Abstract
Background
Cyclophilin A (CyPA) is an immunomodulatory protein, high expression of which correlates with poor outcome of patients with inflammatory diseases. However, its role in inflammatory bowel disease (IBD) has not been studied.
Aim
This study analyzes the correlation between cyclophilin A, matrix metalloproteinase (MMP)-9, and tissue inhibitor of MMP (TIMP)/MMP-9 complexes in the inflamed and non-inflamed colon mucosa of UC and CD patients.
Methods
Serum and biopsy specimens from inflamed and non-inflamed colonic mucosa of 38 patients with IBD (19 with UC and 19 with CD) and 16 controls were included in our study. We measured serum and tissue level of CyPA, and tissue level of TNF-α, MMP-9, TIMP-1/MMP-9, and TIMP-2/MMP-9 using ELISA method.
Results
Our results indicated that serum, but not tissue CyPA is increased in UC, rather than in CD patients, compared to the control. The increase correlated with higher tissue concentration of MMP-9 and TNF-α, especially in the UC group. Moreover, we observed significantly higher level of TIMP-1/MMP-9 in UC and CD group, which overlapped with the change in MMP-9. There was no change in TIMP-2/MMP-9 in the analyzed groups.
Conclusion
The current study suggests that serum CyPA may be an independent additional marker of IBD, especially of UC. Higher CyPA level may be followed by increased MMP-9 in those patients. However, further studies are necessary to verify the role of CyPA in IBD development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a group of chronic disorders primarily consisting of ulcerative colitis (UC) and Crohn’s disease (CD). Both conditions can lead to an extensive damage of the gastrointestinal tract and are manifested by various clinical symptoms, such as visceral pain, diarrhea, rectal bleeding, weight loss and many others, all substantially lowering patients’ quality of life. The clinical distinction between UC and CD is based mostly on the location and morphology of inflammatory lesions [1]. Ulcerative colitis is present in the rectum and colon; the pattern of inflammation is continuous and limited solely to the mucosa and submucosa of the intestine. By contrast, Crohn’s disease can affect entire alimentary tract from mouth to anus; inflammation is usually discontinuous and transmural.
Although UC and CD are well-known inflammatory bowel diseases, the search for reliable disease markers continues [1, 2]. Among those factors is cyclophilin A, high expression of which correlates with poor outcome of patients with inflammatory diseases [3]. CyPA is a 18-kDa cytosolic protein that has an activity of cis–trans isomerase and may play an important role in immunomodulation and cell signaling. In the form of cytokine, CyPA promotes intracellular communication, has chemoattractant effect on inflammatory cells and exacerbates oxidative stress and inflammation [4].
It was showed that in inflammatory diseases CyPA stimulates monocytes production of matrix metalloproteinases (MMP)-9 and MMP-2 [5]. Furthermore, CyPA may regulate the expression of MMP-9 in the esophageal squamous cell carcinoma [6].
Previously Gao et al. [7] have shown that the expression of gelatinases, especially MMP-9, increases in relation to severity of inflammation in both UC and CD. Moreover, the lack of MMP-9 expression reduces inflammation and intestinal mucosa damage in dextran sulfate sodium (DSS)-induced UC mice [8]. Tissue concentration of MMPs is under control of tissue inhibitor of matrix metalloproteinases (TIMP). TIMP-1 and TIMP-2 are responsible for the activity of MMPs, and hence they maintain the correct balance between remodeling and degradation of extracellular matrix. It was showed that TIMP-1 levels tend to be up-regulated in IBD patients, while TIMP-2 levels were shown to be similar between IBD and healthy controls [9, 10]. However, during an ongoing inflammation an excessive amount of MMP-9 is produced by infiltrated neutrophils in response to pro-inflammatory proteins. Therefore, serum CyPA may influence MMPs and TIMPs, and may have prognostic significance in IBD patients.
