and the Sundays estuaries had the highest sRD and instantaneous growth rates (ranging from −0.05 to 0.23 d−1 and −0.02 to 0.22 d−1, respectively) and larvae from the Swartkops Estuary presented the lowest sRD instantaneous growth rate (−0.07 – 0.46 d−1) (Table 1). Ranges of instantaneous growth rates of larvae from the Kariega, Kromme and Great Fish Estuaries were −0.04 – 0.26 d−1, −0.07 – 0.25 d−1 and −0.08 – 0.50 d−1, respectively.
Discussion
The environmental factors affecting nutritional condition, estimated through the RNA-DNA ratio of G. aestuaria larvae, were determined in nursery areas of G. aestuaria in the upper reaches of six warm temperate South African estuaries during the peak summer breeding season (Wooldridge and Bailey 1982; Cyrus et al. 1993). The values of the environmental variables measured in the six estuaries during this study were all within the normal ranges for the summer season in these estuaries (Strydom 2014). Concurrently, the values of sRD obtained in this study are also within an acceptable range for the development and survival of G. aestuaria, according to the work of Meyer et al. (2012), which shows that, with water temperatures above 20 °C, the survival threshold of sRD (the lowest possible biochemical condition sustaining life) for the fish larvae is around 0.5, a value that is lower than what we have found for G. aestuaria larvae at temperatures of 21–24 °C in our study.
Temperature influences the growth of fish larvae (Buckley 1984; Buckley et al. 2008; Costalago et al. 2011; Strydom et al. 2014), but only extreme temperatures have a direct impact on the mortality of fish. However, some authors (Thiel et al. 1995; Esteves et al. 2000) found significant correlations between water temperature and larval fish abundance in estuaries. Since the temperature in the Kariega Estuary was the highest of all estuaries, but the larval condition in this system was significantly lower than in the Gamtoos Estuary, the relationship between temperature and larval fish condition appears especially complex and constrained by other factors such as food availability. Therefore, in our study, although a particular range of water temperature might be important for larval fish occurrence in estuaries linked with summer spawning adults (Strydom 2014), when it comes to explaining the nutritional condition and growth of fish larvae, it is likely that coupled factors play a more determinant role.
Salinity in the areas where G. aestuaria larvae were collected was higher in the Kromme (29.74–30.2), Gamtoos (3.91–32.37) and Sundays (11.12–24.24) estuaries, where G. aestuaria presented the highest sRD and mean growth rates, although the average salinities for the Gamtoos and the Sundays estuaries were within the normal ranges of the highly productive mesohaline zone (5–18), which is where the highest densities of G. aestuaria larvae are known to occur (Strydom 2014). Similarly, in the Swartkops and the Great Fish estuaries, with the lowest sRD and growth rates, salinity was comparatively low (ranging 6.61–12.45 and 1.47–7.03, respectively). The correlation analysis has shown a positive correlation (rs = 0.66) between salinity and sRD. The correlation is non-significant likely because salinity has limited direct effects on fish growth (Gibson 1994). However, it is one of the main physico-chemical variables structuring fish communities in open estuaries, affecting the distribution of several species (Strydom et al. 2003; Harrison 2005) and influencing productivity in estuaries (Wooldridge and Bailey 1982; Strydom et al. 2003). Salinity is known to be positively correlated with the abundance and biomass of G. aestuaria in several South African estuaries (Strydom and Whitfield 2000; Harrison and Whitfield 2006). In this study, the strongest correlations were between sRD and salinity and between sRD and larval abundance. Therefore our results support the hypothesis of salinity as an important regulator of the estuarine ecological structure. Interestingly, the only significant correlation reported in this study was between sRD and larval abundance, suggesting that higher salinity is associated with a higher presence of G. aestuaria larvae and that this positively affects the condition of the individual larvae.
