Summary
Background Previous study indicated that an optional anti-cancer drug for the treatment of small-cell lung cancer (SCLC) is amrubicin. However, no prospective studies have evaluated amrubicin in chemo-naive elderly or poor-risk patients with SCLC. Therefore, this study aimed to evaluate the efficacy of amrubicin as first-line chemotherapy for elderly or poor-risk patients with extensive-disease SCLC (ES-SCLC). Methods Patients with chemotherapy-naive ES-SCLC received multiple cycles of 40 mg/m2 amrubicin for 3 consecutive days every 21 days. The primary endpoint was the overall response rate (ORR), and the secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety. Results Between March 2011 and August 2015, 36 patients were enrolled in this study. Each patient received a median of four treatment cycles (range, 1–6 cycles). ORR was 52.8% [95% confidence interval (CI), 37–69%]. The median PFS and OS periods were 5.0 months (95% CI, 3.4–6.6 months) and 9.4 months (95% CI, 5.2–13.6 months), respectively. Neutropenia was the most common grade 3 or 4 adverse event (69.4%), with febrile neutropenia developing in 13.9% of patients. No treatment-related death occurred. At the time of starting second-line chemotherapy, 19 of 22 patients (86%) had significantly improved or maintained their performance status (PS) relative to their PS at the time of starting amrubicin monotherapy as first-line chemotherapy (P = 0.027). Conclusions The results of the present study suggest that amrubicin could be considered as a viable treatment option for chemotherapy-naive elderly or poor-risk patients with ES-SCLC (Clinical trial registration number: UMIN000011055 www.clinicaltrials.gov).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 15% of all patients with lung cancer have small-cell lung cancer (SCLC). Unlike other types of lung cancer, SCLC is one of the most chemo-sensitive solid tumors [1]. However, after remarkably successful induction therapy, most patients have a relapse as a result of the emergence of drug-resistant tumor cells. While many clinical trials have been performed and reported to provide new drugs including immune check point inhibitors and target based medicine for patients with non-small-cell lung cancer (NSCLC) [2,3,4,5,6,7], the development of new drug for SCLC patients has been delayed in comparison with that of NSCLC. Approximately half of all SCLC patients in Japan are over 70 years of age. The Japan Clinical Oncology Group has reported that carboplatin plus etoposide is an active and less toxic regimen in elderly patients with SCLC [8]. Alternatively, previous clinical trials have indicated that combination chemotherapy consisting of reduced or split doses of cisplatin plus etoposide (SPE) can be safe and effective in elderly or poor-risk patients with SCLC [9, 10]. Subsequently, the Japan Clinical Oncology Group reported the results of a phase III trial indicating that the SPE regimen can be considered as an alternative to the carboplatin plus etoposide regimen for the treatment of elderly and poor-risk patients with extensive-disease small-cell lung cancer (ES-SCLC) [11]. Thus, the carboplatin plus etoposide regimen and SPE have been recognized as standard therapies for elderly Japanese patients with SCLC. Because the proportion of elderly patients with SCLC has been continuously increasing in Japan, novel treatment opportunities for these patients have been becoming increasingly important.
Amrubicin hydrochloride is a completely synthetic 9-aminoanthracycline that is metabolically activated to amrubicinol by a liver enzyme. Both amrubicin and amrubicinol inhibit DNA topoisomerase II and exert a cytotoxic effect by stabilizing a topoisomerase-II-mediated cleavable complex. Their potency as DNA intercalators is approximately one tenth that of doxorubicin [12, 13]. The catatonic activity of amrubicinol in vitro is 18–220 times more potent than that of its parent compound amrubicin [14]. The anti-tumor activity of amrubicin against several human tumor xenografts implanted in nude mice has been found to be more potent than that of the representative anthracycline doxorubicin, with almost no cardiotoxicity [15, 16]. Amrubicin has been shown to be active against previously untreated SCLC [17], and patients in that study had an overall response rate (ORR) of 79% and a median survival time (MST) of 11.0 months. Thus, the results of previous studies support the consideration of amrubicin as a key drug for the treatment of SCLC. However, amrubicin has not been evaluated sufficiently in chemo-naive elderly and poor-risk patients with SCLC. A previous retrospective study that we conducted to estimate the efficacy of amrubicin in these patients revealed an ORR of 70%, progression-free survival of 6.6 months, and an MST of 9.3 months [18]. Considering the elderly and poor-risk population of patients, the starting dose of amrubicin was reduced by 5 mg/m2 from the conventional starting dose of 45 mg/m2/day in the above retrospective study.
