Abstract
Background
Inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) plays vital roles in inflammatory and auto-immune diseases, but its correlations with disease risk and clinical features in inflammatory bowel disease (IBD) need further investigation. The present study intended to explore the correlation of ITIH4 with disease activity and inflammation, as well as its change after treatment in IBD patients.
Methods
Totally, 40 active Crohn’s disease (A-CD) patients, 40 clinical-remission CD (R-CD) patients, 40 active ulcerative colitis (A-UC) patients, 40 clinical-remission UC (R-UC) patients, and 40 health controls (HCs) were enrolled. ITIH4 in serum was assessed by ELISA.
Results
ITIH4 was lower in A-CD, R-CD, A-UC, and R-UC patients than in HCs (P < 0.001). Notably, ITIH4 reduced in A-CD patients than in R-CD patients (P = 0.017), and in A-UC patients compared with R-UC patients (P = 0.010). Besides, in A-CD patients, ITIH4 negatively correlated with tumor necrosis factor-alpha (TNF-α), interleukin (IL)-17A, IL-1β, C-reactive protein (CRP), and clinical disease activity index score (all P < 0.05). In A-UC patients, ITIH4 negatively correlated with TNF-α, IL-17A, IL-1β, IL-6, CRP, and Mayo score (all P < 0.05). However, in R-CD and R-UC patients, these correlations were less obvious than in A-CD and A-UC patients. ITIH4 was increased after treatment (all P < 0.05), and its expression at W12 after treatment was higher in response patients compared with no response patients in A-CD (P = 0.022) and A-UC groups (P = 0.038).
Conclusion
ITIH4 correlates with IBD susceptibility, active risk, inflammation level, and its elevation after treatment relates to clinical response in IBD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is an immune disorder characterized by progressive gastrointestinal inflammation, which primarily consists of Crohn’s disease (CD) and ulcerative colitis (UC) with prevalence of 24.5 and 18.6 cases per 100,000 populations, respectively [1,2,3]. It is caused by interactions between genetic, environmental, and immune factors with a rise in the incidence globally over the past few decades [4, 5]. Besides, advancements in the screening, surveillance, and treatment of IBD have been made and the application of drugs, such as 5-aminosalicylic acid (5-ASA), hormone drug, 6-mercaptopurine (6-MP), methotrexate, and tumor necrosis factor-alpha (TNF-α) inhibitor, are recommended for IBD patients [6,7,8,9]. Although the above treatment options elevate the overall prognosis of IBD patients, there is still large distinction in the response after treatment [10,11,12]. Therefore, it is urgent to find out new biomarkers to predict the treatment response and improve the management of IBD patients.
Inter-alpha-trypsin inhibitor heavy chain 4 (ITIH4) belongs to one of the five inter-alpha-trypsin inhibitor heavy chain (ITIH) proteins, which is reported to function as an anti-inflammatory protein [13]. For instance, in acute ischemic stroke, by binding to cell surface actin on polymorphonuclear cells, ITIH4 inhibits the phagocytic activities, then acting as an anti-inflammatory protein [14, 15]. Besides, in recurrent pregnancy loss patients, through increasing the levels of anti-inflammatory factors, ITIH4 could regulate inflammation in human placental choriocarcinoma cells [16]. Apart from its regulation in inflammatory functions, ITIH4 plays vital roles in immune disease. For example, via activating the complement cascade, ITIH4 may regulate neutrophilic migration and influence arthritis in patients having rheumatoid arthritis (RA) [17]. Clinically, citrullinated ITIH4 negatively associates with disease activity and inflammatory markers in RA patients [18]. Considering that IBD is an immune disease accompanied by inflammatory conditions, we hypothesized that ITIH4 might be used as a potential biomarker for IBD surveillance [19].
The present study was to explore the correlation of ITIH4 with disease activity and inflammation, as well as its change after treatment in IBD patients.
