Summary
Metronomic-chemotherapy (M-CHT) has been rarely assessed in non-Hodgkin-lymphoma (NHL). Therefore, in 2011 we started experimenting a new all-oral M-CHT schedule termed DEVEC (Deltacortene®, etoposide, vinorelbine, cyclophosphamide, +/-Rituximab) in diffuse-large-B-cell lymphoma (DLBCL) patients. Methods Patients with stage Ib-IV were enrolled as follows: 1) treatment-naïve, frail ≥65y, or unfit ≥85y; and 2) relapsed/refractory (R/R) ≥55y. Data were prospectively collected from six Italian centres and compared for efficacy to two reference groups, treated with established iv Rituximab-CHT in 1st and 2nd line respectively. Results from April-2011 to March-2018, 17/51(33%) naïve, 21/51(41%) refractory and 13/51(25.5%) relapsed patients started DEVEC; 39/51(76.5%) were de-novo DLBCL; 10/51(19.6%) transformed-DLBCL and 2/51(3.9%) unclassifiable-DLBCL/classical-Hodgkin-lymphoma. The median age was 85y (range=77-93) and 78y (range=57-91) in naïve and R/R respectively and overall the DEVEC patients had very poor features compared to the reference. The rate of grade≥3 haematological-AEs was 43%(95CI=29-58%): G3-neutropenia was the most frequent; grade≥3 extra-haematological-AEs was 13.7% (95%CI=5.4-25.9%), the most frequent was infection. One-year OS and PFS were 67% and 61% for naive, 60% and 50% for reference-naïve respectively; Cox proportional hazard ratio (Cox-PH-ratio) for OS and PFS were 0.69 (95%CI=0.27-1.76;p=.441) and 0.68 (95%CI=0.28-1.62;p=.381) respectively. One-year OS and PFS were 48% and 39% in the R/R, 36% and 17% in the reference-R/R respectively; Cox-PH-ratio for OS and PFS, were 0.76 (95%CI=0.42-1.40; p=.386) and 0.48 (95%CI=0.28-0.82; p=.007) respectively. Conclusion The favourable activity of DEVEC compared to a real-life series and the convenience of an oral administration, may possibly lay the groundwork for a paradigm-shift in the treatment of elderly DLBCL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Choosing treatment for patients with diffuse large B-cell lymphoma (DLBCL), who are very elderly or frail, requires a thorough evaluation of pros and cons, in almost each subject [1,2,3] Furthermore, as many elderly patients need the availability of caregivers to receive in-hospital treatments, their social condition may be a limiting factor in therapeutic choices.
The largest prospective studies in the very elderly DLBCL, were carried out by Peyrad and colleagues, who showed that nearly 50% of patients ≥80y treated with CHOP administered at 50% reduced dose (mini-CHOP) and anti-CD20 antibodies, may achieve a long term PFS, with acceptable toxicity [4, 5]. In 2015, Tucci and collaborators [1] showed that frailty, appraised through comprehensive geriatric assessment (CGA), [6], is the most important prognostic factor in elderly with DLBCL [7]. In 2018, Storti and co-workers, firstly published the results of a trial that included only frail-elderly patients [8]. In recent years, other schedules have been proposed as a first-line treatment in elderly with comorbidities or other frailty factors [9,10,11,12,13,14]. Although all these protocols have some merits, there is still not a general agreement on the standard 1st line treatment for elderly patients who are not fit [15, 16]
In non-fit elderly with relapse/refractory (R/R) DLBCL, R-bendamustine [17, 18] R-Gemox [19] and R-DHAOX [20]., are very popular schedules. However, the long-term efficacy of chemotherapy, in this subset, is overall unsatisfactory. Metronomic chemotherapy (M-CHT) - the frequent administration of chemotherapeutic drug doses that maintain a low, prolonged and active range of plasma concentrations and a good toxicity profile [21] - has become an emergent treatment modality in solid tumours [22]. Despite, few studies reported on M-CHT in lymphoma, the scant data available, suggests that M-CHT is active even in DLBCL [23,24,25,26]. Coleman and co-workers, firstly devised an effective all-oral metronomic schedule for non-Hodgkin lymphoma (NHL), based on cyclophosphamide (CTX), etoposide (ETO), procarbazine and prednisolone (PDN), +/- Rituximab (R) [23]. In 2011, we devised a new all-oral metronomic schedule, called DEVEC, which was based on the combination of PDN, CTX, ETO [27] and vinorelbine (VRN). In fact, VRN has a significant single agent activity in NHL [28, 29] and pre-clinical and clinical studies have already shown the synergistic effect of CTX and VRN in solid tumours, administered with a metronomic schedule [30,31,32,33,34,35,36,37,38]. DEVEC was initially administered to R/R DLBCL patients, considered unfit for standard in fusional (iv) CHT schedules, which are designed on the concept of delivering the maximum-tolerated-dose (MTD-CHT). Later on, also frail treatment-naïve (naïve) patients were treated with DEVEC. The preliminary data of DEVEC activity were recently published [39] and in this manuscript we will provide evidence this schedule is effective in DLBCL and it compares favourably with standard iv schedules. These results and the convenience of an oral administration may possibly lay the groundwork for a paradigm-shift in this difficult-to-treat subset of NHL patients.
