Abstract
Background
Primary duodenal cancer (PDC) is rare and few studies have addressed it adequately, especially in China. The present study is to evaluate the clinicopathological features and prognosis of PDC in Chinese patients.
Patients and methods
All the consecutive cases confirmed as PDC by histopathological analysis in The First Affiliated Hospital of Sun Yat-sen University between 1995 and 2008 were included. Clinicopathological details were retrospectively analysed and prognostic factors influencing survival were evaluated.
Results
The patient cohort included 53 men and 38 women, accounting for only 0.02% of all in-patients during this period. Esophagogastroduodenoscopy and gastrointestinal barium radiography were mainstay diagnostic tests for PDC; they detected 88.6% and 83.3% of the tumours, respectively. Tumours mainly occurred in the descending portion of the duodenum (67.0%). Abdominal pain was the most frequent symptom (56.0%). Histologically, adenocarcinoma was the most common type (74.7%). The overall 1-, 3- and 5-year survival rates were 62.6%, 43.7% and 33.1%, respectively. Patients survived longer in the curative surgery group (median survival time of 45 months) than those in the palliative group (6 months) (P < 0.001). Nodal metastasis and positive resection margin had a significant negative impact on survival in patients undergoing potentially curative surgery in a univariate and multivariate model (P < 0.05).
Conclusion
Patients with PDC are rare and lack specific presentations. Esophagogastroduodenoscopy and gastrointestinal barium radiography are effective in screening this rare tumour. Nodal metastasis and positive resection margins are associated with a poor prognosis. A curative surgery that achieves complete resection with negative margin should be pursued.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary duodenal cancer (PDC), which arises from the region of duodenum, excluding ampullary regions of the Vater, is exceedingly rare. It represents approximately 0.5% of all gastrointestinal (GI) tract cancers and 30% to 45% of small bowel cancers [1–3]. PDC was first described by Hamburger in 1746, and since then, there have been a few case reports with small numbers of patients [1–4]. In a recent analysis from cancer registries participating in the Surveillance, Epidemiology, and End-Results program, only 6,230 new cases of small bowel carcinoma with 1,110 deaths are estimated in the USA for the year 2009 [5]. In contrast, the incidences of stomach carcinoma and colorectal cancer in the USA are high, being estimated at 21,130 and 146,970 new cases per year, respectively [5]. Due to rarity, the diagnosis and treatment of PDC still challenge most physicians. As a result, patients are usually misdiagnosed or diagnosed at an advanced stage with a poor prognosis. With the development and widespread use of the radiological and endoscopic methods during the past 20 years, patients may have a chance to be diagnosed at a relatively early stage. To the best of our knowledge, there is no data regarding accurate epidemiological and clinicopathological descriptions of PDCs available in China or even in the world. The aim of the present study is to investigate the clinicopathological characteristics and analyse the main factors influencing the prognosis of PDCs based on our 14-year data at a large tertiary institution in South China. The results could help establish the database of the epidemiology, clinicopathological features, treatment and prognosis of PDCs in China.
Patients and methods
Study protocol and data collection
Medical records of patients with PDC dated from January 1995 to December 2008 (14 years) in The First Affiliated Hospital of Sun Yat-sen University, which was the largest hospital in South China, were carefully reviewed. Cases with incomplete records, a history of cancer, an ampullary tumour, tumour of unknown origin or a secondary tumour were excluded. The diagnosis of duodenal cancer was based on the combination of patients’ clinical presentations, adjuvant examinations, surgical findings and the histological results. For patients included in the study, agreement on duodenum being the primary tumour location was made by both the surgeon and the pathologist. All the diagnoses were finally confirmed by histological examination by two experienced pathologists specialised in digestive diseases. Positive margins were defined as tumour present within 1 mm of the margin.
The TNM staging classification was used according to the American Joint Committee on Cancer (AJCC) staging [6]. The World Health Organization (WHO) standard grading system with four categories (well differentiated, moderately differentiated, poorly differentiated and undifferentiated) was used to classify the histological differentiation [6]. A tumour comprising two different degrees of differentiation was recorded as the category of poorer differentiation.
