Abstract
Background
Inflammatory bowel disease (IBD) has been linked to an increased risk of coronary heart disease and stroke. Dyslipidemia is a well-established risk factor for cardiovascular disease. The aim of this study was to investigate the long-term lipid profiles in a large cohort of IBD patients.
Methods
Data of patients from an IBD registry who had more than one measurement of total cholesterol and triglyceride levels during the follow-up period were analyzed. The lipid profiles of IBD patients were compared to those of the general population according to National Health and Nutrition Examination Survey (2009–2012). Quartiles of cholesterol or triglyceride levels in relation to surrogate markers of disease severity were analyzed.
Results
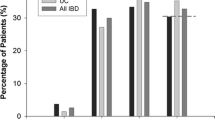
Seven hundred and one IBD patients [54 % Crohn’s disease (CD), 46 % ulcerative colitis (UC)] were included. IBD patients had less frequent high total cholesterol and high LDL cholesterol (6 vs. 13 and 5 vs. 10 %) and more frequent low HDL and high triglycerides (24 vs. 17 and 33 vs. 25 %) compared to the general population (all p < 0.001). Median total cholesterol levels were lower and median triglycerides higher in CD compared to UC (171 vs. 184; 123 vs. 100 mg/dL; both p < 0.001). In the multiple regression analysis, lipid profile was independently associated with hospitalizations (low cholesterol) and IBD surgeries (low cholesterol and high triglycerides).
Conclusions
Low total cholesterol and high triglyceride levels are more frequent in IBD patients (in particular CD) compared to healthy controls and are independently associated with more severe disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyslipidemia is a major risk factor for cardiovascular disease, the leading cause of death in the western world [1, 2]. It is defined as an abnormal amount of lipids in the blood. Causes of dyslipidemia can be primary (genetic) or secondary related to diet, lifestyle, metabolic disorders, among others causes [3]. Chronic inflammation and infection alter the metabolism of lipoproteins leading to various changes in the patient lipid profiles [4, 5]. In the classic chronic inflammatory condition, systemic lupus erythematosus (SLE), a characteristic lipid pattern known as the “lupus pattern,” has been well described and is manifested by increased triglycerides and decreased high-density lipoprotein (HDL) levels [6].
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory condition with gastrointestinal as well as systemic manifestations. Studies have described various lipid profile changes in IBD patients mainly attributed to a complex interaction of inflammatory cytokines, malnutrition, and malabsorption due to intestinal damage or resection [7]. The main lipid pattern presented by some studies include a decrease in total cholesterol and low-density lipoprotein (LDL) levels and an increase in triglyceride and HDL levels compared to healthy controls [8–11]. These changes are more profound in CD patients when compared to UC and likely reflect the bile acid and lipid malabsorption due to ileum involvement [12]. Despite the lower cholesterol levels, recent studies suggest that IBD patients have an increased risk of arterial thromboembolic events such as myocardial infarction and stroke. This is known as the “lipid paradox” in IBD [7].
Furthermore, it is suggested that total cholesterol and LDL cholesterol decrease with disease activity and they increase with IBD treatment with biologics [13]. Existing studies investigating the patterns of lipid profiles in IBD patients are limited, are characterized by small sample size and retrospective data collection, and have shown conflicting results. The aim of this study was to thoroughly describe the long-term lipid profiles of a large cohort of prospectively enrolled and followed IBD patients and investigate their correlation with disease severity.
Methods
Study Design and Patient Population
The IBD registry at the University of Pittsburgh Medical Center consists of a pool of prospectively enrolled and followed patients with a definite IBD diagnosis, based on established criteria. Starting in 2009, the registry currently includes more than 2300 well-phenotyped IBD patients with thorough demographic, clinical, laboratory, endoscopic, pathologic, and radiologic data. Furthermore, medications and treatment practices have also been abstracted prospectively [14–16]. For the purposes of the present study, patients enrolled between January 2009 and October 2014 who had more than one measurement of total cholesterol and triglyceride levels during the follow-up period were analyzed. The IBD registry includes primarily laboratory data of patients seen in the outpatient clinic (clustered data from inpatient care are not included). Data on lipid profiles of US adults aged 20 and over, as captured in the National Health and Nutrition Examination Survey (NHANES; 2009–2012) [17, 18], were utilized for comparison with our IBD cohort.
Disease location and behavior in CD and extent of bowel involvement in UC were classified according to the Montreal classification [19]. The disease activity was also prospectively evaluated using clinical activity scores such as Harvey–Bradshaw index (HBI) for CD [20] and ulcerative colitis activity index (UCAI) for UC [21]. Prospectively collected data on health-related quality of life as measured by the validated short IBD questionnaire (SIBDQ) were also analyzed [22]. Average scores of HBI, UCAI, and SIBDQ as well as average C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin, and albumin levels of the 5-year measurements were calculated and used in the analysis. Additionally, for the multiple lipid profile measurements per patient during the follow-up period, average levels of total cholesterol, LDL, HDL, and triglycerides were included.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile range (IQR), and categorical variables are presented as proportions. Normality of continuous data was assessed using the Kolmogorov–Smirnov test. Comparisons between two groups were made with the Pearson Chi-square test for categorical variables; Student’s t test and Mann–Whitney test were used for continuous variables. Comparisons between multiple groups were made with Chi-square trend test and ANOVA or Kruskal–Wallis test, respectively. A two-sided p value of <0.05 was considered statistically significant.