Methods
This is a multicentre, retrospective study involving six Italian clinical centres. However, data of patients treated with DEVEC were prospectively collected and compared for efficacy, to subjects administered with established iv R-CHT schedules. Only cases with a confirmed diagnosis of aggressive large cell B-cell lymphoma were enrolled [40]. Therefore, all the R/R subjects were re-biopsied before the starting of treatment and 40/51(78.4%) cases were centrally reviewed for histology. Whenever suitable biopsies were available, the Hans’ algorithm was used to classify DLBCL and the cases staining positive for MYC(>40%) and BCL2 (50%) or BCL6 (>40%) expression, were analysed by FISH for the genes split signal. DEVEC foresaw an induction and a deescalated maintenance phase, both consisting of six cycles lasting 21-days, followed by a chemo-free interval of 7-days (Fig. 1). During the first cycle patients were monitored weekly with medical examination, CBC and other blood test. In the event of toxicity, CTX, ETO and VRN were suspended until recovery and the following cycle was started at reduced doses. Only two dose reduction were allowed during the induction (i.e. ETO 1-7 days or ETO withdrawal) and maintenance phase ( i.e. CTX 1-7 days or CTX withdrawal) respectively (details in supplementary data). Four doses of Rituximab (R) 375 mg/m2, were scheduled only during the 1st induction cycle ( days 7, 14, 21, 28). Patients who had already received ≥5 doses of R within the previous six months did not receive R. After, tapering ETO to doses which allowed maintaining haematological values within established threshold, patients were examined every 28-days. Each new cycle should be initiated only if PMN ≥1.500, PLT ≥ 50.000 and haemoglobin (Hb) ≥ 9.5 gr/dL. G-CSF and Erythropoietin were allowed only during the induction cycles. During the first month 300mg OD of allopurinol was administered and following it was tapered to maintain urate levels within normal ranges; Co-trimoxazole prophylaxis was administered at the dose of 960mg 4-times a week during both the induction and the maintenance phase. LMWH and low-dose aspirin were administered to patients at high and low-medium risk of thrombosis respectively. Ciprofloxacin prophylaxis was started if PMN<1.0 x10e9. A post maintenance-phase with VRN and a dose modification schedule, tailored on individual toxicity were provided (Fig S-1, Tables S-1,2,3). Adverse events were recoded basing on the CATCAE v4.03 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03).
The subjects treated with DEVEC, were consecutive patients as follows: 1) treatment-naïve, frail by CGA [6] and ≥65y, or unfit and ≥85y; or 2) R/R ≥55y, considered not suitable for MTD-CHT. Refractory patients were defined as those who did not respond to last chemotherapy or relapsed ≤12 months post-ASCT [41]. Subjects with a malabsorption syndrome, swallow dysfunctions, infected by HIV or with central nervous system involvement, were excluded from the study. Caregivers were required in very old or frail patients in order to guarantee the proper administration of DEVEC. Restaging was scheduled by computerized-tomography (CT) scan between the 2nd and 3rd induction-cycles, at the end of the induction phase by FDG positron-emission CT-scan (CT-PET) [42] and every six months thereafter. The data from patients treated with DEVEC, in 1st or subsequent lines, were compared to two reference groups consisting of 1) naïve patients aged ≥80y and 2) R/R patients aged ≥65y, who started 1st and 2nd line treatment respectively, in the years 2013-2014. Data of the reference patients were retrieved from the academic database of the Lazio Region Lymphoma Network (www.retelazio.net), The reference groups, represented a real-life population who was treated with established iv R-CHT schedules (Table 1 and supplementary table S-5). References and patients were all restaged at the end of treatment with CT-PET scan [42]. All the data were retrieved as of 31th December 2018.
Statistical analysis
The principal end-point of the study was the impact of M-CHT in terms of overall (OS), progression free (PFS) and failure free (FFS) survivals. OS was measured from the date of treatment start until death from any cause or date of last known contact for living patients. PFS was measured from the date of treatment start to either the last follow-up or the occurrence of one of the following events: progression, relapse or death from any cause. FFS was measured from date of treatment start and to either the last follow-up or the occurrence of one of the following events: lack of complete response (CR), relapse after CR or death from any cause. Continuous variables were reported as the median and range. Formal comparisons were performed with the Mann–Whitney test. Categorical variables were reported as absolute and proportion frequencies, and they were compared with the □2 test or Fisher’s exact test. Survival functions were estimated with the Kaplan–Meier method. Statistical comparisons between curves were performed with the log-rank test and the effect of covariate was estimates by means of the Cox proportional hazard (PH) regression analysis, with a confidence interval at 95% (95% CI). The comparative statistical tests was considered as significant if the two-sided p value was less than 0.05. The statistical analysis was performed with Stata 14.2 software (StataCorp LLC, College Station, USA).