Methods
Subjects
From June 2018 to March 2021, this study consecutively enrolled 40 patients with active CD (A-CD), 40 CD patients in clinical remission (R-CD), 40 patients with active UC (A-UC), and 40 UC patients in clinical remission (R-UC). The enrollment criteria were as follows: (i) diagnosed as IBD (including CD and UC) according to 2017 European Society for Clinical Nutrition and Metabolism (ESPEN) guideline [20]; (ii) more than 18 years old; (iii) clinical disease activity index (CDAI) score ≥ 150 points for A-CD patients and < 150 points for R-CD patients, then Mayo score ≥ 3 points for A-UC patients and < 3 for R-UC patients [21]; (iv) willing to provide peripheral blood (PB) samples for study purpose. The patients with the following conditions were excluded from the study: (i) with other inflammatory diseases or immune system diseases; (ii) had active infections; (iii) complicated with hematologic malignancies or cancers. Besides, the study also enrolled 40 healthy subjects with matched age and sex to IBD patients as health controls (HCs). The exclusion criteria for IBD patients were also appropriate for HCs. The written informed consents were gathered from all subjects. The study was permitted by Institutional Review Board.
Treatment
According to the disease status of patients, different medications were administered for the corresponding patients. For A-CD patients and A-UC patients, based on the disease severity, aminosalicylates, steroids, immune modulators, or biologics were administered: 5-aminosalicylate was administered orally at a dose of 2–4.8 g/day; or prednisone was administered orally at a dose of 40–60 g/day; or infliximab was administered intravenously at a dose of 5 mg/kg on week 0, week 2, and week 6. For R-CD patients, azathioprine was administered orally at a dose of 2–3 mg/kg/day. For R-UC patients, 5-aminosalicylate was administered orally at a dose of 1.2–2.4 g/day, and azathioprine was administered orally at a dose of 1.5–2.5 mg/kg/day for the patients who did not stay in remission with aminosalicylates. In addition, based on the evaluation of clinical response at week 12 (W12), for the patients without clinical response, immune modulators and biologics were administered, such as cyclosporine, infliximab, azathioprine, and thalidomide.
Data collection
Basic demographics, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) of all subjects were documented after enrollment. For CD and UC patients specifically, inflammatory cytokines (including TNF-α, interleukin-1beta (IL-1β), IL-6, and interleukin-17A (IL-17A)), CDAI score (used for A-CD patients) and Mayo score (used for A-UC patients) were also recorded.
Sample collection and detection
For all subjects, PB samples were collected after recruitment to isolate serum. ITIH4 in serum was assessed by enzyme-linked immunosorbent assay (ELISA) using Human ITIH4 DuoSet ELISA kit (DY8157-05). For CD and UC patients specifically, inflammatory cytokines in serum were detected by ELISA using Human TNF-alpha DuoSet ELISA (DY210), Human IL-1β DuoSet ELISA (DY201), Human IL-6 DuoSet ELISA (DY206), and Human IL-17A DuoSet ELISA (DY317). To further evaluate the change of ITIH4 during the treatment in A-CD and A-UC patients, PB samples were also collected at week 12 (W12) after treatment, and ITIH4 in serum was assessed by ELISA as well. All ELISA kits were purchased from Bio-Techne China Co., Ltd. (R&D Systems, Shanghai, China). The ELISA procedures were performed according to the instructions from the manufacturer. In brief, 100 µL of diluent mixed with 50 µL of standards, controls, or samples were added to each well. After incubation for 2 h, 100 µL of conjugate was added to each well, and incubated for 1 h. Then, 200 µL of substrate solution was added to each well. After incubation for 30 min at room temperature, 50 µL of stop solution was added to each well, and absorbance at 450 nm was read immediately. Finally, standard curve was fitted, and it was applied to calculate the concentration of unknown samples.
Clinical response assessment
The clinical response was evaluated at W12 after treatment using CDAI score and Mayo score: for A-CD patients, the clinical response was defined as a decline of CDAI score more than 70 points after treatment [21]; for A-UC patients, the clinical response was defined as a decline of Mayo score more than 30% or 3 points after treatment; meanwhile, the rectal bleeding subscore decline over 1 point or the rectal bleeding subscore of 0–1 [22]. Based on the clinical response status at W12, A-CD and A-UC patients were classified as response patients and no response patients.
Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, New York, USA) and GraphPad Prism 7.02 (GraphPad Software Inc., San Diego, CA, USA) were applied for data analysis and graph construction. Comparison of clinical characteristics among groups was determined by one-way analysis of variance (ANOVA) test, Chi-square test, Kruskal–Wallis H rank sum test, and Student’s t test. ITIH4 level between groups was compared by Kruskal–Wallis H rank sum test and adjusted by Bonferroni method. Receiver-operating characteristic (ROC) analysis was used to illustrate the performance of ITIH4 in distinguishing CD patients, UC patients, and HCs. Correlation between ITIH4 and clinical features was evaluated by Spearman’s rank correlation test. Comparison of ITIH4 level between W0 and W12 was analyzed by Wilcoxon signed-rank test, and comparison between response patients and no response patients was assessed using Wilcoxon rank sum test. Multivariable logistic regression analysis was performed to assess the factors affecting clinical response. A P value < 0.05 indicated statistical significance.
Results
Clinical features
A total of 40 A-CD patients, 40 R-CD patients, 40 A-UC patients, 40 R-UC patients, and 40 HCs were recruited in this study, whose detailed characteristics are listed in Table 1. In brief, the mean age and gender (all P > 0.05) were of no difference, but difference was observed in the median value of CRP (P < 0.001) and ESR (P < 0.001) among all subjects; meanwhile, difference was discovered in the median TNF-α (P = 0.001), IL-1β (P = 0.001), and IL-6 (P = 0.022) among A-CD, A-UC, R-CD, and R-UC patients.
ITIH4 in IBD patients and HCs
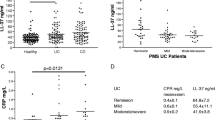
In general, difference was observed in ITIH4 among A-CD patients, R-CD patients, A-UC patients, R-UC patients, and HCs (P < 0.001). ITIH4 was lower in A-CD patients, R-CD patients, A-UC patients, and R-UC patients than in HCs (all P < 0.05). Notably, ITIH4 was decreased in A-CD patients than in R-CD patients (P = 0.017), and in A-UC patients compared with R-UC patients (P = 0.010) (Fig. 1). Besides, the receiver-operating characteristic (ROC) curve revealed that ITIH4 possessed good potential in discriminating A-CD patients from R-CD patients with area under curve (AUC) of 0.704 (95%confidence interval (CI) 0.590–0.817); moreover, ITIH4 possessed certain capability of distinguishing A-UC patients from R-UC patients, of which AUC was 0.714 (95% CI 0.600–0.829) (Fig. 2A, B).
ROC curve analysis. The ability of ITIH4 in distinguishing A-CD patients from R-CD patients, A-CD patients from HCs, and R-CD patients from HCs (A); the potential of ITIH4 in discriminating A-UC patients from R-UC patients, A-UC patients from HCs, and R-UC patients from HCs (B). A-CD, active Crohn’s disease; R-CD, Crohn’s disease in clinical remission; A-UC, active ulcerative colitis; R-UC, ulcerative colitis in clinical remission; HCs, health controls; AUC, area under curve; CI, confidence interval; ITIH4, inter-alpha-trypsin inhibitor heavy chain 4; ROC, receiver-operating characteristic
Association of ITIH4 with disease activity in IBD patients
In patients with A-CD, ITIH4 was negatively associated with CRP (r = − 0.366, P = 0.020) (Fig. 3A) and CDAI score (r = − 0.428, P = 0.006) (Fig. 3B). Meanwhile, in patients with R-CD, ITIH4 was negatively associated with CRP (r = − 0.316, P = 0.047) (Fig. 3C); however, no correlation was discovered in ITIH4 with CDAI score (r = − 0.223, P = 0.167) (Fig. 3D). Regarding patients with A-UC, ITIH4 was negatively correlated with CRP (r = − 0.331, P = 0.037) (Fig. 3E) and Mayo score (r = − 0.367, P = 0.020) (Fig. 3F); however, no association was observed in ITIH4 with CRP (r = − 0.193, P = 0.233) (Fig. 3G) or Mayo score (r = − 0.170, P = 0.293) (Fig. 3H) in patients with R-UC.
