Summary
Purpose: To evaluate the efficacy and safety of amrubicin, (+)-(7S, 9S)-9-acetyl-9-amino-7-[(2-deoxy-β-D-erythro-pentopyranosyl )oxy ]-7,8,9,10-tetrahydro-6,11-dihydroxy-5,12-naphthacenedione hydrochloride, in previously untreated patients with extensive-disease small cell lung cancer (SCLC).
Patients and methods: A total of 35 previously untreated patients with extensive-disease SCLC were entered into the study. Amrubicin was given by daily intravenous infusion at 45 mg/m2/day for 3 consecutive days, every 3 weeks. Unless there was tumor regression of 25% or greater after the first cycle, or 50% or greater after the second cycle, treatment was switched to salvage chemotherapy in combination with etoposide (100 mg/m2, days 1, 2, and 3) and cisplatin (80 mg/m2, day 1).
Results: Of the 35 patients entered, 33 were eligible and assessable for efficacy and toxicity. Of the 33 patients, 3 (9.1%) had a complete response (95% confidence interval [CI], 1.9–24.3%) and 22 had a partial response, for an overall response rate of 75.8% (95% CI, 57.7–88.9%). Median survival time was 11.7 months (95% CI, 9.9–15.3 months), and 1-year and 2-year survival rates were 48.5% and 20.2%, respectively. The most common toxicity was hematologic. Non-hematologic toxicity of grade 3 or 4 was only seen in 3 patients with anorexia (9.1%) and 1 patient with alopecia (3.0%). Salvage chemotherapy was administered to only 6 patients.
Conclusion: Amrubicin was active for extensive-disease SCLC with acceptable toxicity. Further studies in combination with other agents for SCLC are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) is a major cause of cancer deaths and accounts for 15 to 20% of all lung cancers [1]. Although this cancer is initially highly responsive to chemotherapy, the vast majority of patients will ultimately relapse and die of recurrent disease within 2 years [2]. Recently, combination chemotherapy with irinotecan and cisplatin for extensive-disease SCLC produced more survival benefit than etoposide and cisplatin, the worldwide standard regimen since 1981 [3, 4]. Median survival time and 2-year survival rate of the standard regimen is 12.8 months and 19.5%, respectively. Clearly, new and more effective agents against SCLC are needed.
Amrubicin is a totally synthetic 9-aminoanthracycline, (+)-(7S, 9S)-9-acetyl-9-amino-7-[(2-deoxy-β-D-erythro-pentopyranosyl)oxy]-7, 8, 9, 10-tetrahydro-6, 11-dihydroxy-5,12-naphthacenedione hydrochloride, with a chemical structure similar to that of doxorubicin (Fig. 1) [5]. Amrubicin showed more potent antitumor activity than doxorubicin in several human tumor xenografts implanted in nude mice [6]. Acute toxicity of amrubicin is qualitatively similar to that of doxorubicin [7], however, amrubicin shows almost no delayed toxicity (e.g. cardiotoxicity) [8, 9].
Amrubicin is converted to an active metabolite, amrubicinol, by reduction of its C-13 ketone group to a hydroxy group. In vitro cytotoxic activity of amrubicinol was almost equipotent to that of doxorubicin and 20 to 220 times more potent than that of its parent compound, amrubicin [10]. Amrubicinol is considered to be closely associated with the efficacy and toxicity of amrubicin [11].
Despite their similarity in chemical structure, amrubicin has a different mode of action to doxorubicin [12]. Amrubicin and its active metabolite, amrubicinol, are inhibitors of DNA topoisomerase II. Amrubicin and amrubicinol exert cytotoxic effects by stabilizing topoisomerase II-mediated cleavable complexes, while doxorubicin does not inhibit this step of the catalytic cycle of topoisomerase II at concentrations for which it demonstrates cytotoxicity. Doxorubicin is a potent DNA intercalator, and its cytotoxicity is thought to be mainly due to this. Amrubicin and amrubicinol are about one-tenth weaker DNA intercalators than doxorubicin. Therefore, they are similar to etoposide in terms of inhibition of topoisomerase II by stabilizing the cleavable complexes, although etoposide does not show any DNA intercalating activity.