Results
Patients features and cycles administered
From April 2011 to March 2018, 51 subjects started the DEVEC schedule. Seventeen out of 51 (33%) were naïve and 34/51(67%) were R/R patients respectively. Thirty-six out of 51 (70.6%) were de-novo DLBCL; whereas 10/51(19.6%) were transformed from low-grade B-cell lymphomas (T-NHL) and 2/51(3.9%) were unclassifiable lymphomas, with features intermediate between DLBCL and classical Hodgkin lymphoma (cHL/DLBCL), [40, 43]. The median age of the naïve patients was 85y (range 77-93); 15/17 (88%) were frail, inasmuch as 14/17 (82%) had stage III-IV and 13/17 (76%) had PS ≥2 (Table 1). The median age of R/R patients was 78y (range=57-91), 17/34 (50%) were frail, 22/34 (65%) had undergone ≥2 lines of therapies and 21/34 (62%) were refractory [41], (Table 1). R was not administered to 26/51 (51%) patients: 23/34 (67.7%) were R/R subjects, who had already received ≥5 R doses in the previous 6 months, whilst 3/17 (17.7%) were home-bound naïve patients, who did not have the chance to be accompanied to the hospital to receive treatments. The median number of cycles administered were 6 (range 1-43) and the total number 458 respectively (Table S-4 ).
Safety & dose intensity
The safety analysis was available for all 51 patients and the 458 cycles. Thirty-one grade 3-4 haematological AEs were reported in 22 patients (43%, 95%CI=29-58%) the most frequent was grade 3 neutropenia with 19 events (Table 2). Though not mandatory, 23/51 (49%) received at least two doses G-CSF (median=4 doses; range 2-6) and 20/51 (41%) patients received at least one dose of EPO 30.000 IU (median=4 doses; range1-7), while 3/51 (5.8%) required 1 or 2 units of red blood cell transfusions. Severe haematological toxicities (i.e. grade 4 cytopenia lasting for more than 6 days), occurred in 3/51 (5.9%, 95%CI=1.2-16.2%) patients who were heavily pre-treated or with bone marrow-involvement. Seven grade ≥3 extra-haematological AEs (eeAE), were reported in 7/51 patients (13.7%; 95%CI=5.4-25.9%). However 2/7 were not considered therapy-related: one cardiopath patient died of congestive heart failure, another R/R was diagnosed with colonic cancer. The most frequent therapy-related eeAE was bacterial pneumonia in four subjects. Of these 1/4 died within 1 months, 2/4 following pneumonia, become bed-bound and developed multi-organ-failure and died 4 and 9 months after treatment stop respectively, 1/4 discontinued treatment because of progressive disease (PD). One patient who was not on anti-thrombotic prophylaxis, had pulmonary embolism and after recovery continued treatment’ One patient discontinued treatment after the 5th cycle in complete remission (CR), because of therapy-related chronic G2 diarrhoea and started lenalidomide; one R/R patient discontinued DEVEC because was diagnosed with colonic cancer; 7/8 (87%, 95%CI=47-100%) patients, who had grade≥3 infection or neutropenic fever, were frail, with≥2 frail factors, Table S-6). In the first 40 treated patients, ETO was reduced after the occurrence of G≥3 haematological or eeAEs (Tables S-I,II, III). Afterwards, patients who were frail or had received >1 line of chemotherapy or had marrow involvement started with etoposide at a reduced dose (Fig S-1). Overall, 13/51 (25.5%, 95%CI=14.3-39.6%) and 4/51 (7.8%, 95%CI=2.2-18.9%) had 1 and 2 dose reductions respectively. All AE of G≥3 related to treatment, occurred during the induction phase. The dose intensity during induction cycles for ETO, CTX and VRN were 81,9%, 100% and 100% respectively. The direct cost of drugs included into the oral DEVEC schedule was estimated 930 and 817 Euro (year 2017) for a single induction and maintenance cycle, respectively.
Outcome & survival analyses
Outcome: the median follow-up, from treatment beginning, was 24 months and 36 months for naïve (range=10-39) and R/R (range=9-66), respectively. At the time of analysis, 29 (56.8%, 95%CI=42.2-70.7%) patients had died: 22/51 (43%, 95%CI=30.1-58.7%) for PD; 2/51 (3,9%, 95%CI=0.5-13.4%) for an adverse event occurred within 30 days from last treatment administration (heart failure=1 and pneumonia=1) and 4/51 (5.9%) for an adverse event occurred 4, 4, 5 and 9 months after treatment discontinuation (MOF=2, ictus cerebri=1, and alcoholism-related=1). Only1/51 (1,9%) deaths was considered directly -related to treatment toxicity (Table S-4). Although detailed toxicity data was not available for the reference groups, 10/65 (15%, 95CI 4.4-26.5%) deaths were recorded due to an AE and occurring within 30 days from the last treatment administration. This data, compared favourably with only 2/51 (3,9, 95CI 4.8-13.5%) deaths in the DEVEC treated group (p = 0.044).