In the present prospective study, we aimed to estimate the efficacy and safety of amrubicin as first-line chemotherapy for elderly or poor-risk patients with ES-SCLC.
Patients and methods
Study design
This study was designed as a single-arm phase II study to estimate the efficacy and safety of amrubicin monotherapy. The primary endpoint in this study was ORR, which was calculated as confirmed response (Complete response + Partial response) according to independent assessments. The secondary endpoints were PFS, OS, and safety. The study was performed in accordance with the Declaration of Helsinki, the Japanese Pharmaceutical Affairs Law, and the International Conference on Harmonization–Good Clinical Practice guidelines. This study was approved by the institutional ethics review board of the Kitasato University Hospital. Signed informed consent for participation was obtained from all patients. This study was registered at ClinicalTrials.gov (UMIN000011055).
Patient eligibility
The eligibility criteria were as follows: histologically or cytologically proven small cell carcinoma of the lung; stage IIIb (unresectable and unfit for definitive radiotherapy), stage IV (as defined by the Union for International Cancer Control TNM classification, 7th edition); age > 70 years or Eastern Cooperative Oncology Group performance status (PS) > 1; chemotherapy naive; measurable lesion according to the Response Evaluation Criteria In Solid Tumors version 1.0; life expectancy of 3 months; preserved bone marrow function (white blood cell count ≥4000 /mm3, absolute neutrophil count ≥2000 /mm3, platelet count ≥100,000/mm3, and hemoglobin ≥9.5 g/dl), adequate liver function (aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of the normal range and total bilirubin ≤1.5 mg/dl), adequate pulmonary function (PaO2 ≥ 60 Torr or oxygen saturation ≥ 90% in ambient air), and electrocardiogram without abnormal findings requiring treatment.
Patients were excluded from the study if they had any of the following conditions: serious infections or other serious complications; other active cancers; presence of massive pleural effusion, ascites, or pericardial effusion interfering with the administration of chemotherapy; clear evidence of interstitial pneumonia or pulmonary fibrosis on a plain chest radiograph; brain metastasis associated with central nervous system symptoms (patients were eligible for inclusion if the symptoms could be controlled by steroids or other treatments); poorly controlled diabetes mellitus; and a distinct history of drug allergies. Pregnant or lactating women and men who did not intend to use contraception were ineligible.
Treatment
Treatment was started within 1 week after enrollment in this study. Patients were treated every 3 weeks with an intravenous infusion of amrubicin dissolved in 20 mL normal saline on days 1 to 3. The dose of amrubicin was set at 40 mg/m2/day. The treatment regimen was repeated for 4 to 6 cycles at the attending oncologists’ discretion (i.e., after four cycles, the oncologist decided whether a fifth and sixth cycle was appropriate) and continued until disease progression or unacceptable adverse event(s) occurred, or the patient requested to stop treatment. There was no restriction for subsequent chemotherapy after disease progression in this study.
Toxicity assessment and treatment modification
Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. The criteria for dose reduction of amrubicin were as follows: grade 4 neutropenia lasting ≥4 days, febrile neutropenia, grade 4 thrombocytopenia, and grade 3 or severe nonhematologic toxicity, except nausea, anorexia, weight loss, creatinine, hyponatremia, and hyperglycemia. If any of these adverse events occurred, the dose of amrubicin was reduced by 5 mg/m2/day in subsequent cycles. A second dose reduction to 30 mg/m2/day was made in subsequent cycles on the basis of the same criteria. An intra-patient dose escalation was not allowed in this trial. In cases of toxicity that would have required a third dose reduction, the protocol treatment was terminated. The subsequent cycle was started when patients satisfied the organ function eligibility criteria of this trial.