In our analysis, patients were stratified into quartiles based on their average total cholesterol and triglyceride levels. Quartiles of patients were compared in regard to main demographics (age, gender, BMI), inflammatory markers (CRP, ESR), and management practices (medications, surgery, hospitalizations). A multiple regression model was utilized to investigate the independent association of low total cholesterol levels (Q1 vs. Q2, Q3, Q4) and high triglyceride levels (Q4 vs. Q1, Q2, Q3) with the variables being significantly different among quartiles in the univariate analysis. Patients on statins were not included in the above analysis.
Ethical Considerations
The Ethics Committee at the University of Pittsburgh has approved participation of subjects in the IBD research registry (PRO12110117).
Results
Seven hundred and one patients in our registry had a definite IBD diagnosis [380 (54 %) CD and 321 (46 %) UC] along with at least two measurements of total cholesterol and triglyceride levels. Patient demographics, clinical characteristics, disease location/phenotype per Montreal classification, as well as main treatment modalities in CD and UC patients are presented in Table 1. Out of 701 IBD patients, 341 (49 %) were male. Median age was 30 years (IQR 21, 43) and median BMI 26 kg/m2 (IQR 23, 30).
High median total cholesterol (≥240 mg/dL) was noted in 44 patients (6 %) [median HDL < 40 mg/dL in 168/694 (24 %); median LDL ≥ 160 mg/dL in 31/658 (5 %)], and high median triglycerides (≥150 mg/dL) in 231 (33 %). When compared to the general US population, as captured in the NHANES 2009–2012 database, IBD patients had lower rates of elevated total cholesterol [44/701 (6 %) vs. 1214/9338 (13 %); p < 0.001] and lower rates of an elevated LDL cholesterol levels [31/658 (5 %) vs. 484/4745 (10 %); p < 0.001]. IBD patients were noted to more frequently have higher rates of low HDL [168/694 (24 %) vs. 1587/9338 (17 %); p < 0.001] and high triglycerides when compared to the general population [231/701 (33 %) vs. 1225/4881 (25.1 %); p < 0.001].
Serum Biomarkers and Lipid Profiles
Table 2 compares median levels of serum biomarkers and lipids between CD and UC patients. Inflammatory markers, namely CRP and ESR, as well as median albumin levels were similar between CD and UC. Median hemoglobin levels were lower in patients with CD (13.3 mg/dL) when compared to UC patients (13.5 mg/dL; p = 0.045).
Median total cholesterol levels measured 171 mg/dL in CD patients and 184 mg/dL in UC patient (p < 0.001). Median LDL levels were 92 mg/dL in CD vs. 107 mg/dL in UC (p < 0.001), and median HDL levels were 48 vs. 50 mg/dL, respectively (p = 0.054). Median triglyceride levels were higher in CD patients (123 mg/dL) than in UC patients (100 mg/dL; p < 0.001).
With regard to clinical activity scores and health-related quality of life, no significant correlation was found between median lipid levels and HBI or UCAI, as well as SIBDQ (all p > 0.05).
Clinical Characteristics by Quartiles of Total Serum Cholesterol and Triglyceride Levels
Initially, we compared clinical characteristics of IBD patients treated with statins versus those who did not receive this therapy. No statistically significant differences were noted regarding history of IBD surgery (34 vs. 44 %), median CRP levels (0.41 vs. 0.41 mg/dL), use of immunomodulators (40 vs. 47 %), biologics (26 vs. 36 %), steroids (44 vs. 50 %), and median days of hospitalizations (0 vs. 1) between the two groups (all p > 0.05).
After excluding patients on statins (n = 149), we stratified the remaining IBD patients (n = 552) into quartiles based on their median total serum cholesterol and triglyceride levels. Main demographic, clinical characteristics, and treatment practices between quartiles of IBD patients were compared as shown in Tables 3 and 4.
Median age at diagnosis and median BMI increased with higher cholesterol levels (p = 0.002 and 0.004, respectively). In contrast, male gender (p < 0.001), history of surgery for IBD (p < 0.001), use of biologics (p < 0.001), and number of hospitalizations (p < 0.01) were more commonly associated with lower cholesterol levels. No significant difference was noted in median CRP levels, use of immunomodulators, and use of steroids between quartiles (Table 3). Additionally, quartiles were similar with regard to ESR, albumin, hemoglobin, as well as HBI, UCAI, and SIBDQ indices.