Tumour shrinkage was recorded in 14/17 naïve (82.%, 95%CI=62-93) and 24/33 R/R (71.%, 95%CI=57-81) of 50 DEVEC patients who had at least one post-baseline efficacy assessment (Fig. 2). At the end of the induction phase the CRR was 65% and 38% in the naïve and R/R, 40% and 17% in the reference-naïve and reference-R/R respectively (p=.217 and p=.185)
Swimmer-plot of 17 treatment-naive (a) and 34 Relapsed/Refractory (b) diffuse large B-cell lymphoma patients treated with DEVEC. On the left side of the figure are reported the code and the histological diagnosis of patients: DLBCL diffuse large B-cell lymphoma, GC germinal center type, non-GC non germinal center type based on the Hans’ algorithm. IgM-se IgM secreting [43], T-MZL aggressive large cell lymphoma transformed from marginal B-cell lymphoma. T-FL aggressive large cell lymphoma transformed from follicular lymphoma, T-CLL aggressive large cell lymphoma transformed from chronic lymphocytic leukaemia. CHL/DLBCL lymphoma with features classical Hodgkin and diffuse large B-cell lymphoma [40]. DLBCL CD5+ diffuse large B-cell lymphoma CD5 postive. Death, is showed only for patients who died after a treatment-related or unrelated adverse event. Patient #19 discontinued treatment in CR due to chronic diarrhoea of grade G2 and started lenalidomide. Patient #20 after 2 months from treatment discontinuation showed unifocal subcutaneous recurrence of lymphoma. She achieved again a lasting CR after restarting the maintenance phase (cyclophosphamide, vinorelbine, prednisolone for six cycles and then vinorelbine and prednisolone)
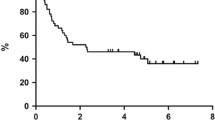
Eight out of eleven (73%) R/R patients, who received R achieved CR compared to only 4/23 (17%) who did not receive it (p=.014, Fig S-2, A-B S4 A-B). However, most R/R patients treated with DEVEC were refractory and had already received ≥2 lines of therapies. Notably, 11/21 (52%, 95%CI=30-74%) patients who were refractory, responded to DEVEC (5 CR; 6 PR, Figure S-4) After the end of the induction, seven patients, who achieved CR, discontinued DEVEC. Four out of 7 were naïve and three of these are still in CR at a median time of 35 months (range=28-38), instead the fourth naïve patient died of ictus cerebri 5 months after DEVEC discontinuation; 3/7 were R/R and they have all relapsed at a median time of 12 months (range 12-14; Fig. 2).
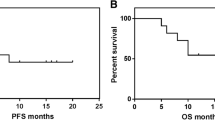
One-year OS, PFS and FFS were 67%, 61% and 55% for DEVEC-naïve, 60% , 50% and 50% for reference-naïve respectively; Cox proportional hazard ratio (Cox-PH-ratio) for OS, PFS and FFS were 0.69 (95%CI=0.27-1.76; p=.441), 0.68 (95%CI=0.28-1.62; p=.381) and 0.69 (95%CI=0.29-1.63; p=.392), respectively. One-year OS, PFS and FFS were 48%, 39% and 34% for the DEVEC-R/R, 36%,17% and 20% for the reference-R/R respectively; Cox-PH-ratio for OS, PFS and FFS were 0.76 (95%CI=0.42-1.40; p=.386), 0.48 (95%CI=0.28-0.82; p=.007) and 0.58 (95%CI=0.33-1.02; p=.056) respectively (Fig. 3 and S-4). Worthy of note 10/35 (28.6%) R/R patients from the reference group, who received DEVEC as a 3rd line, after failure of an iv schedule, were excluded from both the overall and failure free survival analyses. Patients who achieved CR at the end of the DEVEC induction phase, had an estimated one-year PFS of 100% compared to 10% in those who achieved PR (p<.0001; Fig S-3)
Survival and Cox's regression analyses of Naive and Relapsed/Refractory patients treated with DEVEC and standard infusional schedule. Overall (a), Progression free (b) and Failure free (c) Survival analyses by Kaplan-Meier plot with Log-rank test in Naïve and Relapsed/refractory (R/R) patients. Cox regression in Overall (d), Progression free (e) and Failure free (f) Survival in naïve and R/R patients. In OS and FFS analyses ten patients of the reference groups were excluded as these subjects, after the failure of R-chemotherapy, were treated with DEVEC
Discussion
The results of this study show that DEVEC (Fig. 1), a new all-oral-metronomic schedule devised with palliative intent, induced CR and allowed long term remission in a proportion of elderly and frail patients with both treatment-naïve and R/R DLBCL. Furthermore, it compared favourably with two real-life, reference groups of DLBCL patients treated with established R-CHT schedules, in 1st and 2nd line respectively (Table S-V, supplementary data). To our knowledge, this is the largest published series of elderly DLBCL treated with a metronomic schedule [23,24,25,26] Despite the naïve patients treated with DEVEC were considered too frail for receiving iv CHT schedules (Table I), 65% of them achieved a prolonged remission and none has yet relapsed after a median follow-up of 24 months (range 10-39, Fig. 2). Worthy of note, lasting CR was achieved even in three subjects, who did not receive R, as they were homebound, without caregivers who could accompany them to receive in-hospital treatments.
