Abstract
Background
Sporadic nonampullary duodenal epithelial tumors (NADETs) are uncommon, and thus their clinicopathological features have not been fully assessed.
Aims
In this study, we have analyzed a series of early sporadic NADETs, focusing on various immunohistological features.
Methods
We conducted a multicenter retrospective analysis of 68 patients with endoscopically resected sporadic NADETs. Associations between immunohistological features and clinicopathological features were statistically analyzed.
Results
The 68 patients consisted of 46 men (68%) and 22 women (32%) with a mean age of 60.7 ± 12.2 years (range 37–85 years). The 68 tumors were composed of 39 adenomas (57%) and 29 early-stage adenocarcinomas (43%). Duodenal adenocarcinomas were larger in size than adenomas and had papillary architecture in their pathological diagnosis with statistical significance. Duodenal adenocarcinomas also demonstrated a significantly higher expression of gastric markers (MUC5AC and MUC6) and a higher MIB-1 index. Duodenal adenomas were contrastively apt to express intestinal markers (MUC2, CDX1 and CDX2). Of the 68 cases analyzed, there were only 3 tumors positive for p53 staining, all of which were adenocarcinoma. When 7 submucosal invasive cancers and 21 intramucosal cancers were compared, submucosal invasion was positively associated with expression of MUC5AC. Also, submucosal invasion showed strong association with double-positivity of MUC5AC and MUC6.
Conclusions
Our results indicate that immunohistochemical evaluation is useful for predicting malignant potential of NADETs, especially focusing on the expression of gastrointestinal markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small bowel cancers comprise less than 5% of gastrointestinal cancers [1]. Sporadic nonampullary duodenal adenocarcinomas (NADETs) are also uncommon, though duodenal cancers are the most frequent among small bowel cancers (55–82%) [2, 3]. However, it was recently reported that the incidence of duodenal adenocarcinoma has been increasing [4, 5]. According to the latest 2010 WHO classification for tumors of the digestive system, duodenal tumors are classified as tumors of the small intestine [6]. At present, there are no available specific guidelines for the diagnosis and treatment of duodenal tumors worldwide. Though duodenal cancers have been conventionally managed by radical surgery or more conservative local surgical excision, no consensus has been established on the medical treatment policy in duodenal epithelial neoplasms.
Recently, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been performed to treat epithelial tumors of the stomach, esophagus and colorectum [7]. Since endoscopic mucosal resection (EMR) of duodenal neoplasm was first performed in 1992 [8], a few studies concerning the endoscopic resection of duodenal neoplasms have been reported [9,10,11,12]. Even today, endoscopic treatment of duodenal epithelial tumors is very difficult because of narrow lumen of duodenum, thin muscle layer, the presence of Brunner’s gland in submucosal layer, exposure to bile and pancreatic fluid, and so on [13, 14]. Actually, patients undergoing endoscopic resection of duodenal lesions have a much higher complication rate compared to undergoing endoscopic resection of other gastrointestinal lesions [12, 15, 16]. In addition, it is difficult to pre-operatively diagnose whether the duodenal epithelial tumor is adenomatous or cancerous [17, 18]. Endoscopic resection of NADETs is only performed at high-quality institutions worldwide.
Until today, there have been few studies which have clinicopathologically assessed early-stage duodenal adenocarcinoma and precancerous duodenal adenoma based on immunohistochemistry. In this study, we have analyzed a series of 68 cases of early sporadic nonampullary duodenal epithelial tumors, focusing on various immunohistological features.
Methods
Tumor Samples
Sixty-eight endoscopically resected NADETs between May 2003 and July 2013 were evaluated retrospectively. All patients underwent endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) at either the University of Tokyo Hospital, NTT medical center Tokyo or Wakayama Medical University Hospital. In all 3 institutions, lesions with either a histologic or endoscopic diagnosis of intramucosal adenocarcinoma were considered as indications for EMR/ESD. All specimens were aggregated in Fujita Health University school of Medicine. This study was approved by each of the institutional ethical review boards for human investigation in the above three hospitals in 2013.
Definitions
For age, all subjects were categorized into three groups (30–49, 50–69, and ≥ 70 years old), based on the histogram of ages. Lesion sizes were divided into two groups: ≥ 20 mm and < 20 mm, which is based on the absolute criteria of endoscopic submucosal dissection for gastric cancer (< 20 mm) [19]. Color and macroscopic appearance were classified according to the endoscopic images before treatment. Judging from the color tone covered largest area in tumor, color was divided into three groups: whitish, normal, and erythematous [20]. Macroscopic appearance was categorized into three groups: depressed (0-IIc), elevated (0-I, 0-IIa), and mixed (0-IIa + IIc, 0-I + IIc) [20]. Duodenum is anatomically divided into four sections (the first, second, third, and fourth part), but is also divided into the oral and anal side of the papilla [21]. We fixed the boundary between the oral side and the anal side of papilla in order to evaluate the characteristics of NADETs. We thought this boundary reflects the difference more correctly because of boundary of embryological development or the existence of Brunner’s gland.