In a phase I–II study in patients with non-small cell lung cancer, amrubicin was administered as a 5-min intravenous infusion for 3 consecutive days [13]. The maximum tolerated dose (MTD) was 50 mg/m2/day and the dose-limiting toxicities were leukopenia, neutropenia, thrombocytopenia, and gastrointestinal complications. The recommended dose for the phase II study was 45 mg/m2/day for 3 consecutive days every 3 weeks.
Based on these experimental data and preliminary clinical reports indicating that amrubicin may be active against lung cancer, the West Japan Thoracic Oncology Group (WJTOG) evaluated it for use in SCLC. The WJTOG conducted a phase II study in previously untreated extensive-disease SCLC patients as a first-line therapy. Salvage chemotherapy with etoposide and cisplatin and an early cessation rule were set in place as precautionary measures.
Patients and methods
Eligibility criteria
Eligibility criteria included histologically or cytologically proven small cell lung cancer with extensive-disease defined as distant metastasis and/or disease involving the contralateral hilar lymph nodes; no prior treatment; life expectancy of at least 2 months; the Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; at least one bidimensionally measurable lesion; age less than 80; adequate organ function, such as white blood cell (WBC) count of 4000×106/L or greater, hemoglobin level 10 g/dL or greater, platelet count 100×109/L or greater, AST and ALT less than 100 IU/L, bilirubin level 1.5 mg/dL or less, creatinine concentration 1.2 mg/dL or less, electrocardiogram (ECG) findings within normal range, and left ventricular ejection fraction (LVEF) of echocardiogram 60% or greater. All patients gave written informed consent. Ineligibility criteria were: brain or bone metastases requiring radiation; continuous long-term treatment with non-steroidal anti-inflammatory drugs and glucocorticoids; pulmonary fibrosis; serious complications and other active malignancy; or pregnant or nursing subjects.
This study was approved by the institutional review boards at each participating center.
Study design
Amrubicin (Sumitomo Pharmaceuticals Co., Ltd, Osaka, Japan) was dissolved in 20 mL normal saline and administered once intravenously as a 5-min infusion at a dose of 45 mg/m2/day on days 1 to 3, every 3 weeks.
Before treatment, all patients underwent a medical history, physical examination, hematology and serum biochemistry tests, urinalysis, ECG, LVEF, and baseline tumor measurements (chest radiography, CT scans, bone scintigraphy, and other measurements as appropriate). All measurable and assessable lesions were evaluated within 2 weeks before treatment. ECG and LVEF were undertaken within 1 month before treatment.
Complete and differential blood cell counts, platelet counts, hematocrit analysis, biochemical analysis including AST, ALT, alkaline phosphatase, LDH, total bilirubin, BUN, creatinine, serum bilirubin, albumin, total protein, and electrolyte levels (Na, K, Cl, and Ca), and urinalysis (including protein, glucose, urobilinogen, and occult blood) were performed weekly as a rule. When severe myelosuppression was observed, complete and differential blood cell counts plus platelet counts were performed 2 times or more per week. ECG was undertaken every treatment cycle and LVEF every other cycle. Chest radiography and CT scans were carried out every cycle as a rule.
Subjective and objective symptoms were observed and recorded as appropriate.
Dose modifications were made according to WBC and platelet counts. If the WBC count nadir was lower than 1,000×106/L for 4 days or longer and/or the platelet count nadir was lower than 50×109/L, a dose reduction of 5 mg was stipulated in the subsequent treatment course. Treatment was postponed until the WBC and platelet counts recovered to ≥3,000×106/L and≥100×109/L, respectively.
In patients who demonstrated tumor regression of 25% or greater after the first course of chemotherapy, amrubicin treatment was continued. After the second course, patients had to have achieved tumor regression of 50% or greater to continue to receive the drug up to a maximum of 6 courses. Treatment of combination chemotherapy with etoposide (100 mg/m2 on days 1, 2, and 3) and cisplatin (80 mg/m2 on day 1) was recommended for patients who failed to fulfill any of the above criteria.