Few published series so far, have addressed the outcome of very elderly or frail DLBCL patients. In 2011, the Lysarc group [4] reported in 149 patients ≥80y, after R-mini-CHOP, a CRR of 62%, one-year OS and PFS of ~68% and ~60%, respectively. In 2017, Shen and collaborators published the results of a trial which enrolled 60 elderly patients with a median age of 75y, who received R-GEMOX-14 for six cycles. The CRR was 47% and one-year PFS slightly above 60%, [14] . Although, as in both studies, CGA was not carried out, the outcome of frail subjects, could not be assessed. Conversely, in the Benda-Frail trial, which enrolled only elderly-frail patients [8] it was reported a CRR of 53% and a one-year PFS <50%. Worthy of note, although direct comparison is not possible, the results of DEVEC are similar to Rmini-CHOP, which is currently the bench-mark for very elderly DLBCL patients [4].
The R/R subjects treated with DEVEC had very poor prognostic features compared to the reference group (Tables 1, S-5). In addition, as the majority were refractory, only 32% of them received R, compared to 94% of the reference patients (Table S-5). Nonetheless, the outcome of DEVEC patients compared favourably to the reference group (Fig. 3, S-2, S-4). Worthy of note, our results, seem promising also when compared to existing reports of R/R patients treated with established iv regimens [ 18-19, 45] or lenalidomide [44, 45],. Furthermore, the Scholar-1 study [41] showed that standard iv schedule in refractory DLBCL allow a CRR of only 7%, whilst the CRR of refractory patients treated with DEVEC was 23.8%.
In 2017, Zeng and co-workers [24] had already reported, in a small randomized study, that an oral metronomic schedule was more effective than MTD-CHT, in R/R DLBCL patients. Subsequently, two studies gathering 11 and 21 patients respectively, have shown good activity and low toxicity of a trofosfamide-based M-CHT, +/-R in both naïve and R/R elderly subjects [25, 26]
DEVEC was devised empirically, with a combination of drugs, which are known to be active also as single agents in NHL [27,28,29,30,31, 38]. Its formulation was inspired by the PEP-C schedule, which also contains ETO, CTX and PDN [23]. However, as ETO has significant short and long-term toxicities [27], DEVEC was aimed at reducing the administration of this drug. At this purpose, VRN an active drug in NHL [28, 29], which is very well tolerated even in long-term administration, was added at the lowest metronomic dose used in other malignancies such as prostate [46], breast, and non-small cell lung cancer [33, 37]. At odds with the majority of previous metronomic schedules experimented in NHL [23,24,25,26] and solid tumors [34,35,36], which foresaw a continuous administration of M-CHT, DEVEC has short chemo-free breaks within subsequent cycles. This was devised to allow both haematological recovery and to maintain a continuous exposure to different drugs during the cycle, thus limiting the possibility of drug resistance [21]. The administration of only four weekly doses of R was planned to grant both a rapid increase of R concentration to levels which are known to be clinically active [47] and to reduce in-hospital treatment. In fact, lessen hospital admittance is a relevant but often overlooked issue in devising anti-cancer protocols for elderly patients. Several randomized trials have already shown the substantial benefit of adding R to different chemotherapy schedules in improving both PFS and OS in DLBCL patients. Therefore, we believe, but cannot yet prove, that R may have increased also the efficacy of DEVEC. Worthy of note, the anti-lymphoma activity shown by DEVEC, despite only four doses of R were scheduled instead of the 6-8 doses given in standard schedules [4, 8, 14, 18] and observed even in those patients who did not receive it, further highlights the potency of this M-CHT schedule.
Seven out of 23 (30.4%) subjects, who achieved CR, did not proceed with the maintenance cycle after completing the induction phase. The discontinuation was due to a physician’s or patient’s decision as maintenance is not a common practice in DLBCL. Worthy of note, the naïve patients who discontinued are still in lasting CR, while all the R/R relapsed within a year (Fig. 1a, b). Another R/R patient, who after two months from the end treatment had a focal relapse was resumed to lasting CR, by restarting maintenance cycles (Fig. 2b). These observations, possibly suggests that naïve patients may require a different approach and highlights the need for more sensitive methods other than FDG-PET for modulating the duration of M-CHT such as liquid biopsy [48]. Nonetheless, the achievement of a negative PET result at the end of induction, was the most important factor for achieving a lasting PFS in all patients (Figure S-3). Although limited data on the histologic sub-types, were available, both Germinal center (GC), non-GC type, and DLBCL transformed from low-grade B-cell-lymphomas (T-DLBCL) responded to DEVEC. Moreover, two patients with CHL/DLBCL achieved a sustained remission (Fig. 2a, b).
The majority of treatment-related eeAEs of grade ≥3 were infective and occurred almost all in very frail patients. Their incidence was low and seem to compare to other studies in very elderly or frail patients [4, 14, 49].. Haematological toxicity was overall mild, while relevant neutropenia ( i.e. ≥3, lasting ≥ 6 days) or anaemia requiring RBC transfusion occurred only in patients who were heavily pre-treated (>1 lines), or had marrow involvement or who were anaemic. As a result of this experience, we strongly suggest assessing CGA in elderly subjects before starting treatment and to begin DEVEC with a reduced dose of etoposide in frail subjects, in those who have marrow involvement or received >2 lines of chemotherapy or have haemoglobin <100 gr/L (Fig S-1). Surely, even if the DEVEC schedule was generally well tolerated, during the first cycles it is necessary to frequently monitor patients’ compliance and haematological toxicities to promptly adjust doses (Tables S-I,II,III). Furthermore, anti-thrombotic prophylaxis should be given to all patients who are receiving cycles containing ETO. Finally, a high awareness about gastrointestinal symptoms as nausea, vomiting and chronic diarrhoea, related to oral VRN, should be due in case of persistence because drug discontinuation may be necessary. Nonetheless, the DEVEC schedule was very well tolerated overall and most patients evaluated as critical the chance of oral therapy.