In this study, we investigated the concentration of serum and tissue CyPA in inflamed and non-inflamed areas of colon mucosa from UC and CD patients. Furthermore, to investigate whether serum CyPA may influence MMP-9 concentration, we analyzed the concentration of MMP-9 and its inhibitors, TIMP-1 and TIMP-2, in the colon mucosa of inflamed and non-inflamed areas of colon mucosa from UC and CD patients.
Materials and Methods
Patients
Serum and biopsy specimens from the total of 54 patients: 38 patients with IBD (19 with UC and 19 with CD, biopsies from inflamed and non-inflamed colonic mucosa) and 16 controls were included in our study.
In all IBD patients, endoscopic biopsies from the macroscopic inflamed and non-inflamed areas were obtained and all cases were histopathologically confirmed. Tissue was considered non-inflamed if there was an absence of macroscopic or histological evidence of inflammation. The control group included patients with dyspepsia that were diagnosed IBD-free during colonoscopy, and the results were confirmed in the histopathological examination.
Criteria for inclusion in the study groups were based on a diagnosis according to clinical, radiological, endoscopic, and histological criteria recommended by the European Crohn’s and Colitis Organization. The exclusion criteria were low or high grade dysplasia, colorectal cancer, a history of cardiovascular disease, pulmonary and kidney disease, allergy, diabetes, lichen planus, psoriasis, atopic dermatitis, and other autoimmune skin lesions.
The study protocol was approved by the local ethic committee (No. RNN/515/13/KB), and all participants had given written informed consent to participate in the study.
Sample Collection
In each subject undergoing colonoscopy, two endoscopic biopsy specimens were taken from colonic inflamed and non-inflamed areas (UC, CD) and, in the control group, only from non-inflamed areas. Moreover, 5 ml of venous blood was collected from each patient to serum vacuum tubes. Samples were left to clot for 30 min at room temperature before centrifugation at 1,000xg for 15 min. Next, serum was collected and was stored at −80 °C for further biochemical analysis of CyPA.
Tissue Homogenate Preparation
Tissue biopsies from inflamed and non-inflamed colon were homogenized in RIPA lysis buffer (10 mg tissue in 1 ml) (Sigma-Aldrich Co., USA) using tissue raptor (Ultra-Turrax T25, IKA, Germany) at an rpm of 20,000/min for 30 s. Samples were incubated on ice for 15 min and, centrifuged for 10 min (2000×g, +4 °C) and supernatant was collected and stored at −80 °C for further analysis of CyPA, TNF-α, total MMP-9, TIMP-1/MMP-9, and TIMP-2/MMP-9.
Protein Assay of CyPA, TNF-α, MMP-9, TIMP-1/MMP-9, and TIMP-2/MMP-9 Complexes by ELISA
Human CyPA was measured in the serum samples and tissue homogenates (preparation described above), and TNF-α was measured in tissue using commercially available sandwich ELISA kits (Cusabio, USA; R&D Systems, Inc., Minneapolis, USA, respectively) according to the manufacturer’s instructions. The results were presented as mean ± SEM in ng/ml for CyPA or pg/ml for TNF-α after normalization to total protein level.
Levels of MMP-9, TIMP-1/MMP-9 and TIMP-2/MMP-9 complexes were measured in appropriately diluted tissue homogenates using commercially available sandwich ELISA kits (R&D Systems, Inc., Minneapolis, USA) according to the manufacturer’s instructions. The results were presented as mean ± SEM in pg/ml after normalization to total protein level. Each ELISA assay was forerun by total protein concentration assay using the Lowry protocol [11].
Statistical Analysis
Continuous demographic and biochemical data are presented as median, minimum and maximum, or mean ± SEM: demographic categorical data were described with absolute frequencies and percentages. Comparisons between groups were performed using the Kruskal–Wallis test (or non-parametric Mann–Whitney U test) and Chi-square test depending on the normality of distribution. One-way analysis of variance and the Dunns post-test were used to calculate differences. The outliers were excluded using the Grubbs’ test. All p values lower than or equal to 0.05 were considered significant. Statistical analysis was performed using the software package Statistica 9.0.