Based on the type of surgery, patients were grouped into two categories—curative and palliative treatment. Curative resection was defined as a microscopically negative resection with no gross evidence of residual disease (R0 resection). A resection with curative intent was ultimately defined as a palliative intervention if the margin was microscopically (R1) or macroscopically (R2) positive. Palliative treatment also included bypass, partial resection, biopsy and exploration alone. The choice of intervention mainly depended on the extent of the lesion, patients’ physical conditions and surgeon’s opinions. In general, patients with a localised tumour (such as stage I–III) would be given a curative resection as much as possible. Palliative surgery was performed in patients with unresectable tumours, AJCC stage IV, poor physical condition or in emergent situations. However, curative surgery was occasionally attempted on stage IV patients in good physical condition who only had topical liver metastasis (pancreaticoduodenectomy plus partial resections of liver lobe). As a principal, patients who were medically suitable and permissible would be given a potentially curative surgery when they consented. Follow-up information was obtained through follow-up ambulatory visits and telephone contacts with patients or their family members. Survival time was calculated based on the day of histopathological diagnosis, and the closing date for analysis of follow-up was June 30, 2010. The study protocol was approved by the Human Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University.
Statistical methods
Data were analysed using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). The chi-square or Fisher exact tests were used for categorical and ordinal variables. Survival was calculated according to the Kaplan–Meier method. Possible prognostic factors influencing survival, including age, gender, tumour size, tumour location, histological type, histological differentiation, T-stage, N-stage, M-stage and resection margin status were first evaluated by univariate analysis (log-rank test). Only parameters which showed significance in univariate analysis were further analysed by multivariate analysis (Cox proportional hazards test). Statistical significance was determined by a P value of less than 0.05.
Results
Demographics
Of the approximately 27,104,043 out-patient and 441,992 in-patient cases available in our centre during the study period, 8,879 patients were diagnosed with GI cancer. However, only 91 cases were PDCs, accounting for 0.02% of all hospitalised patients. Fifty-three patients were male (58.2%). The ratio of male to female was 1.39. Median age at diagnosis was 55 years (23–89 years). The most common initial presentations were abdominal pain (56.0%), followed by anaemia (haemoglobin <100 g/L) (38.5%), nausea/vomiting (36.3%), weight loss (34.1%) and jaundice (31.9%). Hypoalbuminaemia (albumin <30 g/L), GI bleeding, ileus and abdominal mass were less common in our cases (Table 1). The median duration of the symptoms prior to diagnosis was approximately 3 months.
Tumour location and pathology
Most tumours (67.0%) were located in the second portion (i.e. descending portion) of the duodenum (D2), followed by the bulb portion (D1) (11.0%) (Table 2). The mean diameter of the tumour was 4.2 ± 0.9 cm (range 1.5–9.0 cm). Histologically, adenocarcinoma (74.7%) and gastrointestinal stromal tumour (GIST) (17.6%) were the most common types of PDC. Other histological types included neuroendocrine carcinoma (6.6%) and adeno-squamous carcinoma (1.1%) (Table 3). Based on the WHO standard grading system, 24 (26.4%) cases were well differentiated, 40 (44.0%) were moderately differentiated and 27 (29.7%) were poorly differentiated.
Adjuvant examinations
Esophagogastroduodenoscopy (EGD) examination detected PDC tumours in 62 (88.6%) of the 70 PDC patients. Among the eight false-negative cases, three tumours were located in the third portion of duodenum (D3), two in the distal D2, two in the fourth portion (D4) and one at the D2/D3 junction. GI barium radiography was performed on 24 PDC patients, and a lesion was detected in 20 (83.3%) patients. CT scan of the abdomen identified a lesion in the duodenum in 46 of 62 PDC patients (74.2%). Ultrasonography was also performed on 69 PDC patients and detected a lesion in 33 patients (47.8%). Neither capsule endoscopy nor enteroscopy was used in these cases.
Disease stage
TNM staging was available for all patients. TNM classification was as follows: I—six cases (6.6%), II—34 (37.4%), III—26 (28.6%) and IV—25 (27.5%). Among patients with distant metastases, 14 were found in the liver (56.0%), and 11 were in other sites. Patients with early stage (I + II) were younger than those with advanced stage (III + IV) disease (median age 54 years compared with 59 years), albeit not statistically significant (Z = −0.208, P = 0.835).