Evaluation of response and toxicity
Response was assessed according to the “Criteria for the evaluation of the clinical effects of solid cancer chemotherapy” of the Japan Society for Cancer Therapy [14], which are virtually identical to those of the World Health Organization [15]. A complete response (CR) was defined as disappearance of all lesions for a minimum of 4 weeks. A partial response (PR) was defined as a 50% or greater decrease in the sum of the products of the diameters of measurable lesions for a minimum period of 4 weeks and no new lesions. No change (NC) was defined as a decrease in the tumor mass of less than 25% or any increase of less than 25%. Progressive disease (PD) was defined as an increase in the size of any measurable lesion by 25% or greater or the appearance of new lesions.
Toxicity grading was recorded based on the side effect record form in the “Criteria for the evaluation of the clinical effects of solid cancer chemotherapy” of the Japan Society for Cancer Therapy [14].
Statistical analyses
The estimated sample size was 30 to guarantee that the lower limits of 95% confidence interval would be at least 20% at 40% of expected response rate. An early cessation rule was in place to terminate the study if at least 4 responses had not been seen among 15 patients evaluated. Median overall survival was estimated using the product-limit (Kaplan-Meier) method [16].
Results
Patient characteristics
Of 35 patients entered into this study between May 1995 and January 1997, 33 patients were eligible and assessable for efficacy and toxicity. There were 2 ineligible patients because of serious complications before treatment (cardiac failure and aggravation of hepatitis, respectively), and they did not receive amrubicin. Characteristics of the 33 eligible patients are shown in Table 1. Of the 33 patients, 13 (39%) were 70 years of age or older, 88% were male, and 94% had an ECOG performance status of 0 or 1.
Efficacy
Response to amrubicin is shown in Table 2. The early cessation rule was not imposed to terminate the study, as 10 responses were seen after 15 patients were enrolled. Of 33 patients, 3 achieved a complete response, giving a CR rate of 9.1% (95% CI, 1.9–24.3%), and 22 a partial response, for an overall response rate of 75.8% (95% CI, 57.7–88.9%). Of 7 patients, 6 experiencing no change under amrubicin treatment were switched to salvage chemotherapy. Of these, 2 had partial responses and the others had no change.
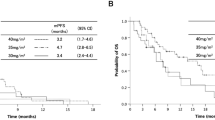
The overall survival curve is shown in Fig. 2. Median survival time was 11.7 months (95% CI, 9.9–15.3 months), and 1-year and 2-year survival rates were 48.5% (95% CI, 31.4–65.5%) and 20.2% (95% CI, 6.4–34.4%), respectively.
Toxicity
The major observed toxicity was hematologic, as shown in Table 3. All patients experienced leukopenia and neutropenia. Grade 3 or 4 leukopenia occurred in 51.5% of patients and grade 3 or 4 neutropenia in 84.8%. Anemia and thrombocytopenia were observed in 78.8% and 39.4% of patients, respectively, both with a frequency of grade 3 or 4 of 21.2%. Despite the severe hematologic toxicity of amrubicin, there was no febrile neutropenia or treatment-related death during the entire treatment of 33 patients. Granulocyte colony-stimulating factor (G-CSF) was used in 55 (40%) of a total of 136 cycles, in 13 patients (39%). Most hematologic toxicity in this trial was well-controlled without dose reduction: 88% of the total treatment cycles were delivered at the planned dosage of amrubicin, 45 mg/m2/day.
Non-hematologic toxicities observed in more than 10% of patients were anorexia (54.5%), nausea and vomiting (57.6%), diarrhea (18.2%), fever (30.3%), alopecia (60.6%), AST increase (15.2%), and ALT increase (27.3%). Most of these were mild (≤grade 2), with only 3 patients (9.1%) experiencing grade 3 anorexia and 1 patient grade 3 alopecia (3.0%). A single patient developed interstitial pneumonia after the second cycle of treatment; however, it was reversibly recovered by steroid therapy and cessation of amrubicin treatment. ECG abnormality was observed in 3 patients (9.4%; supraventricular extrasystole, prolonged QT interval, and T wave flattening in 1 patient each), which did not need any treatment. No LVEF decrease was observed.