Results
Demographic Characteristic
Demographic data are shown in Table 1. The age range and number of women to men between the analyzed groups was comparable (p > 0.05). There was no significant difference in the percentage of smokers in the UC and CD groups (14 and 6%, respectively; p = 0.364). In the control group, none of the subjects received glucocorticosteroids (GCS), azathioprine (AZA), 5-aminosalicylic acid (5-ASA), or sulphasalazine (Sulpha). In the CD group, 2 patients were on GCS, 8 on AZA, 16 on 5-ASA, and 12 on Sulpha, while in the UC group, 3 patients were on GCS, 14 on AZA, 16 on 5-ASA, and 17 on Sulpha (p = 0.768, p = 0.163, p = 0.303, p = 0.308 in CD versus UC, respectively).
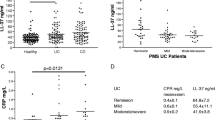
The level of white blood cells (WBC) was statistically higher in UC patients in comparison to control group (9.40 × 106 versus 7.31 × 106 ml p = 0.015), while the CD group had only slightly higher WBC concentration compared to the control; there was no significant difference between UC and CD group (p = 0.145 and p = 0.608, respectively). No sex-related difference in WBC was observed (p > 0.05). Patients in the UC group had statistically higher blood C-reactive protein (CRP) concentration compared to the control group (p = 0.023).
The Serum CyPA Is Increased in UC Patients
Our results indicated that patients with UC had significantly higher level of serum CyPA compared to the control and CD group (p < 0.001 and p = 0.003, respectively; Fig. 1a). There was also a tendency toward higher serum CyPA in the CD group compared to the control group (p = 0.06). The level of tissue CyPA in the inflamed and non-inflamed tissue samples was comparable (Fig. 1b).
Changes of the serum (a) and tissue (b) CyPA in the colon mucosa of UC and CD patients. The results are presented as mean ± SEM in ng/ml of tissue homogenate after normalization to total protein level. CD Crohn’s disease, UC ulcerative colitis. Statistical analysis: Grubb’s test for outliers followed by Kruskal–Wallis test. *An outlier—calculated with Grubb’s test—that was removed from statistical analysis
The UC and CD Patients Have Higher Level of Pro-inflammatory TNF-α in Colon Mucosa
CyPA is known to influence pro-inflammatory cytokine level, such as TNF-α in inflammatory cells [12]. To determine if higher serum CyPA level correlates with elevated cytokine expression in IBD we measured the colon level of TNF-α. The changes are presented in Fig. 2. The results indicated that the level of TNF-α was markedly increased in inflamed areas of the colon mucosa in both UC and CD groups compared to the control (p = 0.04 and p = 0.008, respectively; Fig. 2). Also there was a higher TNF-α level in non-inflamed colon area of the UC group compared to the CD group (p = 0.02; Fig. 2).