Treatment
Fifty-nine patients underwent potentially curative resection, 55 of whom were histologically confirmed as R0 resection. The other four patients with a microscopically positive margin (R1) were considered palliative treatment. The remaining 32 patients were subjected to palliative surgery. Of the 25 patients with stage IV disease, six patients underwent attempted curative surgery with Whipple procedure plus topical liver resection, and the remaining 19 received a palliative bypass, partial resection or underwent biopsy only. Thirteen patients with stage II–III disease received palliative surgery due to the patient’s poor physical condition, emergency (such as gastrointestinal bleeding and intestinal ileus) or unresectable tumours. The median length of stay (LOS) in the hospital was 28 days, ranging from 6 days to 137 days. There were no operative deaths. Three cases with pancreatic fistulae, two with ileus and one with wound infection occurred in the curative resection group. Postoperative complications occurred in five patients who accepted palliative surgery, including two cases of pneumonia, two cases of wound infection and one case of ileus. Postoperative complications were dealt with conservatively. In addition, only three patients underwent chemotherapy (leucovorin calcium and fluorouracil): one in the palliative surgery group and two in the curative resection group. None of the patients received radiation.
Survival
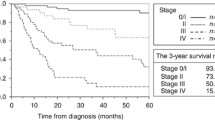
The mean follow-up time of the patients was 27.9 ± 25.4 months (range 1 month to 104 months). The overall 1-, 3- and 5-year survival rates were 62.6%, 43.7% and 33.1%, respectively, with a median survival time of 24 months. Curative resection (R0 resection) was associated with longer survival than palliative resection (non-R0 resection or treatment). The 1-, 3- and 5-year survival rates of patients receiving curative surgery were 81.8%, 62.3% and 49.3% compared with 33.3%, 19.0% and 7.6% for those receiving palliative surgery, respectively. The median survival time for patients undergoing curative surgery (45 months) was significantly longer than those undergoing palliative surgery (6 months) (P < 0.001) (Fig. 1). In a subgroup analysis, patients with stage II tumours with R0 resection (median survival time of 64 months) survived significantly longer than those with non-R0 resection (9 months) (P = 0.007). Patients with stage III tumours with R0 resection (20 months) did not survive significantly longer than those with non-R0 resection (11 months) (P = 0.212) possibly because of the small sample size (26 patients). In addition, the median survival time of patients with R1, R2 and no resection was 6 months, 6 months and 3 months, respectively. There were no significant differences among these three groups (P = 0.052), although patients without receiving any resection appeared to have poorer survival possibly due to the small sample size.
Prognostic factors for the potentially curative resection group
In patients who underwent potentially curative surgery, the effect of possible prognostic factors on survival time was evaluated. In univariate analysis, age, N-stage and resection margin status were found to be significantly associated with survival time (Table 4, Figs. 2 and 3). These parameters were then analysed by multivariate model, where only N-stage (HR = 4.012; 95% CI = 1.899–8.615) and resection margin status (HR = 4.060; 95% CI = 1.379–12.584) were found to be independent predictors for survival (Table 5). Patients with GIST survived longer than those with adenocarcinoma; however, this difference was not statistically significant (P = 0.176) possibly due to the relatively short follow-up time or the small sample size (Fig. 4). Gender, tumour size, location, histological differentiation, T-stage and M-stage did not have a significant effect on survival (Table 4).
Discussion
PDC is a relatively rare cancer compared to the other malignancies of the GI tract. To our knowledge, few studies have addressed PDC adequately, especially in China. Due to its rarity, PDC has been underdiagnosed by most physicians. Some physicians even believe that no tumours will occur in the duodenum or in the small bowel. In our study, only 0.02% of all hospitalised patients were diagnosed with PDC. PDC accounted for about 1.0% of hospitalised patients with GI cancer during this period in our hospital. Several hypotheses have been proposed to explain the low incidence of PDC, including the rapid turnover of duodenal mucosal cells [7, 8], the prompt transit of the duodenal contents and the sparseness of bacterial population that minimises the exposure of potential carcinogens to the duodenum [9, 10]. However, these hypotheses have not been rigorously tested and the actual reasons for the low incidence of PDC remain unclear.