We recognize this study has several limitations: mainly it lacked a phase I and preclinical studies or a direct comparison. In addition, quality of life was not investigated through a questionnaire and CT-PET scan were not centrally reviewed. Whilst in spite of its retrospective nature, the prospective collection of the data may have reduced the impact of this bias.
It is conceivable that the unexpected efficacy of DEVEC in DLBCL may be related to the very short chemo-free intervals of this combination schedule of metronomic chemotherapies, which possibly counteract the proliferative advantage of cancer cells [50] in high-proliferative tumors, such as DLBLC. Indeed, aside the well-known effects on angiogenesis and immunity [22, 51], recently it has been highlighted also the direct antiproliferative activity of chemotherapeutic drugs metronomically administered, such as VRN, on different cancer cells suggesting multiple mechanisms of action for this therapeutic approach [38, 52,53,54].
Although randomized studies are necessary to thoroughly assess the advantages of oral-DEVEC over iv standard protocols, this inexpensive schedule, looks very suited for elderly or frail subjects who need to reduce individual toxicity with tailored-treatments and to limit hospital admittance.
References
Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F et al (2015) Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 56(4):921–926
Dinmohamed AG, Issa DE, Van der Poel MWM, Schouten HC, Lugtenburg PJ, Chamuleau MED et al (2017) Treatment and relative survival in very elderly patients with DLBCL in The Netherlands: a population-based study, 1989 to 2015. Blood Advances 1(21):1839–1841
Buske C, Hutchings M, Ladetto M, Goede V, Mey U, Soubeyran P (2018) ESMO Lymphoma Consensus Conference Panel Members. ESMO Consensus Conference on malignant lymphoma: general perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann Oncol 29(3):544–562
Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S et al (2011) Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 12(5):460–468
Peyrade F, Bologna S, Delwail V, Emile JF, Pascal L, Fermé C et al (2017) Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematology 4:e46–e55
Merli F, Luminari S, Rossi G, Mammi C, Marcheselli L, Ferrari A et al (2014) Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 55(1):38–43
Lin RJ, Behera M, Diefenbach CS (2017) Flowers CR Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood. 130(20):2180–2185
Storti S, Spina M, Pesce EA, Salvi F, Merli M, Ruffini A et al (2018) Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: a phase II multicenter study of the Fondazione Italiana Linfomi. Haematologica. 103(8):1345–1350
Fields PA, Townsend W, Webb A, Counsell N, Pocock C, Smith P et al (2014) De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: a United Kingdom National Cancer Research Institute trial. J Clin Oncol 32(4):282–287
Luminari S, Viel E, Ferreri AJM, Zaja F, Chimienti E, Musuraca G et al (2018) Non pegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol 36(1):68–75
Maybury B, Kimpton G, Otton S (2018) A retrospective multicentre study of COCKLE, an oral chemotherapy regimen, as palliative treatment for high grade lymphoma. British J Haematology. https://doi.org/10.1111/bjh.15637
Rashidi A, Oak E, Carson KR, Wagner-Johnston ND, Kreisel F, Bartlett NL (2016) Outcomes with R-CEOP for R-CHOP-ineligible patients with diffuse large B-cell lymphoma are highly dependent on cell of origin defined by Hans criteria. Leuk Lymphoma 57(5):1191–1193
Spina M, Balzarotti M, Uziel L, Ferreri AJ, Fratino L, Magagnoli M et al (2012) Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 17(6):838–846
Shen QD, Zhu HY, Wang L, Fan L, Liang JH, Cao L et al (2018) Gemcitabine-oxaliplatin plus rituximab (R-GemOx) as first-line treatment in elderly patients with diffuse large B-cell lymphoma: a single-arm, open-label, phase 2 trial. Lancet Haematology 5:e261–e269
Eyre TA, Salisbury R, Eyre DW, Watson C, Collins GP, Hatton CS et al (2016) Results of a large retrospective analysis of the effect of intended dose intensity of R-CHOP on outcome in a cohort of consecutive, unselected elderly patients with de novo diffuse large B cell lymphoma. Br J Haematol 173(3):487–491