Changes of the tissue TNF-α in the colon mucosa of UC and CD patients. The results are presented as mean ± SEM in pg/ml of tissue homogenate after normalization to total protein level. CD Crohn’s disease, UC ulcerative colitis. Statistical analysis: Grubb’s test for outliers followed by Kruskal–Wallis test. *An outlier—calculated with Grubb’s test—that was removed from statistical analysis
Higher Level of MMP-9 and TIMP-1/MMP-9 in Inflamed Areas of Colon Mucosa from the UC and CD Groups
It was previously showed that serum CyPA may directly influence the MMP-9 level [13]. Also MMP-9 plays an important role in IBD’s pathogenesis [14, 15]. In our study we measured the tissue level of MMP-9 alone or in complex with its inhibitors TIMP-1 and TIMP-2 in the inflamed and non-inflamed colon mucosa (Fig. 3). The results indicated that tissue level of MMP-9 was significantly higher in colon of UC and CD patients compared to the control (p < 0.001). Moreover, over-expression of MMP-9 was higher in the inflamed versus non-inflamed tissue in the UC and CD groups (p = 0.01; Fig. 3a). We also observed an increase in TIMP-1/MMP-9 the UC group but only in the inflamed colon areas compared to the control (p = 0.027; Fig. 3b). While in the CD group a significant increase of TIMP-1/MMP-9 was detected in non-inflamed and inflamed colon areas compared to the control (p < 0.001 and p = 0.006, respectively; Fig. 3b). Moreover, we noticed significantly higher level of TIMP-1/MMP-9 in the non-inflamed colon areas of the CD versus UC group (p = 0.021; Fig. 3b). Tissue concentration of TIMP-2/MMP-9 was comparable in all analyzed groups (p > 0.05; Fig. 3c).
The changes of MMP-9, TIMP-1/MMP-9 and TIMP-2/MMP-9 in the colon mucosa of UC and CD patients. The results are presented as mean ± SEM in pg/ml of tissue homogenate after normalization to total protein level. CD Crohn’s disease; UC ulcerative colitis. Statistical analysis: Grubb’s test for outliers followed by Kruskal–Wallis test. *An outlier—calculated with Grubb’s test—that was removed from statistical analysis
Discussion
CyPA is an evolutionarily conserved protein, believed to be present only in the intracellular space; however, recent studies have shown that it is also secreted from cells in response to inflammatory stimuli and oxidative stress [4, 16]. Our results indicated that patients with UC, rather than CD, have higher serum, but not tissue level of CyPA. This effect may be in part explained by excessive concentration of inflammatory cells in the colon mucosa of UC patients. CyPA is also secreted by inflammatory cells during organ damage [17, 18].
Serum CyPA works via its receptor CD147, a single transmembrane glycoprotein, which is widely expressed in all leukocytes, platelets, endothelial cells, and intestinal epithelial cells [19, 20]. Activation of CD147 receptor by CyPA increases phosphorylation of pro-inflammatory extracellular matrix regulated signaling pathway (ERK1/2) [21, 22]. Activation of this pathway may further lead to an increase in MMP-2, MMP-9 and MMP-7 as it was shown in colon cancer cells [23]. Therefore, by acting through its receptor, CyPA increases gene expression of pro-inflammatory cytokines and MMPs including MMP-2 and MMP-9. Earlier study demonstrated that CyPA inhibition significantly decreases MMP-9 and TNF-α in the mouse myocardial inflammation [13] and lung cancer cells [24]. This effect of CyPA on MMP-9 is in line with our results, which showed that serum CyPA correlated with higher tissue concentration of MMP-9 and TNF-α, especially in the UC group.
MMP-9 is synthesized predominantly in inflammatory cells; as a consequence of its release, more leukocytes are drawn to colon mucosa to worsen inflammation [25]. The mechanism behind CyPA influence on MMP-9 in IBD remains unknown; however, among factors that control MMP-9 activity are TIMPs. Previously, Lakatos et al. [14] indicated that patients with UC and CD had elevated MMP-9 and TIMP-1 antigen levels, which correlated with disease activity. Recently Jakubowska et al. [26] showed that MMP-9 and TIMP-1 are over-expressed in the glandular epithelium plus inflammatory infiltration in UC patients and glandular epithelium of CD patients. Also Mao et al. [27] demonstrated a higher expression and activity of MMP-9 in the UC patients.
In our study we noticed a significant increase of MMP-9 in the inflamed colon areas of UC and CD patients. This is in line with previous reports were higher expression and activity of MMP-9 was detected in ulcerated areas compared with non-involved sites of the mucosa in patients with ischemic colitis [28].