Correlation of ITIH4 with CRP and disease activity in IBD patients. Correlation of ITIH4 with CRP (A) and CDAI score (B) in A-CD patients; association of ITIH4 with CRP (C) and CDAI score (D) in R-CD patients; correlation of ITIH4 with CRP (E) and Mayo score in A-UC patients (F); association of ITIH4 with CRP (G) and Mayo score (H) in R-UC patients. A-CD, active Crohn’s disease; R-CD, Crohn’s disease in clinical remission; A-UC, active ulcerative colitis; R-UC, ulcerative colitis in clinical remission; CRP, C-reactive protein; CDAI, clinical disease activity index; ITIH4, inter-alpha-trypsin inhibitor heavy chain 4
Correlation of ITIH4 with inflammatory cytokines in IBD patients
ITIH4 was correlated with TNF-α (r = − 0.353, P = 0.026), IL-1β (r = − 0.320, P = 0.044), and IL-17A (r = − 0.339, P = 0.033) in patients with A-CD, while it was only associated with TNF-α (r = − 0.339, P = 0.032) in patients with R-CD. Besides, ITIH4 was correlated with TNF-α (r = − 0.434, P = 0.005), IL-1β (r = − 0.392, P = 0.012), IL-6 (r = − 0.327, P = 0.039), and IL-17A (r = − 0.336, P = 0.034) in patients with A-UC. No correlation was found in ITIH4 with inflammatory cytokines in patients with R-UC (all P > 0.05) (Table 2).
Changes of ITIH4 after treatment
In A-CD patients, ITIH4 was increased at W12 compared with W0 in total patients (P < 0.001) (Fig. 4A), response patients (P < 0.001) (Fig. 4B), and no response patients (P = 0.044) (Fig. 4C). Additionally, ITIH4 at W12 (P = 0.022), but not at W0 (P = 0.320), was elevated in the response patients compared with no response patients (Fig. 4D, E).
Comparison of ITIH4 before and after treatment. Comparison of ITIH4 before and after treatment in total A-CD patients (A), response A-CD patients (B), no response A-CD patients (C); comparison of ITIH4 at W0 (D) and at W12 (E) between response A-CD patients and no response A-CD patients; comparison of ITIH4 before and after treatment in total A-UC patients (F), response A-UC patients (G), no response A-UC patients (H); comparison of ITIH4 at W0 (I) and at W12 (J) between response A-UC patients and no response A-UC patients. A-CD, active Crohn’s disease; A-UC, active ulcerative colitis; W, week; ITIH4, inter-alpha-trypsin inhibitor heavy chain 4
In terms of A-UC, ITIH4 was increased at W12 than W0 in total patients (P < 0.001) (Fig. 4F) and response patients (P < 0.001) (Fig. 4G). However, no difference was found in ITIH4 between W0 and W12 in no response patients (P = 0.140) (Fig. 4H). Moreover, no difference was discovered in ITIH4 at W0 between response patients and no response patients (P = 0.552) (Fig. 4I), but ITIH4 at W12 in response patients was higher than that in no response patients (P = 0.038) (Fig. 4J).
Furthermore, according to multivariable logistic regression analysis, ITIH4 at W12 was independently correlated with better clinical response in active IBD patients (P = 0.004, odds ratio = 1.021) (Supplementary table 1).