Strydom et al. (2002) concluded that larvae of G. aestuaria are more likely than juveniles to be flushed down to the lower, more saline reaches of the Great Fish Estuary. Therefore, G. aestuaria larvae have difficulty maintaining their position in this highly turbid freshwater rich estuary, and feeding for early larvae may be difficult under strong flow conditions as a result of the inter basin water transfer scheme. Some studies have shown that the patchiness and habitat selection of larval fish are adaptive to assure optimum larval survival (Vlymen 1977; Hewitt 1981; McGurk 1986). If conditions are unfavourable for movement in a highly flushed system like the Great Fish, this may affect feeding opportunities and account for lower body condition.
The values of dissolved oxygen in the Sundays Estuary was among the highest of the six estuaries; however, mean dissolved oxygen did not seem to play a key role in explaining the nutritional condition of larvae, as demonstrated with the Spearman correlation analysis. In line with this, Able et al. (1999) also claimed that low dissolved oxygen was probably not a critical factor for larval fish growth in estuaries, while Gibson (1994) stated that only in highly vegetated and shallow estuaries, or in systems affected by pollution, as is the case in the eutrophic Swartkops Estuary (Lord and Thompson 1998), some fish may suffer oxygen depletion. Although none of the sampled stations presented low levels of dissolved oxygen, it has been reported that fish can easily avoid patches of anoxic areas (Rogers and Lockwood 1990); consequently, even when low oxygen patches are detected, this is not a reason to expect poor nutritional condition in fish.
It is known that larvae of planktivorous fish species rely on their vision for particulate feeding more than the larger individuals due to the lack of well-developed gill rakers –the filtrating apparatus- in the early life stages (Costalago and Palomera 2014; Costalago et al. 2015) Thus, the nutritional condition of planktivorous fish larvae like G. aestuaria would likely be also affected by turbidity, partly explaining why the larvae in the Great Fish Estuary, where the turbidity was significantly higher, were in relatively low condition, in spite of being a very rich fresh water estuary. This hypothesis is also in agreement with Peck et al. (2012), who reviewed the factors that affect the fish larval dynamics and claimed that turbidity affects foraging success of fish larvae, reducing food consumption rates at higher turbidity levels.
In terms of food availability, the freshwater rich Sundays Estuary had very high zooplankton densities. This was supported by good condition larval stage Gilchristella in this study. Sundays River catchment is extensively used for agriculture, a relatively high input of inorganic nutrients, particularly of nitrate, is received by the estuary (Scharler and Baird 2003). Although the extent of the effect of this supply of nutrients on the condition of fish larvae in the Sundays Estuary needs to be further investigated, our results suggest that this input of nitrates might be enhancing the energy flow, through a trophic cascade from plankton to fish.
In South African estuaries, the peak of zooplankton abundance normally occurs between November and February (Wooldridge and Bailey 1982), coinciding with the peak of G. aestuaria larvae. This can be interpreted as an adaptation of G. aestuaria to spawn at the beginning of the period of highest zooplankton abundance [i.e., in October-November (Blaber 1979; Wooldridge and Bailey 1982; Cyrus et al. 1993)] in order for the larvae to have access to a greater food supply, resulting in a better nutritional condition, thus supporting the match-mismatch hypothesis (Cushing 1990) might also be important in estuarine systems, contrarily to what has been previously thought (Newton 1996). While the density of G. aestuaria larvae and the abundance of prey are not correlated, the highest larval densities, in the Gamtoos and, especially, in the Sundays Estuaries, were coinciding with the highest sRD of the larvae. In particular, the larval density in the Sunday Estuary was very high in comparison with what has been found in other studies (Whitfield 1998, 1999; Strydom 2014), and seems to imply that the survival of G. aestuaria larvae is not density dependent, contrarily to what happens to marine species in the ocean, which is a less productive system (Cowan et al. 2000).