Immunohistochemistry
All the specimens were embedded in paraffin wax and were then cut into slices 1–2 μm thick with glass knife. Serial sections were deparaffinized and stained with hematoxylin and eosin (H&E). The mucin expression profile of each case was evaluated by the following immunohistochemical stains: MUC2, MUC5AC, and MUC6. The protein expression profile of each case was evaluated by the following: p53, MIB-1, CDX1, and CDX2. The dilutions are described in Supplementary Table 1. Deparaffinization and endogenous peroxidase inactivation of clinical tissues were performed as described previously [22, 23].
Scoring System for Immunohistochemical Staining
All the immunohistochemical staining of tissue sections was evaluated by 3 evaluators including two expert gastrointestinal pathologists. Immunohistochemical staining was performed on sections of all 68 lesions; then the areas of interest first were pointed out by the pathologists. Immunohistological expression of each lesion and peripheral normal mucosa were each manually evaluated at 400× magnification. MUC2, MUC5AC, MUC6, Ki-67, p53, CDX1, and CDX2 expression were defined as the proportion of positively stained area in the lesion compared to the peripheral normal mucosa. The MIB-1 index was defined as the percentage of MIB-1 positive cells to at least 1000 cells [24, 25]. Cutoff values for each modality of immunohistochemical staining were determined based on a comparison of histograms of adenomas and adenocarcinomas. Cutoff values for MIB-1, MUC2, MUC5AC, MUC6, CDX1 and CDX2 were set as 70, 5, 30, 30, 80 and 80%, respectively. Moreover, we defined the groups of MUC5AC positive cells ≥ 30% and MUC6 positive cells ≥ 30% as “MUC5AC/MUC6 double-positive.” The “presence or absence of papillary architecture (pap)” on the specimen was evaluated with hematoxylin and eosin (H&E) staining.
Statistical Analysis
In the univariate analyses, Chi-square test was used for evaluating differences between adenoma and adenocarcinoma. Fisher’s exact test was used when sample size was small. Cochran–Armitage test was used for statistical evaluation of age. All statistical analyses were performed using the JMP 9.0 or SAS 9.1.3 software (SAS Institute Inc., Cary, NC, USA), and p value of < 0.05 was considered as statistically significant.
Results
Study Population
The 68 patients with sporadic nonampullary duodenal epithelial tumor consisted of 46 men (68%) and 22 women (32%). Their mean age was 60.7 ± 12.2 years (range, 37–85 years). The 68 lesions were comprised of 39 adenomas (57%) and 29 adenocarcinomas (43%).
Associated Background Factors for Duodenal Adenocarcinoma
Univariate analyses were performed to evaluate association between the seven background factors and the histological diagnosis of duodenal epithelial tumors (adenoma or adenocarcinoma, Table 1). “Lesion size” and “the presence of papillary architecture in pathological diagnosis” were positively associated with adenocarcinoma compared to adenoma. On the contrary, age, sex, color, macroscopic appearance and location did not show significant association with the histological type of duodenal epithelial tumor. Statistical power analysis showed that the sample sizes of the two significant factors (lesion size and papillary architecture in pathological diagnosis) were large enough (both effect sizes > 0.3) to assert that their associations with duodenal adenocarcinoma were practically significant. On the contrary, effect sizes of age and sex (0.28 and 0.23) were rather too small to assert their non-significant association with the type of duodenal epithelial tumor.