Discussion
Results of this phase II study demonstrate that amrubicin is an extremely active agent against extensive-disease SCLC. The complete response rate was 9.1% (95% CI, 1.9–24.3%), overall response rate 75.8% (95% CI, 57.7–88.9%), and median survival time 11.7 months (95% CI, 9.9–15.3 months). These results are comparable or even superior to those of the standard combination regimen of cisplatin and etoposide, used as the gold standard of extensive-disease SCLC therapy since 1981 and remaining unchanged over the last 2 decades [4].
SCLC is sensitive to cytotoxic anticancer agents. Of anticancer drugs developed before 1990, a number of agents with response rates of 20% or greater for SCLC were listed as active drugs [17]. Of these drugs, etoposide, cisplatin, carboplatin, doxorubicin, cyclophosphamide, and vincristine, are still currently used as important constituents of combination regimens in the treatment of SCLC. In addition, several drugs with significant activity for SCLC have been developed since 1990. Irinotecan showed a response rate of 33% to 47% even in previously treated patients who are generally less sensitive to chemotherapy [18, 19]. Recently a new combination regimen of irinotecan plus cisplatin was demonstrated to be significantly superior to standard regimen of etoposide plus cisplatin in median survival time (12.8 months vs. 9.4 months, P = 0.002) [3]. In addition, topotecan, paclitaxel, docetaxel, and gemcitabine are reported to have response rates of 26% to 41% for extensive-disease SCLC patients without previous treatment [20–24]. Compared to these agents, amrubicin demonstrated a much higher response rate (75.8%) in this study, indicating it is a promising novel agent with potential to overcome the therapeutic plateau of SCLC.
The major toxicity of amrubicin was hematologic. Grade 3 or 4 leukopenia was frequently observed in 51.5% of patients and grade 3 or 4 neutropenia in 84.8% of patients. Despite such severe hematologic toxicity, 88% of the total treatment cycles could be delivered without dose reduction and non-hematologic toxicities were mild. Although anorexia (54.5%) and nausea and vomiting (57.6%) were frequently observed, there were no episodes of grade 3 or 4 toxicity, except for 3 patients (9.1%) with grade 3 anorexia and 1 patient (3.0%) with grade 3 alopecia. A single patient developed interstitial pneumonia; however, this was reversible with steroid therapy. ECG abnormalities were observed in 3 patients, but they were each reviewed by a medical cardiologist and judged not to be clinically significant. No LVEF decrease was observed. Results show that the toxic profiles of amrubicin are acceptable and favorable in the treatment of extensive-disease SCLC, although due to its hematologic toxicity, in particular neutropenia, G-CSF support is needed.
In conclusion, amrubicin is a very active and promising agent with acceptable toxicity for patients with SCLC. Further studies are warranted in combination with other agents for this disease.
References
Murren J, Glatstein E, Pass H (2001) Small cell lung cancer. In: DeVita VTJ, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology, 6th edn. Lippincott Williams and Wilkins, Philadelphia, pp 983–1018
Aisner J (1996) Extensive-disease small-cell lung cancer: the thrill of victory; the agony of defeat. J Clin Oncol 14:658–665
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N, for the Japan Clinical Oncology Group (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Aisner J, Alberto P, Bitran J, Comis R, Daniels J, Hansen H, Ikegami H, Smyth J (1983) Role of chemotherapy in small cell lung cancer: a consensus report of the International Association for the Study of Lung Cancer workshop. Cancer Treat Rep 67:37–43
Ishizumi K, Ohashi N, Tanno N (1987) Stereospecific total synthesis of 9-aminoanthracyclines: (+)-9-amino-9-deoxydaunomycin and related compounds. J Org Chem 52:4477–4485
Morisada S, Yanagi Y, Noguchi T, Kashiwazaki Y, Fukui M (1989) Antitumor activities of a novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenografts. Jpn J Cancer Res 80:69–76