Food consumption in G. aestuaria adults has been reported to be highest in October-December in the Sundays Estuary (Whitfield and Harrison 1996) and the Swartkops Estuary (Talbot and Baird 1985). Therefore, parental contributions to the condition of larvae might be playing an important role in these estuaries. However, has been poorly studied in estuarine systems worldwide (Gao and Munch 2013). Some studies in coral reefs (Donelson et al. 2008; Green 2008) and others with reared fish (Clemmesen et al. 2003; Probst et al. 2006) concluded that the size of preflexion larvae was significantly positively related with parental body condition. From these studies, it can be suggested that good condition in adult estuarine planktivorous fish might translate into faster growth and higher survival rates of larvae. Although this seems to be the case in the studied estuaries, further research on the adult condition should be performed.
The caloric content of the zooplankton in the studied estuaries did not contribute to the condition of the fish larvae. The Kromme Estuary, where G. aestuaria larvae had the highest sRD and growth rates, was characterised by an elevated value of zooplankton caloric content. On the other hand, the Great Fish Estuary had a significantly higher zooplankton caloric content than any other estuary, but the larvae in this estuary were in relatively poor condition. Copepods are known to be an important source of calories for their predators in comparison with other types of potential prey (Salonen et al. 1976; Davis 1993).
The Sundays Estuary also had G. aestuaria larvae with high sRD and, although the zooplankton caloric content was not as high as in the Kromme or the Great Fish estuaries, likely due to the smaller proportion of copepods in the Sundays Estuary, the total density of the zooplankton was more than twice that of the Kromme Estuary, supporting the hypothesis that food availability plays a very important role in determining the condition of fish larvae in estuaries.
In the Swartkops Estuary, where an important input of nutrients derived from sewage and agricultural activities also takes place, this additional supply of nitrates is accompanied by a variety of pollutants and inorganic dissolved nutrients from the highly industrialized and urbanized catchment area, thus, compromising the water quality in the system (Lord and Thompson 1998) and negatively affecting the condition of the fish larvae. Moreover, this estuary, as explained above, had a very low density of G. aestuaria larvae. As stated by Whitfield and Elliot (2002), new knowledge on larval fish dynamics in estuaries will be extremely useful for future ecological and environmental health indices, which are, as particularly seen with the Swartkops Estuaries, highly impacted systems that might show clear effects on important fish species like G. aestuaria.
In conclusion, larvae of G. aestuaria were in better nutritional condition in the freshwater rich Gamtoos and Sundays estuaries than the larvae from other estuaries. Larvae from the Swartkops Estuary presented the worst nutritional condition of all studied larvae. This estuary receives a moderate supply of freshwater and has historically been a good fish nursery area (Strydom et al. 2003), but on-going eutrophication from sewerage spills may be altering water quality. The Great Fish Estuary is characterized by particularly large volumes of fresh water derived via an inter-basin transfer. Strydom et al. (2002) demonstrated that G. aestuaria larvae spawned and residing in the upper reaches of a high-flow estuary, like the Great Fish Estuary, are at a much greater risk of being flushed out to sea as a result of the excessive amount of river. Although freshwater inputs into estuarine systems are vital for the nursery function of South African estuaries (Whitfield 1994), excessive supply, and the high turbulence associated with it, can potentially alter the larval composition and the larval feeding success in the upper reaches.
Food quantity was an important was positively correlated with the nutritional condition of G. aestuaria larvae in these warm temperate estuaries. This supports findings from marine studies where the RNA-DNA ratios of fish larvae have also been related to food density (Buckley 1984; Clemmesen 1994; Rooker and Holt 1996; Clemmesen et al. 2003; Catalán et al. 2007). This study thus suggests that G. aestuaria larvae can survive and thrive in particularly variable environments, and especially within very wide ranges of salinity (4.46–30.07) and turbidity (0–52.62), inferring that estuarine fish species have remarkable adaptive capabilities (Whitfield 2015). This work is the first of its kind in South Africa and much is still needed to fully understand the drivers of a successful nursery. Food patch dynamics are poorly studied in South Africa and further research is required. Cost of biochemical analyses are however an impediment for larger sample sizes and longer term studies but the use of RNA-DNA ratio derived body condition assessment appears to be a step in the right direction to fully understand food patch dynamics in estuaries and larval fish survival.