Comparisons of Duodenal Adenoma and Adenocarcinoma Based on Immunohistochemical Properties
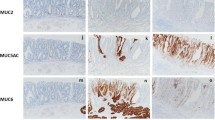
Using the 68 specimens, immunohistochemical analyses with seven antibodies were performed. Figure 1 shows typical cases of gastric-type markers being positive or negative for MUC5AC and MUC6 expression. Figure 2 shows typical cases of intestinal-type markers of positive or negative immunostaining for CDX2 antibodies and MUC2 expression. As shown in Supplementary Fig. 1, other results of immunohistochemistry
Typical expression images of the gastric-type mucin in the duodenal epithelial tumors. Photomicrographs show typical examples of positive (a, b) and negative (c, d) cases for mucin expression. In all the specimens, nuclei were counterstained with hematoxylin. Scale bars indicate 50 μm in a–d. (a, c) In the MUC5AC-positive case, strong expression of MUC5AC was observed in the glands of the duodenal adenocarcinoma. In the MUC5AC-negative tumor case, only a small portion of superficial layer and some part of the glandular epithelium (endocrine cells) were stained. (b, d) In the MUC6-positive case, MUC6 immunoreactivity was seen in the cytoplasm of cancer cells. In the MUC6-negative case, few cells in the glandular epithelium of the tumor were stained. In contrast, Brunner’s glands were intensely stained
Typical immunohistochemical staining of CDX2 and MUC2 in the duodenal epithelial tumors. Photomicrographs show typical examples of positive (a, b) and negative (C and D) cases for CDX2 and MUC2 staining. In all the specimens, nuclei were counterstained with hematoxylin. Scale bars indicate 50 μm in a–d. (a, c) In the CDX2-positive case, almost all the nuclei of the tumor cells were stained intensely. In the CDX2-negative case, only a smart of cellular nuclei in the tumor were stained. (b, d) In the MUC2-positive case, epithelial cells in the tumor were sparsely stained from the basal layer to the upper layer. In the MUC2-negative case, almost none of the intraductal cells in the tumor were stained
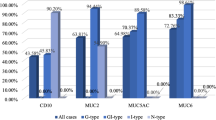
Table 2 provides association between the results of immunohistochemistry and the histological feature of duodenal epithelial tumors. Univariate analyses showed that intestinal types (expression of MUC2, CDX1 and CDX2) were positively correlated with adenomatous histology. In contrast, gastric mucin types (MUC5AC and MUC6) were positively correlated with histology of adenocarcinoma. All the seven lesions positive for both MUC5AC and MUC6 (MUC5AC/MUC6 double-positive) were adenocarcinoma (Table 2). Such five factors as erythematous appearance, oral-side location, adenocarcinoma, the presence of papillary architecture, and submucosal invasion were significantly associated with the seven cancerous lesions showing double-positive for MUC5AC and MUC6 (data not shown). MIB-1 index was positively associated with adenocarcinoma. Of the 68 duodenal epithelial tumors examined, only three lesions showed strong p53 expression, all of which were malignant tumors.
All the immunohistochemical factors except for p53 expression showed significant difference between the duodenal adenocarcinoma and adenoma, and most of their effect sizes were large enough to be considered as reliable (Table 2). About p53 expression, Fisher’s exact test showed no significant difference between duodenal adenoma and adenocarcinoma (p = 0.073), but the power and effect size were not large enough for practical evaluation.
Significant Background Factors Associated with the Depth of Duodenal Adenocarcinoma
In the 29 cases of adenocarcinomas, the depth of invasion could not be assessed in one case due to coagulation necrosis of the tissue. Of the 28 remaining lesions, 7 cases (25%) had submucosal invasion and 21 cases (75%) were intramucosal cancer (Table 3). When the seven background factors were analyzed, no factors showed significant association with the depth of duodenal adenocarcinoma. Of the analyzed seven factors, p value for the presence of papillary architecture was rather small (p = 0.063), and its power and effect size were not so high (0.62 and 0.43).
Correlation Between the Depth of Cancer Invasion and Immunohistochemical Characteristics of Duodenal Cancer
Table 4 summarizes the results of immunohistochemistry in the 28 duodenal adenocarcinoma cases. Expression of gastric marker mucin (MUC5AC) was significantly associated with submucosal invasion of the cancer. Similar to the comparison between adenoma and adenocarcinoma (Table 2), double-positive feature for MUC5AC and MUC6 was the significant risk factor for submucosal invasion of duodenal adenocarcinoma. Though MUC6 did not show meaningful association with submucosal invasion, effect size was not so large (0.43) and therefore might have some influence on this result. Submucosal invasion was not associated with MUC2, CDX1, CDX2, MIB-1 index and p53 immunostaining.
Discussion
This study demonstrated clinicopathological features of 68 sporadic NADETs in Japan. Through immunohistological evaluation of sporadic NADETs, significant association between malignant potential (tumor invasion) and expression of gastric markers (MUC5AC and MUC6) were demonstrated.