Patients received supportive care as required, including transfusion of blood products. The protocol specified that 50 μg/m2/day or 2 μg/kg/day recombinant human granulocyte colony-stimulating factor (G-CSF) should be used in accordance with the national health insurance coverage of Japan. Indications for G-CSF administration were as follows: (a) when fever (in principle, body temperature over 37.5 °C) was observed with a neutrophil count of ≤1000/mm3; (b) when a neutrophil count of 500/mm3 was observed; and (c) during the previous course, if fever (in principale, body temperature over 37.5 °C) with a neutrophil count of ≤1000/mm3 was observed, or if a neutrophil count of 500/mm3 was observed, then after completing the same chemotherapy, if a neutrophil count of ≤1000/mm3 was observed.
Response evaluation
Before the start of treatment, the following examinations were performed: hematologic examinations and serum chemical analysis, urinalysis, measurement of tumor marker levels, measurement of percutaneous oxygen saturation, and electrocardiography. Lesions were evaluated by performing plain chest radiography, computed tomography (CT) of the chest and abdomen, positron emission tomography (PET) or bone scintigraphy, and CT or magnetic resonance imaging (MRI) of cranium. After initiation of treatment, hematologic examinations and serum chemical analyses were carried out at 1- to 2-week intervals. When absolute neutrophil count and leukocyte count were <500/mm3 and <1000/mm3, respectively, the complete blood count was repeated every day until recovery of the values to the normal range. To evaluate the tumor lesions, CT of the chest and abdomen was performed at least every two cycles. Confirmation of complete or partial response was required at least 4 weeks after the first documentation of a response. PET or bone scintigraphy and CT or MRI of the cranium were performed either when patients had significant symptoms associated with tumor lesions or at 6-month intervals. Tumor shrinkage was assessed in accordance with the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1). All evaluations of the recorded responses were confirmed by an independent evaluator.
Statistical analyses
All data were analyzed with a cut-off date of February 1, 2017. The primary endpoint of this study was ORR, which was calculated as confirmed response (complete response and partial response) according to an independent review. The sample size was set as N = 32 to achieve a power of at least 80% with a one–sided alpha of 0.1, and expected and threshold values for the primary endpoint of 70% and 50%, respectively. PFS was defined as the time interval from the date of enrollment to disease progression or patient death. OS was defined as the time interval from the date of enrollment to patient death or the last follow-up. We evaluated time-to-event/Kaplan-Meier curves for analysis of PFS and OS. We also evaluated a change of PS of each patient who received second-line chemotherapy, and used the Wilcoxon signed-rank test to analyze differences in PS. The statistical analyses were performed using the SPSS software program, version 23.0 (SPSS Inc., Chicago, Illinois), for Windows. P-values less than 0.05 were considered significant.
Results
Patient characteristics
From March 2011 to August 2015, a total of 36 patients were enrolled in this study. All these patients were included in the efficacy and safety analyses. The demographic data of the patient population are shown in Table 1. Twenty-eight patients were men and eight were women, 20 patients had a PS of 2 or 3, and the median patient age was 74 years (range, 51–90). A total of 125 cycles of treatment were administered, and the number of treatment cycles administered per patient ranged from 1 to 6 (median, 4 cycles). While the dose was reduced to 35 mg/m2/day in five patients (grade 4 neutropenia lasting ≥4 days in two patients, febrile neutropenia in three patients), a second dose reduction to 30 mg/m2/day was not required. The delay in the administration of a subsequent cycle did not exceed 14 days in any patient. Reasons for off-protocol included disease progression (n = 35) and patient refusal (n = 1). The protocol treatment was not terminated because of severe toxicities in any patient.
Response
An independent review of tumor response was performed for each patient. Of the 36 patients, a partial response was observed in 19 patients, stable disease in six patients, and progressive disease in 10 patients. The tumor response was not evaluable in one patient because of early termination of the treatment protocol due to the patient’s refusal, and he refused further treatment after one cycle of amrubicin treatment. The overall response rate was 52.8% (95% CI: 37–69%, Table 2). Of the 36 patients, 22 (61%) received second-line chemotherapy that consisted of carboplatin plus etoposide in 19 patients and cisplatin plus irinotecan in three patients. Among the 22 patients, partial response was observed in nine patients by the second-line chemotherapy (40.9% of response rate), including two responders to cisplatin plus irinotecan.