References
Able KW, Manderson JP, Studholme AL (1999) Habitat quality for shallow water fishes in an urban estuary: the effects of man-made structures on growth. Mar Ecol Prog Ser 187:227–235
Able KW, Fahay MP, Witting DA, McBride RS, Hagan SM (2006) Fish settlement in the ocean vs. estuary: comparison of pelagic larval and settled juvenile composition and abundance from southern New Jersey, USA. Estuar Coast Shelf Sci 66(1):280–290
Amara R, Selleslagh J, Billon G, Minier C (2009) Growth and condition of 0-group European flounder, Platichthys flesus as indicator of estuarine habitat quality. Hydrobiologia 627(1):87–98
Baumann H, Peck MA, Götze HE, Temming A (2007) tarving early juvenile sprat Sprattus sprattus (L.) in western Baltic coastal waters: evidence from combined field and laboratory observations in August and September 2003. J Fish Biol 70(3):853–866
Beck MW, Heck KL Jr, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 51(8):633–641
Berdalet E, Roldán C, Olivar MP (2005) Quantifying RNA and DNA in planktonic organisms with SYBR green II and nucleases. Part B. Quantification in natural samples. Sci Mar 69:17–30
Bergeron JP (1997) Nucleic acids in ichthyoplankton ecology: a review, with emphasis on recent advances for new perspectives. J Fish Biol 51(sA):284–302
Blaber SJ (1979) The biology of filter feeding teleosts in Lake St Lucia, Zululand. J Fish Biol 15:37–59
Buckley LJ (1984) RNA-DNA ratio: an index of larval fish growth in the sea. Mar Biol 80:291–298
Buckley LJ, Durbin EG (2006) Seasonal and inter-annual trends in the zooplankton prey and growth rate of Atlantic cod (gadus morhua) and haddock (Melanogrammus aeglefinus) larvae on Georges Bank. Deep Sea Res II Top Stud Oceanogr 53(23):2758–2770
Buckley L, Caldarone EM, Ong TL (1999) RNA/DNA ratio and other nucleic acid-based indicators for growth and condition of marine fishes. In: Zehr JP, Voytek MA (eds) Molecular ecology of aquatic communities. Hydrobiol 401:265–277
Buckley LJ, Caldarone EM, Clemmesen C (2008) Multi-species larval fish growth model based on temperature and fluorometrically derived RNA/DNA ratios: results from a meta-analysis. Mar Ecol Prog Ser 371:221–232
Caldarone EM, Wagner M, St Onge-Burns J, Buckley LJ (2001) Protocol and guide for estimating nucleic acids in larval fish using a fluorescence microplate reader. Northeast Fisheries Science Center Reference Document 1
Caldarone EM, Clemmesen C, Berdalet E, Miller TJ, Folkvord A, Holt GJ, Olivar MP, Suthers IM (2006) Intercalibration of four spectrofluorometric protocols for measuring RNA/DNA ratios in larval and juvenile fish. Limnol Oceanogr Methods 4:153–163
Catalán IA, Berdalet E, Olivar MP, Roldán C (2007) Response of muscle-based biochemical condition indices to short-term variations in food availability in post-flexion reared sea bass Dicentrarchus labrax (L.) larvae. J Fish Biol 70:391–405
Chícharo MA, Amaral A, Faria A, Morais P, Mendes C, Piló D, Ben-Hamadou R, Chícharo L (2012) Are tidal lagoons ecologically relevant to larval recruitment of small pelagic fish? An approach using nutritional condition and growth rate. Estuar Coast Shelf Sci 112:265–279
Clemmesen C (1994) The effect of food availability, age or size on the RNA/DNA ratio of individually measured herring larvae: laboratory calibration. Mar Biol 118:377–382
Clemmesen C, Bühler V, Carvalho G, Case R, Evans G, Hauser L, Svaasand T (2003) Variability in condition and growth of Atlantic cod larvae and juveniles reared in mesocosms: environmental and maternal effects. J Fish Biol 62(3):706–723