Our results were consistent with previous findings showing that PDCs occurred most often in men and in elderly patients [4, 11–14]. The median age at diagnosis in this study was 55 years. The median LOS was 28 days, which was similar to previous findings [12, 15]. Clinically, the majority of patients with PDCs usually presented with nonspecific signs and symptoms. In our study, the most common symptoms were abdominal pain, anaemia and nausea/vomiting, in agreement with previous reports [12–14, 16, 17]. Nonspecific or insidious signs and symptoms often lead to a delayed diagnosis or misdiagnosis. In our study, the median time of symptom presentation prior to diagnosis was approximately 3 months, which was shorter than reported in some other studies [4, 18–20]. There might be two major reasons. First, the presenting symptoms associated with PDC are usually nonspecific. Thus, they are often attributed to other upper GI problems, such as peptic ulcer and gastric cancers. In China, in particular, gastric cancer is more prevalent than in the USA and Europe. Second, the cost of EGD examination is much lower in China (about $40 US) than that in the USA and Europe. Therefore, EGD is routinely chosen as a screening tool to exclude upper GI cancers for patients who present with similar complaints. EGD is a valuable diagnostic procedure to detect PDCs, because EGD allows the visualisation of the entire duodenum, especially the bulb and proximal descending regions. Recent studies have found that EGD detects about 90% of the duodenal cancers [4, 16–23]. In our study, EGD diagnosed 88.6% of PDC cancers. GI barium radiography is another important diagnostic method used to detect bowel abnormalities and reportedly detects 70–90% of duodenal lesions [4, 17–19, 21, 23]. In our study, it was able to detect a tumour in 83.3% of patients subsequently diagnosed with PDC. The role of CT scans in diagnosing duodenal cancer has not been thoroughly addressed, although it was reported to be useful in detecting bowel disease, including tumours [24]. It identified a lesion in the duodenum in 74.2% PDC patients in our study. One retrospective study found that CT scans were valuable in predicting malignancy of a duodenal lesion and might play an important role in staging the tumour preoperatively and postoperatively [25, 26]. Therefore, CT scan cannot only be used to demonstrate sites of distant metastases but also be used to investigate bowel abnormalities more generally. However, its value in detecting PDCs still requires further clinical study. Ultrasonography could only detect tumours in 47.8% of patients ultimately diagnosed with PDC. This suggested that it had a poor sensitivity for PDC, although it might be occasionally valuable in making differential diagnosis.
Previous reports showed that tumours occurred more frequently in the second portion of the duodenum [12, 15, 16, 23, 27], which was further confirmed in our study, where we found that 67.0% of tumours occurred in D2. It has been speculated that the high concentration of PDC in the descending portion might implicate bile as a possible carcinogenic factor, although the accurate mechanism is still unclear [28, 29]. Adenocarcinoma was the most common type of PDCs (74.7%) and was always better differentiated (70.4%), which was similar to the findings of previous reports [4, 12, 16, 19, 27].
The overall 5-year survival rate was 33.1% in our study, in accordance with previous reports ranging from 7.9% to 47% [4, 12, 13, 15–22, 26, 27, 30]. The survival rates might depend on the stage and operative modality of patients included. In our study, the R0 resection rate was 60.4%, similar to other reports [14–19, 21, 22, 26]. Recent studies reported that 5-year survival rates ranged from 25% to 54% for patients who accepted curative surgery [14, 15, 17, 18, 22, 27]. In our study, survival rate after curative surgery was comparable with previous reports, with 3- and 5-year survival rates of 62.3% and 49.3%, respectively, and was significantly higher than those patients who underwent palliative treatment (median survival 45 months vs. 6 months). Consistent with previous findings, the survival rates suggested that curative surgery might provide more favourable outcomes than palliative operations [12–22, 26, 27, 30]. Resection is the only method of cure. Pancreaticoduodenectomy remains the standard procedure of choice in resection of duodenal cancer, especially for the proximal duodenum, mostly likely due to the theoretical advantage of en bloc resection with lymphadenectomy. In addition, wide segmental resection was reported to be appropriate for locally distal tumours (especially for the third and fourth portions of the duodenum). It could also acquire a good outcome with a lower morbidity [15, 17, 18, 21, 22, 31]. Therefore, curative surgery should be pursued to acquire a favourable outcome. In addition, patients with R1/R2 appeared to survive longer than those without any resection, which might be related to patients’ physical conditions and clinical stage. However, no significant difference was achieved possibly due to the small sample size.