Kumar A, Fraz MA, Usman M, Malik SU, Ijaz A, Durer C, Durer S, Tariq MJ, Khan AY, Qureshi A, Faridi W, Nasar A, Anwer F (2018 Sep 1) Treating Diffuse Large B Cell Lymphomain the Very Old or Frail Patients. Curr Treat Options in Oncol 19(10):50
Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N et al (2013) Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 31(17):2103–2109
Arcari A, Chiappella A, Spina M, Zanlari L, Bernuzzi P, Valenti V et al (2016) Safety and efficacy of rituximab plus bendamustine in relapsed or refractory diffuse large B-cell lymphoma patients: an Italian retrospective multicenter study. Leuk Lymphoma 57(8):1823–1830
Corazzelli G, Capobianco G, Arcamone M, Ballerini PF, Iannitto E, Russo F et al (2009) Long-term results of gemcitabine plus oxaliplatin with and without rituximab as salvage treatment for transplant-ineligible patients with refractory/relapsing B-cell lymphoma. Cancer Chemother Pharmacol 64(5):907–916
Machover D, Delmas-Marsalet B, Misra SC, Gumus Y, Goldschmidt E (2001 Oct) Schilf A, et al Dexamethasone, high-dose cytarabine, and oxaliplatin (DHAOx) as salvage treatment for patients with initially refractory or relapsed non-Hodgkin's lymphoma. Ann Oncol 12(10):1439–1443
Bocci G, Kerbel R (2016) Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol 13(11):659–673
Natale G, Bocci G (2018) Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett 432:28–37
Coleman M, Martin P, Ruan J, Furman R, Niesvizky R, Elstrom R et al (2008) Prednisone, etoposide, procarbazine, and cyclophosphamide (PEP-C) oral combination chemotherapy regimen for recurring/refractory lymphoma: low-dose metronomic, multidrug therapy. Cancer. 112(12):2228–2232
Zeng J, Yang L, Huang F, Hong T, He Z, Lei J et al (2016) The metronomic therapy with prednisone, etoposide, and cyclophosphamide reduces the serum levels of VEGF and circulating endothelial cells and improves response rates and progression-free survival in patients with relapsed or refractory non-Hodgkin's lymphoma. Cancer Chemother Pharmacol 78(4):01–08
Schelker RC, Herr W, Reichle A, Vogelhuber M (2018) Low-dose trofosfamide plus rituximab is an effective and safe treatment for diffuse large B-cell lymphoma of the elderly: a single center experience. BMC Cancer 18(1):1000
Witte HM, Riecke A, Mayer T, Bartscht T, Rades D (2019 Jan) Lehnert H et alTrofosfamide in the treatment of elderly or frailpatients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol 145(1):129–136
Thompson DS, Hainsworth JD, Hande KR, Holzmer MC, Greco FA (1993) Prolonged administration of low-dose, infusional etoposide in patients with etoposide-sensitive neoplasms: a phase I/II study. J Clin Oncol
Balzarotti M, Santoro A, Tondini C, Fornier M, Bonadonna G (1996 Nov) Activity of singleagent vinorelbine in pretreated non-Hodgkin's lymphoma. Ann Oncol 7(9):970–972
Rule S, Tighe M, Davies S, Johnson S (1998 Sep) Vinorelbine in the treatment of lymphoma. Hematol Oncol 16(3):101–105
Man S, Bocci G, Francia G, Green SK, Jothy S (2002 May 15) Hanahan D et al Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res 62(10):2731–2735
Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS (2003 Aug 1) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effectson the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63(15):4342–4346
Rozados VR, Sánchez AM, Gervasoni SI, Berra HH, Matar P, Graciela SO (2004 Oct) Metronomic therapy with cyclophosphamide induces rat lymphoma and sarcomaregression, and is devoid of toxicity. Ann Oncol 15(10):1543–1550
Briasoulis E, Aravantinos G, Kouvatseas G, Pappas P, Biziota E, Sainis, I, et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study BMC Cancer 2013;13:263.
Minard-Colin V, Ichante JL, Nguyen L, Paci A, Orbach D, Bergeron C et al (2012 Oct) Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma--a report from the Société Française des Cancers et leucémies del'Enfant et de l'adolescent (SFCE). Eur J Cancer 48(15):2409–2416
Mavroeidis L, Sheldon H, Briasoulis E, Marselos M, Pappas P, Harris AL (2015) Metronomic vinorelbine: Anti-angiogenic activity in vitro in normoxic and severe hypoxic conditions, and severe hypoxia-induced resistance to its anti-proliferative effect with reversal by Akt inhibition. Int J Oncol
Launay S, Sabatier R, Brunelle S, Esterni B, Tarpin C, Viret Fet al . METRO1: A Phase I Study of Metronomic Chemotherapy in Adults with Advanced Refractory Solid Tumors. Anticancer Res 2016 Jan;36(1):293-299.