According to the recent studies describing the clinical characteristics of duodenal tumors, age of the patients ranged from 54 to 67 years [2, 10, 11, 20, 26,27,28,29], men are more likely to have duodenal epithelial tumors [2, 10, 11, 20, 26,27,28,29,30], and the predilection site of the tumor is first or second portion of the duodenum [10, 26,27,28]. About the location of duodenal epithelial tumors, there must be a bias that oral-side lesions tend to be frequently included, because most studies analyzed the lesions endoscopically resected. Considering the studies of endoscopically resected lesions only, average sizes of the tumors are ranged from 10 to 27 mm [9,10,11, 20, 27, 31]. In our analysis, a mean age of the study subjects is 61 ± 12 years, men (68%) are more likely to have duodenal epithelium tumors, and the mean size of lesions is 15 ± 10 mm (2–45 mm); all of these are consistent with the previous reports. All the 68 lesions were located in the first or second portion of duodenum. Though not statistically significant, it should be noteworthy that 6 out of 7 lesions on the oral side of papilla are submucosal invasive adenocarcinomas (86%). It is interesting that proximal lesion tends to invade more deeply despite its accessibility by endoscopy. It may be due to the frequent breaks in the muscularis mucosae accompanied with Brunner’s glands.
The role of Brunner’s glands for duodenal tumorigenesis is barely elucidated, but it is well known that Brunner’s glands express not MUC5AC but MUC6 [32]. As shown in Tables 2 and 4, expression of MUC6 in duodenal epithelial tumors tends to be associated with more malignant property of duodenal tumors. There is a possibility that Brunner’s glands may become cancerous.
Ushiku et al. [28] recently reported the immunohistochemical study of sporadic and malignant NADETs. They classified the 38 lesions based on morphologic features as follows: gastric type, intestinal type, pancreaticobiliary type and others. They reported that all gastric-type adenocarcinomas were located in the proximal duodenum, whereas other type lesions were located both in the proximal and distal duodenum. They also showed that intestinal type was associated with more favorable overall survival and disease-free survival compared to other non-intestinal phenotypes. In our current study, we compared 39 cases of adenoma and 29 cases of adenocarcinoma in the duodenum, all of which were endoscopically resected. We showed that expression of gastric markers is significantly associated with adenocarcinomas, and expression of intestinal markers is associated with adenomas (Table 2). We further found that invasion activity of duodenal adenocarcinoma is significantly associated with expression of gastric markers (Table 4). Considering Ushiku’s report together [28], it is indicated that higher grade of atypia and deeper invasive cancers tend to exhibit stronger expression of gastric markers. To put it the other way around, it is indicated that less malignant duodenal epithelial tumors have a tendency to show stronger expression of intestinal markers.
Although our results suggested that MUC5AC, MUC6 and MUC2 are useful immunohistochemical markers for duodenal adenoma/adenocarcinoma as well as gastric cancer [33], these mucin markers cannot be used for blood/plasma test. To search blood biomarkers for NADETs, we are planning to perform comprehensive gene expression analysis of duodenal adenoma/adenocarcinoma. One of our goals is to identify good blood markers which can reflect the difference in gene expression between duodenal tumor cells and normal adjacent cells.
Concerning the typical markers of malignancy, higher MIB-1 index and intense staining of p53 generally show significant correlation with adenocarcinoma [34, 35]. Our result expectedly showed these cancer-related markers would help to distinguish between adenoma and adenocarcinoma in duodenum.
Of the 29 early-stage duodenal adenocarcinomas, only 3 tumors were positive for p53 staining. This suggests that p53-independent pathways should play an important role in the first step toward malignant transformation of duodenal tumors. However, we think that disordered p53 function also can lead to malignant property of duodenal tumors, since all the lesions with strong p53 expression were unexpectedly malignant. In addition to these, expression analyses of gastric and intestinal differentiation markers can contribute to not only predicting malignant potential of lesions but also deciding the treatment strategy against duodenal epithelial tumors.
Even today, little is known about the expression of gastric markers in NADETs. A few papers reported that duodenal epithelial tumors expressing gastric markers are originated from Brunner’s gland or gastric metaplasia in duodenum [36,37,38]. It is well known that abnormal differentiation sometimes increases the risk of cancer [22, 23]. Similar to intestinal metaplasia in stomach [39] or Barrett’s dysplasia at the esophagogastric junction [40], ectopic expression of gastric markers obviously indicates the unstable differentiation status of duodenum. We speculate that unstable differentiation status of duodenum between gastric and intestinal property must play an essential role of malignant transformation of duodenal epithelial cells.
The current study has several limitations that should be recognized. First, this study design is a retrospective cross-sectional study and the data were originated from institutions in Japan only. Second, due to rarity of the disease, the number of study subjects is small. Third, the mechanism of upregulated gastric marker expression in duodenal tumorigenesis has not been elucidated.
References
Neugut AI, Jacobson JS, Suh S, et al. The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomark Prev. 1998;7:243–251.