Survival
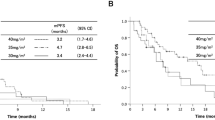
The median PFS for all patients was 5.0 months (95% CI: 3.4–6.6 months, Fig. 1a). The median OS for all patients was 9.4 months (95% CI: 5.2–13.6 months, Fig. 1b). The median follow-up time was 10.4 months. In addition, the median OS was 13.5 months (95% CI, 9.0–18.0) in the 22 patients who could receive the platinum-based chemotherapy as second-line chemotherapy.
Toxicity
The profile of grade 3 or higher toxicities is summarized in Table 3. The most common adverse events were hematological toxicities, including grade 3 or 4 neutropenia (69.4%), leukopenia (38.9%), and thrombocytopenia anemia (16.7%; Table 3). Grade 3 febrile neutropenia developed in five patients (13.9%). Non-hematological toxic effects were relatively mild. Grade 3 pneumonitis occurred in one patient after administration of the fourth cycle of amrubicin. Neither discontinuation of treatment because of unacceptable toxicity nor treatment-related death was observed.
Change in PS of patients receiving second-line chemotherapy
Figure 2 shows the change in PS for 22 patients receiving platinum-based chemotherapy as a second-line chemotherapy after amrubicin monotherapy. At start of the second-line chemotherapy, 19 of 22 patients (86%) had improved or maintained PS relative to the PS at the start of the amrubicin monotherapy as first-line chemotherapy. The change in PS was significant according to the Wilcoxon signed rank test (P = 0.027).
Discussion
To the best of our knowledge, this is the first report to estimate the efficacy of amrubicin as first-line chemotherapy for elderly or poor-risk patients with ES-SCLC. It is noteworthy that the amrubicin monotherapy had a clinical response rate of 52.8%, median PFS of 5.0 months, and median OS of 9.4 months. Prior to this phase II study, we reported a retrospective study indicating a response rate of 70%, median PFS of 6.6 months, and OS of 9.3 months after treatment of elderly or poor-risk patients with ES-SCLC with 35 or 40 mg/m2 of amrubicin [18]. While the response rate did not reach the expected value of 70% in the present study, the median PFS of 5.0 months and OS of 9.4 months were consistent with those of carboplatin plus etoposide in elderly and poor-risk patients with ES-SCLC, as found in recent phase II or III studies [10, 11, 19, 20] and our above mentioned retrospective study [18]. Therefore, this regimen may represent a viable treatment option because its treatment outcomes were equivalent to those of the carboplatin plus etoposide regimen, which has been recognized as a standard treatment for elderly or poor-risk patients with ES-SCLC. The anti-tumor mechanism of amrubicin differs from that of platinum and topoisomerase I inhibitors. Accordingly, amrubicin has mainly been used for the treatment of previously treated SCLC patients in clinical practice. Onoda et al. [21] found that amrubicin at 40 mg/m2 had significant activity and acceptable toxicity in previously treated SCLC patients. However, Kato et al. [22] found that amrubicin at 45 mg/m2 had promising activity but a severe and unacceptable toxicity profile in previously treated SCLC patients. Thus, when we conducted this phase II trial, we selected 40 mg/m2 as the starting dose of amrubicin on the basis of the results of these previous studies [21, 22] and our retrospective study [18].
Of the 36 patients in this study, 22 (61%) could receive second-line chemotherapy on the basis of the improvement or maintenance of PS during amrubicin monotherapy, and three of these 22 patients surprisingly received cisplatin plus irinotecan, suggesting that the clinical usefulness of amrubicin as first-line chemotherapy for ES-SCLC patients with poor PS. In general, a preservation of PS is indispensable to an effective chemotherapy regimen that results in prolonged patient survival. A further important point is that the number of and effective anti-cancer drugs for the treatment of SCLC is limited compared to those available for NSCLC. Consequently, in addition to amrubicin, platinum-based chemotherapy is the primary treatment regimen available to SCLC patients. Therefore, providing platinum-based chemotherapy to patients without an exacerbation of PS is extremely important during all treatment processes.
