Abstract
Purpose
Vitamin A is a lipid-soluble compound that is critical in maintaining phototransduction. Ocular manifestations of hypovitaminosis A may present with anterior segment signs of xeropthalmia, with advanced cases also causing classic retinal and electrophysiologic changes of vitamin A deficiency retinopathy. We present a case of vitamin A deficiency retinopathy, with corresponding retinal imaging and electrophysiology, in an adult patient with celiac disease and liver fibrosis.
Methods
A single case report was conducted in Toronto, Canada.
Results
A 77-year-old male with known celiac disease and liver fibrosis presented progressively worsening vision noticed primarily when driving. Vision was 20/50 OD and 20/200 OS. Bitot spots were noted on anterior segment examination. Fundus photography demonstrated bilateral peripheral macular hypopigmentation and far-peripheral granular retinal hypopigmentation with focal yellow dots and hyper-pigmented deposits. Optical coherence tomography (OCT) imaging demonstrated indistinct outer retinal banding with mild outer nuclear layer thinning, focal hyper-reflective deposits, and a thin choroid bilaterally. Full-field electroretinography (ERG) testing demonstrated reduced rod-isolated and combined rod-cone response amplitudes, and multifocal ERG testing demonstrated blunted individual responses throughout the field. The patient was treated with pulse vitamin A therapy. After 6 months of therapy, ERG responses were back within reference range, and the outer retinal changes reversed; visual acuity improved to 20/30 OD and 20/40 OS.
Conclusion
This case represents the classic findings of vitamin A deficiency retinopathy on fundus examination and electrophysiologic testing secondary to gastrointestinal pathology. Prompt treatment of high dose vitamin A supplementation led to improvement of full-field and multifocal ERG results, as well as reconstitution of outer retinal architecture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin A is a lipid-soluble compound that plays a crucial role in cellular development, immune system integrity, and vision. Primary hypovitaminosis A is relatively rare in the developed world due to access to natural sources of fish, leafy greens, and milk products; however, cases are increasing due to malabsorption syndromes and gastric bypass procedures [1]. In impoverished regions worldwide, vitamin A deficiency still remains a public health concern as it leads to significant morbidity, and if left untreated, mortality [2]. Vitamin A constitutes a group of molecules, including retinaldehyde, retinol, retinoic acid and beta-carotene [3]. Retinal (11-cis retinaldehyde) plays a crucial role in phototransduction by combining with opsin in photoreceptor outer segments to produce rhodopsin. Following photoreceptor hyperpolarization, retinal isomerizes to all-trans retinal, and the visual cascade is initiated. In states of hypovitaminosis A, characteristic photoreceptor and overall retinal changes have been demonstrated in animal models and case reports [4]. We present a case of vitamin A deficiency retinopathy in the setting of celiac disease with multi-modal imaging and characteristic electrophysiology results. The patient’s vision and retinal function improved after correct identification of hypovitaminosis A status and subsequent supplementation.
Case presentation
A 77-year-old male was referred to a tertiary retina clinic in Toronto, Canada for evaluation for possible inherited retinal dystrophy. At night, the patient had more difficulty with driving, in particular, appreciating a subjective full-field of vision. He did not have difficulty with oncoming headlights and had no concerns with dark-adaptation. He had normal colour vision, normal reading vision, and had no issues with vision in bright or well-lit conditions. The patient had no past ophthalmic history and no family history of eye disease. His past medical history included gout, celiac disease, non-cirrhotic portal hypertension and moderate liver fibrosis. His gastroenterologists were concerned that this might be secondary to non-alcoholic steatohepatitis versus nodular regenerative hyperplasia. His only medications were Betamethasone cream and Terbinafine cream as needed. He described experimentation with a 100% gluten-free diet for the past two years supplemented with vitamin D. His previous BMI was over 30, and he endorsed significant and rapid intentional weight loss over the preceding year.
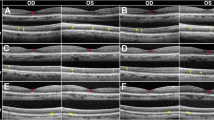
On examination, best-corrected distance visual acuity (BCVA) was 20/50 in the right eye (OD) and 20/200 in the left eye (OS). Intraocular pressure (IOP) was 20 mmHg OD and 21 mmHg OS. There was no relative afferent pupillary defect. External examination revealed dry, scaly skin with ulcerations, nail chipping and pitting bilaterally. Slit lamp examination revealed bilateral Bitot spots, moderate punctate epithelial erosions, and moderate nuclear sclerotic cataracts. Fundus examination demonstrated normal optic discs and inner retinal vasculature in both eyes (OU). The macula of both eyes showed preserved foveal pigmentation but diffuse peripheral macular hypopigmentation. The retinal periphery in both eyes showed granular peripheral retinal hypopigmentation with focal yellow dots and hyper-pigmented deposits in the far-peripheral retina (Fig. 1A–D, Optos Daytona, Optos Plc). Fundus autofluorescence imaging demonstrated preserved physiologic foveal hypo-autofluorescence, with peripheral macular hypo-autofluorescence, as well as mid-peripheral hypo-autofluorescence (Fig. 1E and F). Optical coherence tomography (OCT) imaging of the macula demonstrated indistinct outer retinal banding (external limiting membrane (ELM) and ellipsoid zone (EZ)), with mild outer nuclear layer (ONL) thinning, focal hyper-reflective deposits, and a thin choroid bilaterally; the inner retinal lamination in the fovea and peripheral macula was preserved (Fig. 1G and H, Zeiss Cirrus 6000, Carl Zeiss AG).