Gender, age, tumour size, location, histological types, histological differentiation, T-stage, N-stage, M-stage and resection margin were considered possible prognostic factors associated with survival time for patients who received potentially curative surgery. In our study, gender, age, tumour size, location, histological differentiation and T-stage did not have a significant effect on the survival time. However, not all of our findings were consistent with previous reports, especially for histological differentiation, T-stage and tumour size [13–16, 18, 19, 22, 26, 27, 30]. In regard to the histological type, adenocarcinoma and GIST were two main types of PDC. They originate from different tissues—epithelium and mesenchyma, respectively. Patients with GIST survived longer than those with adenocarcinoma; however, the difference was not statistically significant possibly due to the relatively short follow-up time or the small sample size. Further study is needed to elucidate possible effects of the histological type on survival time. Distant metastasis was a poor prognosis for survival [30]. They were always subjected to palliative surgery. However, six patients with stage M1 but with singular liver lobe metastasis and tumours which could be completely resected received curative surgery in our study. In the potentially curative surgery group, patients with stage M0 survived longer than those with stage M1, but without significant difference, possibly due to a small sample of M1 patients. The role of nodal metastasis in patients with resected duodenal lesions remains unknown. It was reported to be associated with poor prognosis by some studies [22, 27, 32–34], although others did not find a significant result [4, 13–16, 18, 30]. In our study, for patients who underwent potentially curative surgery, nodal metastasis was associated with decreased survival time, confirmed by both univariate and multivariate analysis. The median survival time for a patient with positive nodes was 17 months compared to 64 months for node-negative patients. This indicates that early detection without lymph node involvement would result in a better prognosis. However, the association between nodal status and survival still requires a large-scale multicentre evaluation. In addition, resection margin involvement was generally believed to be critical to survival in duodenal adenocarcinoma [13, 22, 30]. Our study confirmed that a negative margin (R0 resection) was another favourable prognostic factor in both univariate and multivariate models, suggesting that a clean resection margin is important for survival. Complete resection (R0) with a negative margin should be pursued when possible.
Conclusion
Our analysis of patients from a single tertiary centre in South China found that PDCs were rare. We found that there were no specific signs and symptoms for PDCs. Both low incidence and nonspecific presentations contributed to the delayed or missed diagnoses. PDCs occurred mostly in the second portion of the duodenum, and adenocarcinoma was the most common histological type. EGD and GI barium radiography were valuable for diagnosis and might ultimately be considered as a routine screening test for this rare tumour. Improved awareness and aggressive use of many different diagnostic adjuvant examinations will result in an earlier diagnosis of the disease, which improves chances for resectability. Patients’ long-term survival is directly associated with nodal status and resection margins, and can be improved significantly by early detection and curative treatment, which achieves complete resection with negative margin.
References
Spira IA, Ghazi A, Wolff WI (1977) Primary adenocarcinoma of the duodenum. Cancer 39:1721–1726
Kerremans RP, Lerut J, Penninckx FM (1979) Primary malignant duodenal tumors. Ann Surg 190:179–182
Alwmark A, Andersson A, Lasson A (1980) Primary carcinoma of the duodenum. Ann Surg 191:13–18
Delacore R, Thomas JH, Forster J, Hermreck AS (1993) Improving resectability and survival in patients with primary duodenal carcinoma. Am J Surg 166:626–630
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Greene FL, Compton CC, Fritz AG, Shah J, Winchester DP (2002) AJCC cancer staging manual, 6th edn. Springer, New York
Kim SH, Roth KA, Moser AR, Gordon JI (1993) Transgenic mouse models that explore the multistep hypothesis of intestinal neoplasia. J Cell Biol 123:877–893
Kitchen PA, Walters JR (2001) Molecular and cellular biology of small-bowel mucosa. Curr Opin Gastroenterol 17:104–109
Lowenfels AB (1973) Why are small-bowel tumors so rare? Lancet 1:24–26
Arber N, Neugut AI, Weinstein IB, Holt P (1997) Molecular genetics of small bowel cancer. Cancer Epidemiol Biomark Prev 6:745–748
Bal A, Joshi K, Vaiphei K, Wig JD (2007) Primary duodenal neoplasms: a retrospective clinico-pathological analysis. World J Gastroenterol 13:1108–1111