Poultsides GA, Huang LC, Cameron JL, et al. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928–1935.
Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97–104.
Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71.
Lu Y, Fröbom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol. 2012;36:e158–e163.
Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, 4th edn.
Fernández-Esparrach G, Calderón A, de la Peña J, et al. Endoscopic submucosal dissection. Endoscopy. 2014;46:361–370.
Obata S, Suenaga M, Araki K, et al. Use of strip biopsy in a case of early duodenal cancer. Endoscopy. 1992;24:232–234.
Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754–759.
Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy. 2013;45:138–141.
Sohn JW, Jeon SW, Cho CM, et al. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc. 2010;24:3195–3200.
Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135.
Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26:46–49.
Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389–395.
Bourke MJ. Endoscopic resection in the duodenum: current limitations and future directions. Endoscopy. 2013;45:127–132.
Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11.
Kakushima N, Kanemoto H, Sasaki K, et al. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21:5560–5567.
Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26:23–29.
Japanese GCA. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123.
Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2010;106:357–364.
Maruoka D, Arai M, Ishigami H, et al. Sporadic nonampullary duodenal adenoma/carcinoma is associated with not only colon adenoma/carcinoma but also gastric cancer: association of location of duodenal lesions with comorbid diseases. Scand J Gastroenterol. 2015;50:333–340.
Yamamichi N, Inada K, Ichinose M, et al. Frequent loss of Brm expression in gastric cancer correlates with histologic features and differentiation state. Cancer Res. 2007;67:10727–10735.
Konno-Shimizu M, Yamamichi N, Inada K, et al. Cathepsin E is a marker of gastric differentiation and signet-ring cell carcinoma of stomach: a novel suggestion on gastric tumorigenesis. PLoS ONE. 2013;8:e56766.
Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664.
Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220.
Zhang S, Cui Y, Zhong B, et al. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis. 2011;26:219–226.
Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386.
Ushiku T, Arnason T, Fukayama M, et al. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol. 2014;38:1484–1493.
Solaini L, Jamieson NB, Metcalfe M, et al. Outcome after surgical resection for duodenal adenocarcinoma in the UK. Br J Surg. 2015;102:676–681.
Park SM, Ham JH, Kim BW, et al. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Pract. 2015;2015:692492.
Arai T, Murata T, Sawabe M, et al. Primary adenocarcinoma of the duodenum in the elderly: clinicopathological and immunohistochemical study of 17 cases. Pathol Int. 1999;49:23–29.
Bartman AE, Buisine MP, Aubert JP, et al. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998;186:398–405.
Ushiku T, Arnason T, Ban S, et al. Very well-differentiated gastric carcinoma of intestinal type: analysis of diagnostic criteria. Mod Pathol. 2013;26:1620–1631.
Saleh HA, Aburashed A, Bober P, et al. P53 protein immunohistochemical expression in colonic adenomas with and without associated carcinoma. Am J Gastroenterol. 1998;93:980–984.
Hong MK, Laskin WB, Herman BE, et al. Expansion of the Ki-67 proliferative compartment correlates with degree of dysplasia in Barrett’s esophagus. Cancer. 1995;75:423–429.
Matsumoto T, Iida M, Nakamura S, et al. Depressed adenoma of the duodenum in patients with familial adenomatous polyposis: endoscopic and immunohistochemical features. Cancer. 1999;86:1414–1420.
Kushima R, Stolte M, Dirks K, et al. Gastric-type adenocarcinoma of the duodenal second portion histogenetically associated with hyperplasia and gastric-foveolar metaplasia of Brunner’s glands. Virchows Arch. 2002;440:655–659.
Kushima R, Ruthlein HJ, Stolte M, et al. ‘Pyloric gland-type adenoma’ arising in heterotopic gastric mucosa of the duodenum, with dysplastic progression of the gastric type. Virchows Arch. 1999;435:452–457.
Yamamichi N, Inada K, Furukawa C, et al. Cdx2 and the Brm-type SWI/SNF complex cooperatively regulate villin expression in gastrointestinal cells. Exp Cell Res. 2009;315:1779–1789.
Zhang HY, Spechler SJ, Souza RF. Esophageal adenocarcinoma arising in Barrett esophagus. Cancer Lett. 2009;275:170–177.
Acknowledgments
The authors thank Dr. Yosuke Muraki from Wakayama Medical University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minatsuki, C., Yamamichi, N., Inada, Ki. et al. Expression of Gastric Markers Is Associated with Malignant Potential of Nonampullary Duodenal Adenocarcinoma. Dig Dis Sci 63, 2617–2625 (2018). https://doi.org/10.1007/s10620-018-5179-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5179-0