Widefield colour photograph (Optos Daytona, Optos Plc) of the A right eye and B left eye at presentation demonstrating symmetric diffuse peripheral macular hypopigmentation and granular far-peripheral retinal hypopigmentation, with small yellow dots in the mid-periphery. C and D are high magnification images of the far-periphery OU of the regions noted in A and B, respectively. Black arrows demonstrate the small yellow dots, and white arrows demonstrated hypopigmentation in the far periphery OU. E Widefield fundus autofluorescence imaging at presentation of the right eye and F left eye demonstrating preserved physiologic foveal hypo-autofluorescence, peripheral macular hypo-autofluorescence (with reflectance from RPE loss and thin choroid), and mid-peripheral hypo-autofluorescence. G Optical coherence tomography imaging (Zeiss Cirrus 6000, Carl Zeiss AG) of the right eye and H left eye macula at presentation demonstrating indistinct outer retinal banding including the external limiting membrane and ellipsoid zone (white arrows) with outer nuclear layer thinning, hyper-reflective deposits (red arrows) and choroidal thinning
Full-field electroretinography (ERG) testing was performed (ISCEV standard) and demonstrated reduced rod-isolated and combined rod-cone response amplitudes, but cone-isolated responses were within reference range for amplitude and timing (Fig. 2A, ColorDome full-field ERG System). Multifocal ERG testing performed (ISCEV standard) with 61 hexagons demonstrated blunted, but detectable individual responses throughout the field (Fig. 2A, LCD Pattern Stimulation Multifocal ERG System). A review of his recent laboratory work revealed elevated beta-2-globulin and gamma globulin on a serum protein electrophoresis, 2 months prior to presentation. His IgG/IgA/IgM levels were also elevated. Recent liver ultrasonography revealed hepatic steatosis with mild splenomegaly consistent with moderate fibrosis. Gastroscopy three years prior with duodenal biopsies were consistent with celiac disease. A CT abdomen 6 months prior demonstrated findings consistent with cirrhosis and portal hypertension, with no evidence of hepatoma. After his visit in the eye clinic, Vitamin A serology was ordered by the ophthalmology team and revealed a level of < 0.4 µmol/L (reference range: 1.3–3.8).
A Full-field electroretinography (ERG, ColorDome full-field ERG System)at presentation demonstrating reduced amplitude of rod-isolated and combined rod-cone responses, withcone-isolated responses within reference range. Multifocal electroretinography (ERG) performed with 61 hexagons (LCD Pattern Stimulation Multifocal ERG System) demonstrating blunted, but detectable individual responses throughout the field. The average ring amplitudes were below reference ranges, and the timings were delayed. B Full-field ERG at 2-month follow-up on vitamin A therapy, demonstrating a 170% increase in scotopic response amplitude OD and 210% increase OS; photopic responses were stably normal. Multifocal ERG testing at the 2-month follow-up demonstrating improvement in response amplitudes closer to expected limits at all eccentricities, with peak times within expected limits. C Full-field ERG at the 6-month follow-up results demonstrating all responses within reference range, without evidence of electrophysiologic dysfunction. Multifocal ERG testing at the 6-month follow-up visits demonstrating response amplitudes and peak times within expected limits at all eccentricities. Ring average responses are also within reference range for amplitude and timing. D Full-field ERG and multifocal ERG results for age-matched controls. (Note: The left eye is displayed for the full-field ERG and multifocal ERG images. Green dashed-line traces represent ERG response to a single trial stimulus. ERGs performed with ISCEV standard protocols; flash intensity units are in cd·s/m2, amplitudes are measured in uV, and timings in ms)
After discussion and review with the gastroenterology and hepatology team, the patient was started on vitamin A replacement therapy of 10,000 IU/day. He was also advised to use UV-light protection and engage in a healthy, diverse diet. Two months after beginning vitamin A supplementation, the patient’s visual acuity improved to 20/30 OD and 20/40 OS. He subjectively felt his visual acuity had improved, notably when driving. His fundus examination and fundus autofluorescence imaging was stable from his initial presentation. OCT images demonstrated trace improvement in the definition of parafoveal outer retinal banding (ELM and EZ) in both eyes (more prominently in the left eye compared to the right eye; two-month OCTs not shown). Repeat full-field ERG demonstrated a 170% increase in amplitude for scotopic responses OD and 210% increase OS; photopic responses were stable (Fig. 2B). Repeat multifocal ERG testing demonstrated improvement in ring average response amplitudes closer to expected limits at all eccentricities, with peak times within expected limits (Fig. 2B).