The principal severe adverse event associated with amrubicin monotherapy in this study was hematological toxicity in the form of neutropenia and leucopenia. Non-hematologic adverse events were mild and consistent with historical data [18, 21]. The number of patients requiring dose reduction and a delay in drug administration was few because of appropriate G-CSF support, indicating that the 40 mg/m2 dose of amrubicin was acceptable for elderly or poor-risk patients with ES-SCLC. While five patients required a dose reduction to 35 mg/m2, all patients could perform confirmed partial response. This result is supported by previous reports indicating that amrubicin was efficacious against recurrent ES-SCLC at a dose between 35 mg/m2 and 40 mg/m2 [23, 24]. Furthermore, a previous study reported that leukopenia and neutropenia induced by an amrubicin dose of 45 mg/m2 were severe, with grade 3 to 4 leukopenia occurring in as many as 80% of patients, febrile neutropenia occurring in 34% of elderly SCLC patients, and treatment-related deaths from infection associated with neutropenia occurring in two patients [25]. Another phase II study also revealed a high incidence of amrubicin-induced febrile neutropenia at a dose 45 mg/m2 and concluded that the dose of 45 mg/m2 may not be recommended in pretreated patients with SCLC [26]. In our previously retrospective study, amrubicin at a dose of 35–40 mg/m2 was well tolerated in patients aged >75 years, without treatment-related mortality [18]. Another retrospective study comparing 30 and 40 mg/m2 amrubicin monotherapy between elderly and younger patient groups found no between-group differences in the mean dose and interval of amrubicin administration, and the severity of hematologic toxicity [27]. A previous study summarized seven cases of interstitial lung disease (ILD) induced by amrubicin monotherapy from among a review of 100 cases of SCLC treated with this anti-cancer agent [28]. The incidence rates of ILD were 3% and 33% in patients with or without pre-existing pulmonary fibrosis [28]. Thus, the incidence of drug-induced ILD in our present study (2.8%) was consistent with the data of this previous study.
This study has several limitations. It was performed in a single institute, and accordingly the enrollment of patients in this clinical trial was slow. Although the individuals included in this study were elderly or poor-risk patients, data regarding their quality of life were not evaluated.
In conclusion, amrubicin monotherapy for chemo-naive elderly or poor-risk patients with ES-SCLC resulted in moderate tumor response, PFS, and OS, and acceptable toxicity. The results of this study suggest that amrubicin is a viable option for the treatment of chemo-naive elderly or poor-risk patients with ES-SCLC. Further prospective studies comparing this regimen with carboplatin plus etoposide are warranted.
References
Morita T (2002) A statistical study of lung cancer in the annual of pathological autopsy cases in Japan, from 1958 to 1997, with reference to time trends of lung cancer in the world. Jpn J Cancer Res 93(1):15–23. doi:10.1111/j.1349-7006.2002.tb01195.x
Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H, Tamura T (2017) Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investig New Drugs 35(2):207–216. doi:10.1007/s10637-016-0411-2
Shimizu T, Seto T, Hirai F, Takenoyama M, Nosaki K, Tsurutani J, Kaneda H, Iwasa T, Kawakami H, Noguchi K, Shimamoto T, Nakagawa K (2016) Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Investig New Drugs 34(3):347–354. doi:10.1007/s10637-016-0347-6