Hung FC, Kuo CM, Chuah SK, Kuo CH, Chen YS, Lu SN, Chang Chien CS (2007) Clinical analysis of primary duodenal adenocarcinoma: an 11-year experience. J Gastroenterol Hepatol 22:724–728
Sohn TA, Lillemoe KD, Cameron JL et al (1998) Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg 2:79–87
Hurtuk MG, Devata S, Brown KM, Oshima K, Aranha GV, Pickleman J, Shoup M (2007) Should all patients with duodenal adenocarcinoma be considered for aggressive surgical resection? Am J Surg 193:319–324
Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA (2000) Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg 135:1070–1074
Rotman N, Pezet D, Fagniez PL, Cherqui D, Celicout B, Lointier P (1994) Adenocarcinoma of the duodenum: factors influencing survival. Br J Surg 81:83–85
Han SL, Cheng J, Zhou HZ, Zeng QQ, Lan SH (2009) The surgical treatment and outcome for primary duodenal adenocarcinoma. J Gastrointest Cancer 40:33–37
Hu JX, Miao XY, Zhong DW, Dai WD, Liu W, Hu W (2006) Surgical treatment of primary duodenal adenocarcinoma. Hepatogastroenterology 53:858–862
Santoro E, Sacchi M, Scutari F, Carboni F, Graziano F (1997) Primary adenocarcinoma of the duodenum: treatment and survival in 89 patients. Hepatogastroenterology 44:1157–1163
Heniford BT, Iannitti DA, Evans P, Gaqner M, Henderson JM (1998) Primary nonampullary/periampullary adenocarcinoma of the duodenum. Am Surg 64:1165–1169
Barnes G Jr, Romero L, Hess KR, Curley SA (1994) Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol 1:73–78
Bakaeen FG, Murr MM, Sarr MG et al (2000) What prognostic factors are important in duodenal adenocarcinoma? Arch Surg 135:635–641
Solej M, D’Amico S, Brondino G, Ferronato M, Nano M (2008) Primary duodenal adenocarcinoma. Tumori 94:779–786
Freeman AH (2001) CT and bowel disease. Br J Radiol 74:4–14
Kazerooni EA, Quint LE, Francis IR (1992) Duodenal neoplasms: predictive value of CT for determining malignancy and tumor resectability. AJR Am J Roentgenol 159:303–309
Adedeji OA, Trescoli-Serrano C, Garcia-Zarco M (1995) Primary duodenal carcinoma. Postgrad Med J 71:354–358
Lee HG, You DD, Paik KY, Heo JS, Choi SH, Choi DW (2008) Prognostic factors for primary duodenal adenocarcinoma. World J Surg 32:2246–2252
Neugut AI, Jacobson JS, Suh S, Mukherjee R, Arber N (1998) The epidemiology of cancer of the small bowel. Cancer Epidemiol Biomark Prev 7:243–251
Ross RK, Hartnett NM, Bernstein L, Henderson BE (1991) Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer 63:143–145
Rose DM, Hochwald SN, Klimstra DS, Brennan MF (1996) Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg 183:89–96
Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS (2000) Extent of resection in the management of duodenal adenocarcinoma. Am J Surg 179:37–41
Sarela AI, Brennan MF, Karpeh MS, Klimstra D, Conlon KC (2004) Adenocarcinoma of the duodenum: importance of accurate lymph node staging and similarity in outcome to gastric cancer. Ann Surg Oncol 11:380–386
Struck A, Howard T, Chiorean EG, Clarke JM, Riffenburgh R, Cardenes HR (2009) Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol 100:144–148
Gold JS, Tang LH, Gönen M, Coit DG, Brennan MF, Allen PJ (2007) Utility of a prognostic nomogram designed for gastric cancer in predicting outcome of patients with R0 resected duodenal adenocarcinoma. Ann Surg Oncol 14:3159–3167
Acknowledgements
The authors greatly thank Dr Brandon Steelman from Stanford University, Tracy Zhu from Chinese University of Hong Kong and Mr Steven Lee from Indiana University–Purdue University Indianapolis for their kind critical reading and linguistic revisions of the manuscript. We thank Dr Zijin Weng and Prof. Yu Tao from Division of Pathology of Sun Yat-sen University for helpful suggestions on the pathological typing and skilful technical assistance. We also thank Prof. Yuantao Hao from Department of Statistics and Epidemiology, Sun Yat-sen University for his kind help on statistics. In addition, we appreciate all the doctors and students for their great work in building the gastrointestinal tumour database.
Conflicts of interest
None.
Funding source
This study is sponsored by Science and Technology Foundation of Guangdong Province (no. 2008B030301298, 2009B030801174), Yat-sen innovative talents’ training program of outstanding supervisor of Sun Yat-sen University and International Program Fund of 985 Project of Sun Yat-sen University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presentation of meeting: The abstract of this article was accepted as poster presentation by the meeting of GASTRO 2009, UEGW/WCOG in London.
Shenghong Zhang and Yi Cui have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Zhang, S., Cui, Y., Zhong, B. et al. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis 26, 219–226 (2011). https://doi.org/10.1007/s00384-010-1063-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-010-1063-x