Pleased with his progress, he continued vitamin A supplementation over the next 4 months. At his 6-month follow-up, he continued to have improvement in his subjective vision. His BCVA was stable, with regression of punctate epithelial erosions, and unchanged fundus findings. Full-field ERG and multifocal ERG responses were now within reference range for amplitude and timing (Fig. 2C); in addition, multifocal ERG response amplitudes had continued to improve by 23% OD and 66% OS from the previous testing at 2 months (Fig. 2C). Consistent with his electrophysiologic results, OCT macula imaging demonstrated continued improvements in parafoveal outer retinal band definition in both eyes compared to the 2-month imaging (Fig. 3A–D).
Optical coherence tomography imaging of the A right eye and B left eye at presentation with white arrows demonstrating indistinct outer retinal banding including the external limiting membrane and ellipsoid zone. C Optical coherence tomography imaging of the right eye and D left eye macula at the 6-month follow-up demonstrating increased thickness and definition of outer retinal banding including the external limiting membrane and ellipsoid zone (red arrows)
Discussion
Xerophthalmia refers to the spectrum of ocular diseases secondary to vitamin A deficiency [4]. The World Health Organization provides a grading system, ranging from early signs such as conjunctival xerosis, to more advanced signs such as corneal ulceration and keratomalacia. Early detection of xerophthalmia is crucial to initiate prompt vitamin A treatment, as late stages of xerophthalmia carry a poor prognosis [4]. Our patient did present with anterior segment signs of hypovitaminosis A, notably Bitot spots, which are desquamated, keratinized conjunctival epithelial cells, and an irregular ocular surface and dry eye symptoms [4]. Our patient also presented with typical signs of vitamin A deficiency retinopathy, including deep yellow-white granular deposits in the peripheral retina, disorganization of photoreceptor outer segments and photoreceptor loss seen as ellipsoid zone loss on OCT imaging, and evidence of generalized retinal electrophysiologic dysfunction [5]. A case series of two patients with vitamin A deficiency retinopathy presented by Berkenstock et al. in 2020 demonstrated similar characteristic changes in outer retinal changes at presentation and following treatment [6]. Both patients demonstrated outer nuclear layer thinning and poor integrity of the ellipsoid zone on OCT imaging, with improved reconstitution of the photoreceptor layers following high-dose vitamin A supplementation [6]. This improvement in ellipsoid zone integrity correlated to improved visual acuity in a similar fashion to our patient. A new OCT biomarker for vitamin A deficiency retinopathy, proposed by Breazzano et al. in 2023, is the “double carrot” sign, seen as increased subfoveal ellipsoid zone reflectance [7]. This “double carrot” sign is not as well-defined in our patient, which may reflect the chronicity of the hypovitaminosis A state in our patient compared to the patient presented by Breazzano et al. [7]. Retinal hypopigmented spots have also been described in vitamin A deficiency retinopathy, as seen in our patient, which are hypothesized to be caused by rod photoreceptor outer segment degradation and phagocytosis by underlying RPE cells; these flecks were not found to change during the treatment course unlike the outer retinal banding changes seen [8].
Vitamin A deficiency retinopathy also shows changes on electrophysiologic testing, which can aid in the diagnosis and treatment monitoring for patients. Vitamin A deficiency preferentially affects the electrophysiologic function of rod over cone photoreceptors, and presents early with reduced scotopic, and preserved photopic, responses on full-field ERG. This was seen in our patient, with the full-field ERG demonstrating reduced amplitude of rod-isolated and combined rod-cone responses, but preserved cone-isolated responses. Multiple cases, such as Saker et al. in 2015 and Breazzano et al. in 2023, demonstrate that central cone function can be affected in late-stage vitamin A deficiency retinopathy, and cone photoreceptor function has delayed recovery compared to generalised rod function [7, 9]. While generalized cone dysfunction was not noted in our patient on full-field ERG, there was some evidence of reduced macular function on multifocal ERG, which suggests at least some compromised central cone function, consistent with the patient’s reduced visual acuity and central macular anatomic disorganization. Therefore, early detection of hypovitaminosis A related ocular disease is crucial to prevent irreversible vision loss. As previously documented, there was a dramatic improvement on full-field ERG testing following high-dose vitamin A supplementation after just 2 months of therapy [7,8,9]. Prompt treatment in coordination with the team managing the underlying malabsorptive disorder should be initiated to aid in visual recovery.