Discussion
Until now, few studies have been performed on the expression of ITIH4 in patients with immune diseases; only one study shows that serum citrullinated ITIH4 is increased in patients with RA compared with HCs [18]. However, research on the expression of ITIH4 in patients with other immune diseases, including IBD, has not been conducted previously. This study observed that ITIH4 was declined in IBD patients than in HCs; meanwhile, it was reduced in A-CD than in R-CD patients, and in A-UC patients compared with R-UC patients. Possible reasons might be that (1) ITIH4 might downregulate neutrophilic migration by suppressing the complement cascade, subsequently modulating inflammatory responses and affecting the development of IBD [17, 23]. Therefore, ITIH4 was lower in IBD patients than in HCs; (2) ITIH4 was an anti-inflammatory protein [24], and a higher inflammatory level could cause elevated disease activity; thus, ITIH4 was lower in A-CD patients than that in R-CD patients, and in A-UC patients in comparison with R-UC patients.
Concerning the correlation of ITIH4 with disease activity and inflammatory markers in patients getting autoimmune disease, only one previous study shows that citrullinated ITIH4 negatively correlate with disease activity and inflammatory markers in RA patients [18]. However, there is no study performed on the correlation of ITIH4 with disease activity and inflammation, so we conducted this correlation analysis in our study, which discovered that ITIH4 was negatively correlated with disease activity and inflammation in patients with active IBD, while the association was slight in IBD patients at clinical remission. Possible explanations could be that (1) ITIH4 might negatively regulate the T-helper 1 and T-helper 2 cytokines, subsequently participating in anti-inflammatory activities, then modulating the inflammatory response and resulting in reduced disease activity and inflammation [16, 17]; (2) regulated by plasma kallikrein (KLKB1) via IL-6 signaling cascade, ITIH4 might inhibit the production of pro-inflammatory cytokines [16]; and (3) as inflammation in patients with remission would be milder than patients with active disease, ITIH4 was correlated with inflammation in patients with active disease, but not those with remission.
In terms of the increase of ITIH4 after treatment in patients with immune disease, no previous studies have been performed on this. In our study, we conducted research in this point and found that ITIH4 was increased in patients with active IBD after treatment; besides, ITIH4 was higher in response patients at W12, which could be explained by that ITIH4 could act as an anti-inflammatory marker; therefore, its increase meant the attenuation of inflammation. Besides, as mentioned above, ITIH4 was negatively associated with disease activity and inflammation; thus, ITIH4 was increased after treatment in IBD patients [21, 25]. These findings implied that ITIH4 might serve as a potential biomarker for evaluating the treatment response of patients with IBD.
Although a lot of findings were identified in this study, there still existed several limitations. Firstly, the sample size was relatively small, which could be expanded to improve the statistical power. Secondly, this study did not investigate the molecular mechanism of ITIH4 involved in the IBD progression; thus, in vivo and in vitro experiments might be needed. Thirdly, this study enrolled UC and CD patients, while patients with indeterminate colitis might also be included to further evaluate the correlation of ITIH4 with general disease conditions of IBD patients. Fourthly, an additional data on cohort disease control might be collected in the future study to better illustrate the correlation of ITIH4 with IBD risk. Fifthly, we did not evaluate the response to treatment by the endoscope due to lacking complete data; thus, response to treatment by the endoscope should be evaluated in the subsequent study.
In conclusion, ITIH4 correlates with IBD susceptibility, active risk, inflammation level, and its elevation after treatment relates to clinical response in IBD patients. ITIH4 might serve as a potential biomarker for predicting treatment response and improving the management of IBD patients.