Horinouchi H, Yamamoto N, Fujiwara Y, Sekine I, Nokihara H, Kubota K, Kanda S, Yagishita S, Wakui H, Kitazono S, Mizugaki H, Tokudome T, Tamura T (2015) Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Investig New Drugs 33(4):881–889. doi:10.1007/s10637-015-0243-5
Mizugaki H, Yamamoto N, Murakami H, Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T, Takahashi T (2016) Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumors. Investig New Drugs 34(5):596–603. doi:10.1007/s10637-016-0371-6
Miura S, Maemondo M, Iwashima A, Harada T, Sugawara S, Kobayashi K, Inoue A, Nakagawa T, Takiguchi Y, Watanabe H, Ishida T, Terada M, Kagamu H, Gemma A, Yoshizawa H (2017) A phase II study of carboplatin plus weekly paclitaxel with bevacizumab for elderly patients with non-squamous non-small-cell lung cancer (NEJ016). Investig New Drugs 35(2):227–234. doi:10.1007/s10637-017-0436-1
Thomas M, Sadjadian P, Kollmeier J, Lowe J, Mattson P, Trout JR, Gargano M, Patchen ML, Walsh R, Beliveau M, Marier JF, Bose N, Gorden K, Schneller F 3rd (2017) A randomized, open-label, multicenter, phase II study evaluating the efficacy and safety of BTH1677 (1,3–1,6 beta glucan; Imprime PGG) in combination with cetuximab and chemotherapy in patients with advanced non-small cell lung cancer. Investig New Drugs 35(3):345–358. doi:10.1007/s10637-017-0450-3
Okamoto H, Watanabe K, Nishiwaki Y, Mori K, Kurita Y, Hayashi I, Masutani M, Nakata K, Tsuchiya S, Isobe H, Saijo N (1999) Phase II study of area under the plasma-concentration-versus-time curve-based carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J Clin Oncol 17(11):3540–3545. doi:10.1200/JCO.1999.17.11.3540
Souhami RL, Spiro SG, Rudd RM, Ruiz de Elvira MC, James LE, Gower NH, Lamont A, Harper PG (1997) Five-day oral etoposide treatment for advanced small-cell lung cancer: randomized comparison with intravenous chemotherapy. J Natl Cancer Inst 16;89(8):577–580. doi:doi:10.1093/jnci/89.8.577
Murray N, Grafton C, Shah A, Gelmon K, Kostashuk E, Brown E, Coppin C, Coldman A, Page R (1998) Abbreviated treatment for elderly, infirm, or noncompliant patients with limited-stage small-cell lung cancer. J Clin Oncol 16(10):3323–3328. doi:10.1200/JCO.1998.16.10.3323
Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T, Saijo N (2007) Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 97(2):162–169. doi:10.1038/sj.bjc.6603810
Ishizumi K, Ohashi N, Tanno N (1987) Stereospecific total synthesis of 9-aminoanthracyclines: (+)-9-amino-9-deoxydaunomycin and related compounds. J Org Chem 52(20):4477–4485. doi:10.1021/jo00229a010
Hanada M, Mizuno S, Fukushima A, Saito Y, Noguchi T, Yamaoka T (1998) A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res 89(11):1229–1238. doi:10.1111/j.1349-7006.1998.tb00519.x
Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y (1998) Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 89(10):1067–1073. doi:10.1111/j.1349-7006.1998.tb00498.x
Morisada S, Yanagi Y, Noguchi T, Kashiwazaki Y, Fukui M (1989) Antitumor activities of a novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenografts. Jpn J Cancer Res 80(1):69–76. doi:10.1111/j.1349-7006.1989.tb02247.x
Noda T, Watanabe T, Kohda A, Hosokawa S, Suzuki T (1998) Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs. Investig New Drugs 16(2):121–128. doi:10.1023/A:1006088907271
Yana T, Negoro S, Takada M, Yokota S, Takada Y, Sugiura T, Yamamoto H, Sawa T, Kawahara M, Katakami N, Ariyoshi Y, Fukuoka M; West Japan Thoracic Oncology Group (2007) Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan thoracic oncology group (WJTOG) study. Investig New Drugs 25(3):253–258. doi:10.1007/s10637-006-9012-9
Igawa S, Ryuge S, Fukui T, Otani S, Kimura Y, Katono K, Takakura A, Kubota M, Mitsufuji H, Katagiri M, Yanase N, Masuda N (2010) Amrubicin for treating elderly and poor-risk patients with small-cell lung cancer. Int J Clin Oncol 15(5):447–452. doi:10.1007/s10147-010-0085-2