Morisada S, Yanagi Y, Kashiwazaki Y, Fukui M (1989) Toxicological aspects of a novel 9-aminoanthracycline, SM-5887. Jpn J Cancer Res 80:77–82
Suzuki T, Minamide S, Iwasaki T, Yamamoto H, Kanda H (1997) Cardiotoxicity of a new anthracycline derivative (SM-5887) following intravenous administration to rabbits: comparative study with doxorubicin. Invest New Drugs 15:219–225
Noda T, Watanabe T, Kohda A, Hosokawa S, Suzuki T (1998) Chronic effects of a novel synthetic anthracycline derivative (SM-5887) on normal heart and doxorubicin-induced cardiomyopathy in beagle dogs. Invest New Drugs 16:121–128
Yamaoka T, Hanada M, Ichii S, Morisada S, Noguchi T, Yanagi Y (1998) Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 89:1067–1073
Noguchi T, Ichii S, Morisada S, Yamaoka T, Yanagi Y (1998) In vivo efficacy and tumor-selective metabolism of amrubicin to its active metabolite. Jpn J Cancer Res 89:1055–1060
Hanada M, Mizuno S, Fukushima A, Saito Y, Noguchi T, Yamaoka T (1998) A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res 89:1229–1238
Negoro S, Fukuoka M, Nakamura S, Ikegami H, Sugiura T, Ariyoshi Y, Takada M, Yana T, Ogawa M (1995) Phase I–II study of amrubicin (SM-5887), a novel 9-aminoanthracycline, by iv administration for 3 consecutive days in patients with advanced non-small-cell lung cancer (abstract). Proc Am Soc Clin Oncol 14:361
Japan Society for Cancer Therapy (1993) Criteria for the evaluation of the clinical effects of solid cancer chemotherapy. J Jpn Soc Cancer Ther 28:101–130
World Health Organization (1979) Handbook for reporting results of cancer treatment (WHO Offst Publication No. 48). World Health Organization, Geneva, Switzerland
Kaplan WH, Meier P (1952) Nonparametric estimation from incomplete observations. J Am Atat Assoc 53:583–612
Grant SC, Gralla RJ, Kris MG, Orazem J, Kitsis EA (1992) Single-agent chemotherapy trials in small-cell lung cancer, 1970 to 1990: the case for studies in previously treated patients. J Clin Oncol 10:484–498
Negoro S, Fukuoka M, Niitani H, Suzuki A, Nakabayashi T, Kimura M, Motomiya M, Kurita Y, Hasegawa K, Kuriyama T, Nishiwaki Y, Ogawa M, Nakao I, Saijo N, Obo K, Furue H, Ariyoshi Y, Shimokata K, Furuse K, Nakajima S, Irie K, Kimura I, Ogura T, Fujii M, Hara N, Hara Y, Nakano N, Araki J, Miyata Y, Taguchi T (1991) A phase II study of CPT-11, a camptothecin derivative, in patients with primary lung cancer. CPT-11 Cooperative Study Group. Jpn J Cancer Chemother 18:1013–1019
Masuda N, Fukuoka M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M (1992) CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10:1225–1229
Schiller JH, Kim K, Hutson P, DeVore R, Glick J, Stewart J, Johnson D (1996) Phase II study of topotecan in patients with extensive-stage small-cell carcinoma of the lung: an Eastern Cooperative Oncology Group Trial. J Clin Oncol 14:2345–2352
Ettinger DS, Finkelstein DM, Sarma RP, Johnson DH (1995) Phase II study of paclitaxel in patients with extensive-disease small-cell lung cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 13:1430–1435
Kirschling R, Jung S, Jett J (1994) A phase II study of taxol and GCSF in previously untreated patients with extensive stage small cell lung cancer (SCLC) (abstract). Proc Am Soc Clin Oncol 13:326
Hesketh P, Crowley J, Burris HA 3rd, Williamson SK, Balcerzak SP, Peereboom D, Goodwin JW, Gross HM, Moore DF Jr, Livingston RB, Gandara DR (1999) Evaluation of docetaxel in previously untreated extensive-stage small cell lung cancer: a Southwest Oncology Group phase II trial. Cancer J Sci Am 5:237–241
Cormier Y, Eisenhauer E, Muldal A, Gregg R, Ayoub J, Goss G, Stewart D, Tarasoff P, Wong D (1994) Gemcitabine is an active new agent in previously untreated extensive small cell lung cancer (SCLC). A study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 5:283–285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yana, T., Negoro, S., Takada, M. et al. Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs 25, 253–258 (2007). https://doi.org/10.1007/s10637-006-9012-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-9012-9