References
Abraham BP, Ahmed T, Ali T (2017) Inflammatory bowel disease: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol 239:115–146
Flynn S, Eisenstein S (2019) Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am 99(6):1051–1062
Park J, Park S, Lee SA et al (2021) Improving the care of inflammatory bowel disease (IBD) patients: perspectives and strategies for IBD center management. Korean J Intern Med 36(5):1040–1048
Wallace KL, Zheng LB, Kanazawa Y, Shih DQ (2014) Immunopathology of inflammatory bowel disease. World J Gastroenterol 20(1):6–21
Mak WY, Zhao M, Ng SC, Burisch J (2020) The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol 35(3):380–389
Sood A, Ahuja V, Midha V et al (2020) Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease. Intest Res 18(4):355–378
Mitoma H, Horiuchi T, Tsukamoto H, Ueda N (2018) Molecular mechanisms of action of anti-TNF-alpha agents - comparison among therapeutic TNF-alpha antagonists. Cytokine 101:56–63
Chande N, Townsend CM, Parker CE, MacDonald JK (2016) Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 10(10):CD000545
Clarke WT, Feuerstein JD (2019) Colorectal cancer surveillance in inflammatory bowel disease: practice guidelines and recent developments. World J Gastroenterol 25(30):4148–4157
Gecse KB, Vermeire S (2018) Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol 3(9):644–653
Oligschlaeger Y, Yadati T, Houben T (2019) Inflammatory bowel disease: a stressed “gut/feeling”. Cells 8(7):659
Misselwitz B, Juillerat P, Sulz MC et al (2020) Emerging treatment options in inflammatory bowel disease: Janus kinases, stem cells, and more. Digestion 101(Suppl 1):69–82
Chandler KB, Brnakova Z, Sanda M et al (2014) Site-specific glycan microheterogeneity of inter-alpha-trypsin inhibitor heavy chain H4. J Proteome Res 13(7):3314–3329
Kashyap RS, Nayak AR, Deshpande PS et al (2009) Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta 402(1–2):160–163
Choi-Miura NH, Takahashi K, Yoda M et al (2000) The novel acute phase protein, IHRP, inhibits actin polymerization and phagocytosis of polymorphonuclear cells. Inflamm Res 49(6):305–310
Li L, Choi BC, Ryoo JE et al (2018) Opposing roles of inter-alpha-trypsin inhibitor heavy chain 4 in recurrent pregnancy loss. EBioMedicine 37:535–546
Osada A, Matsumoto I, Mikami N et al (2021) Citrullinated inter-alpha-trypsin inhibitor heavy chain 4 in arthritic joints and its potential effect in the neutrophil migration. Clin Exp Immunol 203(3):385–399
Kawaguchi H, Matsumoto I, Osada A et al (2018) Identification of novel biomarker as citrullinated inter-alpha-trypsin inhibitor heavy chain 4, specifically increased in sera with experimental and rheumatoid arthritis. Arthritis Res Ther 20(1):66
Gersemann M, Wehkamp J, Stange EF (2012) Innate immune dysfunction in inflammatory bowel disease. J Intern Med 271(5):421–428
Forbes A, Escher J, Hebuterne X et al (2017) ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 36(2):321–347
Nie J, Zhao Q (2020) Lnc-ITSN1-2, derived from RNA sequencing, correlates with increased disease risk, activity and promotes CD4(+) T cell activation, proliferation and Th1/Th17 cell differentiation by serving as a ceRNA for IL-23R via sponging miR-125a in inflammatory bowel disease. Front Immunol 11:852
Farkas K, Rutka M, Golovics PA et al (2016) Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis 10(11):1273–1278
Filippi MD (2019) Neutrophil transendothelial migration: updates and new perspectives. Blood 133(20):2149–2158
Garcia-Gil FA, Lampreave F, Fuentes-Broto L et al (2010) Inter-alpha-trypsin inhibitor heavy chain 4 as a marker of acute rejection in pancreas allotransplantation in pigs. Transplant Proc 42(8):3063–3069
Shi X, Ohta Y, Liu X et al (2019) Acute anti-inflammatory markers ITIH4 and AHSG in mice brain of a novel Alzheimer’s disease model. J Alzheimers Dis 68(4):1667–1675
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
The written informed consents were gathered from all subjects. The study was permitted by Institutional Review Board.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, N., Zhao, N., Xu, H. et al. Serum inter-alpha-trypsin inhibitor heavy chain 4 in patients with inflammatory bowel disease: correlation with disease risk, inflammation, activity, and its variation after treatment. Ir J Med Sci 191, 2105–2111 (2022). https://doi.org/10.1007/s11845-021-02837-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02837-3