Evans WK, Radwi A, Tomiak E, Logan DM, Martins H, Stewart DJ, Goss G, Maroun JA, Dahrouge S (1995) Oral etoposide andcarboplatin. Effective therapy for elderly patients with small cell lung cancer. Am J Clin Oncol 18(2):149–155
Quoix E, Breton JL, Daniel C, Jacoulet P, Debieuvre D, Paillot N, Kessler R, Moreau L, Coëtmeur D, Lemarié E, Milleron B (2001) Etoposide phosphate with carboplatin in the treatment of elderly patients with small cell lung cancer: a phase II study. Ann Oncol 12(7):957-962. Doi:doi:10.1023/a:1011171722175
Onoda S, Masuda N, Seto T, Eguchi K, Takiguchi Y, Isobe H, Okamoto H, Ogura T, Yokoyama A, Seki N, Asaka-Amano Y, Harada M, Tagawa A, Kunikane H, Yokoba M, Uematsu K, Kuriyama T, Kuroiwa Y, Watanabe K; Thoracic Oncology Research Group Study 0301 (2006) Amrubicin for treatment of refractory or relapsed small-cell lung cancer: a phase II thoracic oncology research group study 0301. J Clin Oncol 24(34):5448–5453. doi:10.1200/JCO.2006.08.4145
Kato T, Nokihara H, Ohe Y, Yamamoto N, Sekine I, Kunitoh H, Kubota K, Nishiwaki Y, Saijo N, Tamura T (2006) Phase II trial of amrubicin in patients with previously treated small cell lung cancer (SCLC). J Clin Oncol 24(18):7061–7061. doi:10.1200/jco.2006.24.18
Igawa S, Yamamoto N, Ueda S, Ono A, Nakamura Y, Tsuya A, Murakami H, Endo M, Takahashi T (2007) Evaluation of the recommended dose and efficacy of amrubicin as second- and third-line chemotherapy for small cell lung cancer. J Thorac Oncol 2(8):741–744. doi:10.1097/JTO.0b013e31811f46f0
Okamoto I, Hamada A, Matsunaga Y, Sasaki J, Fujii S, Uramoto H, Yamagata H, Mori I, Kishi H, Semba H, Saito H (2006) Phase I and pharmacokinetic study of amrubicin, a synthetic 9-aminoanthracycline, in patients with refractory or relapsed lung cancer. Cancer Chemother Pharmacol 57(3):282–288. doi:10.1007/s00280-005-0051-2
Sekine I, Okamoto H, Horai T, Nakagawa K, Ohmatsu H, Yokoyama A, Katakami N, Shibuya M, Saijo N, Fukuoka M (2014) A randomized phase III study of single-agent amrubicin vs. carboplatin/etoposide in elderly patients with extensive-disease small-cell lung cancer. Clin Lung Cancer 15(2):96–102. doi:10.1016/j.cllc.2013.11.006
Asao T, Nokihara H, Yoh K, Niho S, Goto K, Ohmatsu H, Kubota K, Yamamoto N, Sekine I, Kunitoh H, Fujiwara Y, Ohe Y (2015) Phase II study of amrubicin at a dose of 45 mg/m2 in patients with previously treated small-cell lung cancer. Jpn J Clin Oncol 45(10):941–946. doi:10.1093/jjco/hyv107
Nakao M, Oguri T, Suzuki T, Kunii E, Tomita Y, Iwashima Y, Miyazaki M, Maeno K, Sato S, Ueda R (2010) Amrubicin monotherapy for elderly patients with previously treated lung cancer. Intern Med 49(17):1857–1862. doi:10.2169/internalmedicine.49.3606
Yoh K, Kenmotsu H, Yamaguchi Y, Kubota K, Ohmatsu H, Goto K, Niho S, Ohe Y, Saijo N, Nishiwaki Y (2010) Severe interstitial lung disease associated with amrubicin treatment. J Thorac Oncol 5(9):1435–1438. doi:10.1097/JTO.0b013e3181e369a8
Acknowledgements
The authors thank all participants of this study. The authors would like to thank Dr. Akihiro Tamiya (Department of Internal Medicine, Kinki-Chuo Chest Medical Center, Osaka) for evaluating the eligibility, evaluability, and responses in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of this study declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Igawa, S., Otani, S., Ryuge, S. et al. Phase II study of Amrubicin monotherapy in elderly or poor-risk patients with extensive disease of small cell lung cancer. Invest New Drugs 35, 642–648 (2017). https://doi.org/10.1007/s10637-017-0482-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0482-8