Orecchioni S, Talarico G, Labanca V, Calleri A, Mancuso P, Bertolini F (2018 May) Vinorelbine, cyclophosphamide and 5-FU effects on the circulating andi ntratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer 118(10):1329–1336. https://doi.org/10.1038/s41416-018-0076-z
Orlandi P, Di Desidero T, Salvia G, Muscatello B, Francia G, Bocci G (2018) Metronomic vinorelbine is directly active on Non Small Cell Lung Cancer cells and sensitizes the EGFRL858R/T790M cells to reversible EGFR tyrosine kinase inhibitors. Biochem Pharmacol 152:327–337
Cox MC, Musuraca G, Battistini R, Casaroli I, Zoli V, Anticoli-Borza P et al (2018) Aggressive lymphomas of the elderly: the DEVEC metronomic chemotherapy schedule fits the unfit. Br J Haematol 183(5):819–822
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 127(20):2375–2390
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. Blood. 2018 Feb 1;131(5):587-588
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Cox MC, Di Napoli A, Scarpino S, Salerno G, Tatarelli C, Talerico C et al (2014) Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One 9:e93903
Zinzani PL, Pellegrini C, Argnani L, Broccoli A (2016) Prolonged disease-free survival in elderly relapsed diffuse large B-cell lymphoma patients treated with lenalidomide plus rituximab. Haematologica. 101:e385–e386. https://doi.org/10.3324/haematol.2016.147256
Houot R, Cartron G, Bijou F, de Guibert S, Salles GA., Fruchart C, et al. Obinutuzumab plus Lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study. Leukemia. 2018; https://doi.org/10.1038/s41375-018-0282-y.
Di Desidero T, Derosa L, Galli L, Orlandi P, Fontana A, Fioravanti A et al (2016) Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients. Investig New Drugs 34(6):760–770
NL Berinstein, AJ Grillo-López, CA White, et al: Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma Ann Oncol 9: 995– 1001,1998
Rossi D, Diop F, Spaccarotella E, Monti S, Zanni M, Rasi S et al (2017) Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood. 129(14):1947–1957
Park SI, Grover NS, Olajide O, Asch AS, Wall JG, Richards KL et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untreated diffuse 10.1111/bjh.14232. Epub 2016 Jul 22. PubMed PMID: 27448091; PubMed Central PMCID:
Ciccolini J, Barbolosi D, Meille C, Lombard A, Serdjebi C, Giacometti S et al (2017) Pharmacokinetics and Pharmacodynamics-Based Mathematical Modeling Identifies an Optimal Protocol for Metronomic Chemotherapy. Cancer Res 77(17):4723–4733
Kerbel RS (2017) Shaked Y The potential clinical promise of 'multimodality' metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett 400:293–304
Cerrito MG, De Giorgi M, Pelizzoni D, Bonomo SM, Digiacomo N, Scagliotti A et al (2018) Metronomic combination of Vinorelbine and 5Fluorouracil is able to inhibit triple-negative breast cancer cells. Results from the proof-of-concept VICTOR-0 study. Oncotarget. 9(44):27448–27459
André N, Tsai K, Carré M, Pasquier E (2017 May) Metronomic Chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer 3(5):319–325
Buckstein R, Kerbel RS, Shaked Y, Nayar R, Foden C, Turner R et al (2006) High-Dose celecoxib and metronomic "low-dose" cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin's lymphoma. Clin Cancer Res 12(17):5190–5198
Acknowledgments
M Christina Cox : performed the research, designed the research study, analysed the data, wrote the paper.
Sabrina Pelliccia: performed the research, analysed the data, wrote the paper
Luigi Marcheselli: designed the research study, analysed the data, wrote the paper
Roberta Battistini: performed the research, wrote the paper
Annalisa Arcari: performed the research, designed the research study, wrote the paper
Paola Anticoli Borza: performed the research, wrote the paper
Caterina Patti: performed the research, wrote the paper
Ivana Casaroli: performed the research, wrote the paper
Francesca di Landro: performed the research, wrote the paper
Arianna Di Napoli: performed the research, wrote the paper wrote the paper
Francesca Fabbri: performed the research, wrote the paper
Matteo Caridi: analysed the data, wrote the paper
Agostino Tafuri: performed the research, wrote the paper
Guido Bocci: designed the research study, analysed the data, wrote the paper
Gerardo Musuraca: performed the research, designed the research study, analysed the data, wrote the paper
Funding
The work was supported by the Department of Oncological science, Azienda Ospedaliera-Universitaria Sant’Andrea, Rome, Italy
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Author M Christina Cox declares that she has no conflict of interest;
Author Sabrina Pelliccia, declares that she has no conflict of interest,
Author Luigi Marcheselli, declares that he has no conflict of interest;
Author Roberta Battistini, declares that she has no conflict of interest;
Author Annalisa Arcari, declares that she has no conflict of interest;
Author Paola Anticoli Borza, declares that she has no conflict of interest;
Author Caterina Patti , declares that she has no conflict of interest;
Author Ivana Casaroli , declares that she has no conflict of interest;
Author Francesca di Landro , declares that she has no conflict of interest;
Author Arianna Di Napoli, declares that she has no conflict of interest;
Author Francesca Fabbri, declares that she has no conflict of interest;
Author Matteo Caridi, declares that he has no conflict of interest;
Author Agostino Tafuri , declares that he has no conflict of interest;
Author Guido Bocci , declares that he has no conflict of interest;
Author Gerardo Musuraca,, declares that he has no conflict of interest;
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Sapienza ethics committee (EC approval n° 4640).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cox, M.C., Pelliccia, S., Marcheselli, L. et al. The metronomic all-oral DEVEC is an effective schedule in elderly patients with diffuse large b-cell lymphoma. Invest New Drugs 37, 548–558 (2019). https://doi.org/10.1007/s10637-019-00769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00769-5