Coetzee DJ (1982) Stomach content analyses of Gilchristella aestuarius and Hepsetia breviceps from the Swartvlei system and Groenvlei, southern Cape. S Afr J Zool 17:59–66
Costalago D, Palomera I (2014) Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: from late larvae to adults. Sci Mar 78(1):41–54
Costalago D, Tecchio S, Palomera I, Álvarez-Calleja I, Ospina-Álvarez A, Raicevich S (2011) Ecological understanding for fishery management: condition and growth of anchovy late larvae during different seasons in the Northwestern Mediterranean. Estuar Coast Shelf Sci 93:350–358
Costalago D, Strydom N, Frost C (2014) Nutritional condition of fish larvae in South African estuaries: an appraisal of three biochemical methods. Afr J Mar Sci 36(3):377–386
Costalago D, Garrido S, Palomera I (2015) Comparison of the feeding apparatus and diet of European sardines Sardina pilchardus of Atlantic and Mediterranean waters: ecological implications. J Fish Biol 86(4):1348–1362
Cowan JH Jr, Rose KA, DeVries DR (2000) Is density-dependent growth in young-of-the-year fishes a question of critical weight? Rev Fish Biol Fish 10:61–89
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81(1):201–219
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293
Cyrus DP, Wellmann EC, Martin TJ (1993) Diet and reproduction activity of the estuarine round-herring Gilchristella aestuaria in Cubhu, a freshwater coastal lake in northern Natal, South Africa. S Afr J Aquat Sci 19:3–13
Davis ND (1993) Caloric content of oceanic zooplankton and fishes for studies of salmonid food habits and their ecologically related species. (NPAFC Doc.) FRI-UW-9312. Fisheries Research Institute, School of Fisheries, College of Ocean and Fishery Sciences, University of Washington, Seatle
Donelson JM, McCormick MI, Munday PL (2008) Parental condition affects early life-history of a coral reef fish. J Exp Mar Biol Ecol 360(2):109–116
Esteves E, Pina T, Chícharo MA, Andrade JP (2000) The distribution of estuarine fish larvae: nutritional condition and co-occurrence with predators and prey. Acta Oecol 21(3):161–173
Ferron A, Leggett WC (1993) An appraisal of condition measures for marine fish larvae. Adv Mar Biol 30:217
Gao J, Munch SB (2013) Genetic and maternal variation in early growth in the Atlantic silverside Menidia menidia. Mar Ecol Prog Ser 485:211–222
Gibson RN (1994) Impact of habitat quality and quantity on the recruitment of juvenile fishes. Neth J Sea Res 32:191–206
Green BS (2008) Maternal effects in fish populations. Adv Mar Biol 54:1–105
Grote B, Ekau W, Stenevik EK, Clemmesen C, Verheye HM, Lipinski MR, Hagen W (2012) Characteristics of survivors: growth and nutritional condition of early stages of the hake species Merluccius paradoxus and M. capensis in the southern Benguela ecosystem. ICES J Mar Sci 69(4):553–562
Haigh EH, Whitfield AK (1993) Larval development of Gilchristella aestuaria (Gilchrist, 1914) (Pisces: Clupeidae) from southern Africa. Afr Zool 28:168–172
Harrison TD (2005) Ichthyofauna of South African estuaries in relation to the zoogeography of the region. Smithiana 6:2–27
Harrison TD, Whitfield AK (2006) Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar Coast Shelf Sci 66:335–345
Harrison TD, Whitfield AK (2012) Fish trophic structure in estuaries, with particular emphasis on estuarine typology and zoogeography. J Fish Biol 81:2005–2029
Heck KL, Hays G, Orth RJ (2003) Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar Ecol Prog Ser 253:123–136