The expression of MMP-9 is not only changed in tissue but also changed in serum of patients with IBD. Although we did not measure serum level of MMP-9 in our study, there are numerous reports that in IBD serum MMP-9 is elevated [10, 14]. Recently a large study on changes in serum level of MMP-9 in IBD patients described a significant elevation of serum MMP-9 in patients with UC, but only a tendency in CD [29]. Those authors also propose serum MMP-9 as a supportive marker for differentiation between active and inactive IBD.
The MMP-9 released from macrophages may be stimulated by CyPA via CD147 receptor and NF-κB pathway as it was shown in rheumatoid arthritis [30]. Another pathway stimulated by CyPA is ERK1/2, whose activation increases transcription of MMP-9 and TIMP-1, but not TIMP-2 in the biliary atresia [31]. This is in line with our results, where higher concentration of TIMP-1/MMP-9, but not TIMP-2/MMP-9, was observed. Moreover, in our study TIMP-1/MMP-9 seemed to balance higher MMP-9 in both groups. These observations may be explained by the mechanism behind TIMP-1 regulation which involves CyPA. It was shown previously that the expression of TIMP-1, rather than TIMP-2 may be regulated by CD147, the CyPA receptor, in the in vitro studies [32]. Blockage of CD147 with siRNA decreases secretion of MMP-9 and MMP-2 in carcinoma cells and hepatocytes [32, 33]. Also, CD147 regulates TIMP-2 production in rats during inflammation [34]. However, the direct link between CyPA–CD147–TIMP in inflammatory bowel disease needs to be verified.
In conclusion, the current study presents for the first time that patients with UC, rather than CD, have higher serum, but not tissue CyPA level. Higher level of CyPA in serum may influence tissue level of MMP-9 and TIMP-1/MMP-9; however further studies are necessary to verify the role of CyPA in IBD development.
References
Matricon J, Barnich N, Ardid D. Immunopathogenesis of inflammatory bowel disease. Self Nonself. 2010;1:299–309.
Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604.
Suzuki J, Jin ZG, Meoli DF, et al. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817.
Nigro P, Pompilio G, Capogrossi MC. Cyclophilin A: a key player for human disease. Cell Death Dis. 2013;4:e888.
Wang L, Wang CH, Jia JF, et al. Contribution of cyclophilin A to the regulation of inflammatory processes in rheumatoid arthritis. J Clin Immunol. 2010;30:24–33.
Li Y, Guo H, Dong D, et al. Expression and prognostic relevance of cyclophilin A and matrix metalloproteinase 9 in esophageal squamous cell carcinoma. Diagn Pathol. 2013;8:207.
Gao Q, Meijer MJ, Kubben FJ, et al. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005;37:584–592.
Santana A, Medina C, Paz-Cabrera MC, et al. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol. 2006;12:6464–6472.
La Pietra V, Marinelli L, Cosconati S, et al. Identification of novel molecular scaffolds for the design of MMP-13 inhibitors: a first round of lead optimization. Eur J Med Chem. 2012;47:143–152.
Meijer MJ, Mieremet-Ooms MA, van der Zon AM, et al. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn’s disease phenotype. Dig Liver Dis. 2007;39:733–739.
Cadman E, Bostwick JR, Eichberg J. Determination of protein by a modified Lowry procedure in the presence of some commonly used detergents. Anal Biochem. 1979;96:21–23.
Yuan W, Ge H, He B. Pro-inflammatory activities induced by CyPA–EMMPRIN interaction in monocytes. Atherosclerosis. 2010;213:415–421.
Heinzmann D, Bangert A, Muller AM, et al. The novel extracellular cyclophilin A (CyPA)—inhibitor MM284 reduces myocardial inflammation and remodeling in a mouse model of troponin I—Induced Myocarditis. PLoS ONE. 2015;10:e0124606.
Lakatos G, Hritz I, Varga MZ, et al. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30:289–295.
O’Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm. 2015;2015:964131.
Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin–CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160:305–317.
Song T, Yang M, Chen J, et al. Prognosis of sepsis induced by cecal ligation and puncture in mice improved by anti-Clonorchis Sinensis cyclopholin a antibodies. Parasit Vectors. 2015;8:502.
Dear JW, Leelahavanichkul A, Aponte A, et al. Liver proteomics for therapeutic drug discovery: inhibition of the cyclophilin receptor CD147 attenuates sepsis-induced acute renal failure. Crit Care Med. 2007;35:2319–2328.
Zhu X, Song Z, Zhang S, et al. CD147: a novel modulator of inflammatory and immune disorders. Curr Med Chem. 2014;21:2138–2145.
Saksena S, Theegala S, Bansal N, et al. Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G878–G885.
Jin ZG, Lungu AO, Xie L, et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191.
Bahmed K, Henry C, Holliday M, et al. Extracellular cyclophilin-A stimulates ERK1/2 phosphorylation in a cell-dependent manner but broadly stimulates nuclear factor kappa B. Cancer Cell Int. 2012;12:19.
Xie Z, Qu Y, Leng Y, et al. Human colon carcinogenesis is associated with increased interleukin-17-driven inflammatory responses. Drug Des Devel Ther. 2015;9:1679–1689.
Qian Z, Zhao X, Jiang M, et al. Downregulation of cyclophilin A by siRNA diminishes non-small cell lung cancer cell growth and metastasis via the regulation of matrix metallopeptidase 9. BMC Cancer. 2012;12:442.
Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41.
Jakubowska K, Pryczynicz A, Januszewska J, et al. Expressions of matrix metalloproteinases 2, 7, and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis Markers. 2016;2016:9895721.
Mao JW, He XM, Tang HY, Wang YD. Protective role of metalloproteinase inhibitor (AE-941) on ulcerative colitis in rats. World J Gastroenterol. 2012;18:7063–7069.
Medina C, Santana A, Paz-Cabrera MC, et al. Increased activity and expression of gelatinases in ischemic colitis. Dig Dis Sci. 2006;51:2393–2399.
Matusiewicz M, Neubauer K, Mierzchala-Pasierb M, et al. Matrix metalloproteinase-9: its interplay with angiogenic factors in inflammatory bowel diseases. Dis Markers. 2014;2014:643645.
Yang Y, Lu N, Zhou J, et al. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:1299–1310.
Iordanskaia T, Malesevic M, Fischer G, et al. Targeting extracellular cyclophilins ameliorates disease progression in experimental biliary atresia. Mol Med. 2015;21:657–664.
Xu Q, Cao X, Pan J, et al. Extracellular matrix metalloproteinase inducer (EMMPRIN) remodels the extracellular matrix through enhancing matrix metalloproteinases (MMPs) and inhibiting tissue inhibitors of MMPs expression in HPV-positive cervical cancer cells. Eur J Gynaecol Oncol. 2015;36:539–545.
Calabro SR, Maczurek AE, Morgan AJ, et al. Hepatocyte produced matrix metalloproteinases are regulated by CD147 in liver fibrogenesis. PLoS ONE. 2014;9:e90571.
Attia M, Huet E, Gossard C, et al. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med. 2013;41:908–917.
Acknowledgments
Supported by the grants from the Medical University of Lodz (#503/1-156-04/503-01 to JF). Aleksandra Piechota-Polanczyk is currently working at the Department of Medical Biotechnology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, Krakow, Poland. Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Center (KNOW) supported by the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Piechota-Polanczyk, A., Włodarczyk, M., Sobolewska-Włodarczyk, A. et al. Serum Cyclophilin A Correlates with Increased Tissue MMP-9 in Patients with Ulcerative Colitis, but Not with Crohn’s Disease. Dig Dis Sci 62, 1511–1517 (2017). https://doi.org/10.1007/s10620-017-4568-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4568-0