The incidence of celiac disease is growing in the developing world, which has led to an increase in malabsorptive disorders; therefore, understanding the impact of these disorders on ophthalmic health may become more relevant to comprehensive practice. Our patient was appropriately supplementing his gluten-free diet with vitamin D, the primary molecule which has been found in low serum levels for these patients. However, our patient was not supplementing with vitamin A. Hypovitaminosis A has been seen in patients with underlying celiac disease, which have manifested as irreversible xerophthalmia [10]. Vitamin A is also primarily stored in hepatic stellate cells. However, in a state of hepatic insult, liver cells lose vitamin A during conversion to myofibroblasts which ultimately leads to liver fibrosis [11]. Our patient’s past medical history of non-cirrhotic portal hypertension and moderate liver fibrosis placed him at an increased risk of hypovitaminosis A. Patients with visual decline in this setting should have a prompt eye examination to rule out vitamin A deficiency retinopathy and initiate replacement therapy to prevent irreversible xerophthalmia and vision loss.
In summary, ophthalmic manifestations of vitamin A deficiency present with both characteristic ocular surface irregularities and fundus findings of retinopathy, including hyper-reflective dots in the mid-periphery, outer retinal thinning and loss of the ellipsoid zone. Electrophysiologic testing demonstrates preferential rod photoreceptor insult and is an important ancillary for both diagnostic confirmation of retinal dysfunction and monitoring of response to therapy. Treatment with vitamin A supplementation can help reverse outer retinal changes and improve electrophysiologic visual function. Patients with celiac disease or underlying liver disease should be advised on appropriate supplementation to avoid fat-soluble vitamin deficiency, and have a dedicated retinal examination if they present with visual disturbances in order for therapy to be initiated prior to advanced or irreversible disease.
Data availability
Not applicable.
Code availability
Not applicable.
References
D’Ambrosio DN, Clugston RD, Blaner WS (2011) Vitamin A metabolism: an update. Nutrients 3(1):63–103
Wirth JP, Petry N, Tanumihardjo SA et al (2017) Vitamin A supplementation programs and country-level evidence of vitamin A deficiency. Nutrients 9(3):190
George W (2001) The discovery of the visual function of vitamin A. J Nutr 131(6):1647–1650
Gilbert C (2013) The eye signs of vitamin A deficiency. Community Eye Health 26(84):66–67
Aleman TS, Garrity ST, Brucker AJ (2013) Retinal structure in vitamin A deficiency as explored with multimodal imaging. Doc Ophthalmol 127(3):239–243
Berkenstock MK, Castoro CJ, Carey AR (2020) Outer retina changes on optical coherence tomography in vitamin A deficiency. Int J Retin Vitr 6:23
Breazzano MP, Oh JK, Batson SA et al (2023) Vitamin A deficiency and the retinal “double carrot” sign with optical coherence tomography. Eye 37:1489–1495
Genead MA, Fishman GA, Lindeman M (2009) Fundus white spots and acquired night blindness due to vitamin A deficiency. Doc Ophthalmol 119(3):229–233
Saker S, Morales M, Jhittay H, Wen Y, Amoaku W (2015) Electrophysiological and microperimetry changes in vitamin A deficiency retinopathy. Doc Ophthalmol 130(3):231–240
Alwitry A (2000) Vitamin A deficiency in coeliac disease. Br J Ophthalmol 84:1075
Freund C, Gotthardt DN (2017) Vitamin A deficiency in chronic cholestatic liver disease: is vitamin A therapy beneficial? Liver Int 37(12):1752–1758
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to manuscript planning, organization, writing and review. Dr. Ballios provided supervision throughout the study.
Corresponding author
Ethics declarations
Conflict of interest
No authors have conflicts of interests to declare relative to this study.
Ethics approval
No research ethics board approval was required for the anonymized publication of clinical information.
Informed consent
Informed consent was obtained for this case.
Statement of human rights
All procedures in human participants were performed in accordance with the ethical standards of the Department of Ophthalmology of the Institute of Medicine, Tribhuvan University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This is a human-subject study. It does not involve animals, so the statement on the welfare of animals is not appropriate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, A., Wright, T., Weisbrod, D. et al. Vitamin A deficiency retinopathy in the setting of celiac disease and liver fibrosis. Doc Ophthalmol (2024). https://doi.org/10.1007/s10633-024-09978-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10633-024-09978-7