Hewitt R (1981) The value of pattern in the distribution of young fish. Rapp Process Verbaux Reunions Cons Int Explor Mer 178:229–236
Houde ED (2008) Emerging from Hjort’s shadow. J Northwest Atl Fish Sci 41:53–70
Lord DA, Thompson GA (1998) The Swartkops estuary: pollution status. In: Baird D, Marais JFK, Martin AP (eds) The Swartkops Estuary. Proceedings of a Symposium Held on the 14 and 15 Sept. 1987 at the University of Port Elizabeth, S Afr Nat Sci Prog Rep 156. CSIR, Pretoria, pp 16–24
McGurk MD (1986) Natural mortality of marine pelagic fish eggs and larvae: role of spatial patchiness. Mar Ecol Prog Ser 34:227–242
Meyer S, Caldarone EM, Chícharo MA, Clemmesen C, Faria AM, Faulk C, Folkvord A, Holt GJ, Hoie H, Kanstinger P, Malzahn A, Moran D, Peteriet C, Stottrup JG, Peck MA (2012) On the edge of death: rates of decline and lower thresholds of biochemical condition in food-deprived fish larvae and juveniles. J Mar Syst 93:11–24
Newton GM (1996) Estuarine ichthyoplankton ecology in relation to hydrology and zooplankton dynamics in salt-wedge estuary. Mar Fresh Res 47(2):99–111
O’Connell CP, Raymond LP (1970) The effect of food density on survival and growth of early post yolk-sac larvae of the northern anchovy Engraulis mordax (Girard) in the laboratory. J Exp Mar Biol Ecol 5(2):187–197
Paulsen M, Hammer C, Malzahn AM, Polte P, von Dorrien C, Clemmesen C (2014) Nutritional situation for larval Atlantic herring (Clupea harengus L.) in two nursery areas in the western Baltic Sea. ICES J Mar Sci 71(4):991–1000
Peck MA, Huebert KB, Llopiz JK (2012) Intrinsic and extrinsic factors driving match–mismatch dynamics during the early life history of marine fishes. Adv Ecol Res 47:177–302
Probst WN, Kraus G, Rideout RM, Trippel EA (2006) Parental effects on early life history traits of haddock Melanogrammus aeglefinus. ICES J Mar Sci 63(2):224–234
Rogers SI, Lockwood SJ (1990) Observations on coastal fish fauna during a spring bloom of Phaeocystis pouchetii in the eastern Irish Sea. J Mar Biol Assoc UK 70(2):249–253
Rooker JR, Holt GJ (1996) Application of RNA: DNA ratios to evaluate the condition and growth of larval and juvenile red drum (Sciaenops ocellatus). Mar Freshw Res 47:283–290
Salonen K, Sarvala J, Hakala I, Viljanen ML (1976) Relation of energy and organic carbon in aquatic invertebrates. Limnol Oceanogr 21(5):724–730
Scharler UM, Baird D (2003) The nutrient status of the agriculturally impacted Gamtoos Estuary, South Africa, with special reference to the river‐estuarine interface region (REI). Aquat Conserv Mar Freshwat Ecosyst 13(2):99–119
Smith JLB (1965) The sea fishes of Southern Africa. Central News Agency, Cape Town
Strydom NA (2014) Patterns in larval fish diversity, abundance, and distribution in temperate South African estuaries. Estuar Coast 37:1–17
Strydom NA, Whitfield AK (2000) The effects of a single freshwater release into the kromme estuary. 4: larval fish response. Water SA 26(3):319–328
Strydom NA, Whitfield AK, Paterson AW (2002) Influence of altered freshwater flow regimes on abundance of larval and juvenile Gilchristella aestuaria (Pisces: Clupeidae) in the upper reaches of two South African estuaries. Mar Freshw Res 53(2):431–438
Strydom NA, Whitfield AK, Wooldridge TH (2003) The role of estuarine type in characterizing early stage fish assemblages in warm temperate estuaries, South Africa. Afr Zool 38:29–43
Strydom NA, Sutherland K, Wooldridge TH (2014) Diet and prey selection in late-stage larvae of five species of fish in a temperate estuarine nursery. Afr J Mar Sci 36(1):85–98
Summers JK, Paul JF, Robertson A (1995) Monitoring the ecological condition of estuaries in the United States. Toxicol Environ Chem 49(1–2):93–108
Suthers IM (1992) The use of condition indices in larval fish, Larval biology. Australian Society for Fish Biology workshop, Hobart. Australian Government Publishing Service, Canberra, p 49
Talbot MF, Baird D (1985) Feeding of the estuarine round herring Gilchristella aestuarius (Stolephoridae). J Exp Mar Biol Ecol 87:199–214
Thiel R, Sepulveda A, Kafemann R, Nellen W (1995) Environmental factors as forces structuring the fish community of the Elbe Estuary. J Fish Biol 46(1):47–69
Vasconcelos RP, Reis-Santos P, Maia A, Fonseca V, Franc AS, Wouters N, Costa MJ, Cabral HN (2010) Nursery use patterns of commercially important marine fish species in estuarine systems along the Portuguese coast. Estuar Coast Shelf Sci 86:613–624
Vasconcelos RP, Reis-Santos P, Costa MJ, Cabral HN (2011) Connectivity between estuaries and marine environment: integrating metrics to assess estuarine nursery function. Ecol Indic 11(5):1123–1133
Vlymen WJ (1977) A mathematical model of the relationship between larval anchovy (Engraulis mordax) growth, prey microdistribution, and larval behavior. Environ Biol Fish 2(3):211–233
Vos JH, Ooijevaar MAG, Postma JF, Admiraal W (2000) Interaction between food availability and food quality during growth of early instar chironomid larvae. Interaction 19(1):158–168
Whitfield AK (1992) A characterization of southern African estuarine systems. S Afr J Aquat Sci 18(1–2):89–103
Whitfield AK (1994) Abundance of larval and 0+ juvenile marine fishes in the lower reaches of three southern African estuaries with differing freshwater inputs. Mar Ecol Prog Ser 105:257
Whitfield AK (1998) Biology and Ecology of Fishes in southern African Estuaries. Ichthyological Monographs of the J.L.B. Smith Institute of Ichthyology 2. J.L.B. Smith Institute of Ichthyology, Grahamstown
Whitfield AK (1999) Ichthyofaunal assemblages in estuaries: a South African case study. Rev Methods Technol Fish Biol Fish 9:151–186. doi:10.1023/A:1008994405375
Whitfield AK (2010) A century of fish research in South African estuaries. Afr J Aquat Sci 35(3):211–225
Whitfield AK (2015) Why are there so few freshwater fish species in most estuaries. J Fish Biol 86(4):1227–1250
Whitfield AK, Harrison TD (1996) Gilchristella aestuaria (Pisces: Clupeidae) biomass and consumption of zooplankton in the Sundays Estuary. S Afr J Mar Sci 17:49–53
Whitfield AK, Elliott M (2002) Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J Fish Biol 61(sA):229–250
Wooldridge TH, Bailey C (1982) Euryhaline zooplankton of the Sundays Estuary and notes on trophic relationships. S Afr J Zool 17:151–163
Acknowledgments
We thank Dr. Isabel Palomera and Sonia Sánchez-Gómez for their assistance in the analysis of the zooplankton caloric content, and Matthias Paulsen for his valuable comments. Funding was provided by the National Research Foundation of South Africa. DC’s postdoctoral fellowship was granted by the National Research Foundation, South Africa, and Nelson Mandela Metropolitan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costalago, D., Strydom, N., Frost, C. et al. Preliminary insight into the relationship between environmental factors and the nutritional condition and growth of Gilchristella aestuaria larvae in the upper reaches of South African estuaries. Environ Biol Fish 98, 2367–2378 (2015). https://doi.org/10.1007/s10641-015-0447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-015-0447-8