When IBD patients categorized by their median triglyceride levels were compared, median BMI, history of IBD surgery, median CRP levels, and number of hospitalizations all increased with higher triglycerides (p = 0.04). Median age at diagnosis, gender, and use of immunomodulators, biologics, or steroids were similar among quartiles (Table 4). Median ESR, albumin, hemoglobin, as well as HBI or UCAI, SIBDQ, use of bile acid sequestrants or levothyroxine also did not differ between quartiles.
In the multiple regression model, controlling for age, gender, BMI, IBD surgery, use of biologics, and number of hospitalizations, low median total serum cholesterol levels (≤149 mg/dL) were independently associated with male gender (p = 0.001), low BMI (p = 0.001), more IBD-related surgeries (p = 0.002), and more hospitalizations (p = 0.04).
In a similar multivariate analysis controlling for age, gender, BMI, IBD surgery, median CRP levels, and number of hospitalizations, high median serum triglyceride levels (≥160) were independently associated with older age (p = 0.047), male gender (p = 0.003), higher BMI (p < 0.001), and more IBD surgeries (p < 0.001).
Discussion
To our knowledge, this is the largest study in the USA reporting long-term changes in lipid profiles of prospectively enrolled and followed IBD patients and investigating their correlation with disease severity. We showed that IBD patients have lower total cholesterol and higher triglyceride levels when compared to the general US population. This lipid pattern is more prominent in CD than in UC patients. Furthermore, we found that median total serum cholesterol levels ≤149 mg/dL and median serum triglyceride levels ≥160 were independently associated with more severe IBD as reflected by higher number of IBD-related surgeries and hospitalizations.
Lipid profile alterations in IBD patients have been presented in previous studies. First, Becker et al. [11] showed an association of lower cholesterol and higher triglyceride levels with intestinal resections in CD patients. In 2000, Levy et al. [8] reported lower total cholesterol and higher triglyceride levels in 22 children with CD compared to 10 healthy controls. Piquer et al. [9] showed the same lipid pattern in 21 adults with IBD but also with more prominent alterations during the active phase of disease where inflammation leads to reduced cholesterol efflux from the cells. Finally, in 2010 a US study by Sappati Biyyani et al. [12] concluded that IBD patients have lower total and HDL cholesterol and higher LDL cholesterol and triglycerides when compared to controls from the NHANES 2005–2006 database. Our results are in agreement with these findings adding the significant association that we found between lipid profile changes and surrogate markers of disease severity. Furthermore, our findings were based on multiple lipid profile measurements spanning over a multiyear follow-up period. This supports that the described lipid pattern in IBD patients is not only noted during a single hospitalization or disease flare but persists over time.
Regarding the effect of IBD medications on lipid profile, treatment with infliximab increased total cholesterol and HDL levels in a study of 22 IBD patients [15]. However, a subsequent study on 128 IBD patients receiving anti-TNF agents (infliximab or adalimumab) showed no significant changes in lipid profile after 1 and 3 years of treatment [23]. The present study also does not support any independent effect of IBD treatment on patients’ lipid profile.
Use of statins for dyslipidemia has recently been associated with reduced use of steroids in IBD patients, suggesting a potential protective role of statins [24]. In our study, statin use tended to decrease the steroid and anti-TNF use, hospitalizations, and IBD surgeries but failed to show a protective role since the results were not statistically significant.
Bowel resection in IBD patients and especially in CD is considered to have an impact on lipid metabolism [11]. However, in a series of 24 CD patients by Romanato et al. [25], it was demonstrated that lipid profile 6 months after intestinal resection was stable and only an increase in HDL cholesterol was observed. Interestingly, in the same study, CD recurrence, but not the extent of bowel resection, was the main predictor of changes in lipid profile. In our study, IBD-related surgery was the most important factor associated with changes in lipid profile, but we cannot conclude whether this effect is due to intestinal resection or to the disease severity, although it is reasonable to suggest that both mechanisms are involved.
Plasma triglycerides are mediated by apolipoprotein C-III and play an important role in atherogenesis [26]. The inflammation-related hypertriglyceridemia is caused by increased hepatic lipoprotein production and decreased lipoprotein clearance. Our data suggest that in IBD patients, there is a hypertriglyceridemic state that is associated with disease severity and is possibly linked to the increased cardiovascular risk.
Many studies have shown that chronic inflammation is linked to changes in lipid profile and cardiovascular events. This can be explained by the down-regulation of the lipolytic enzyme activity from the inflammatory cytokines including tumor necrosis factor, interleukin-6, and interferon-γ. In IBD, the pathophysiology behind altered lipid profiles is complex given that besides chronic inflammation, malnutrition and lipid malabsorption are involved as well. Total and HDL cholesterol have been found to be inversely associated with CRP levels in IBD [27]. Although our study showed a significant association of low total cholesterol and high triglyceride levels with surrogate markers of disease severity, including IBD-related surgeries and number of patient hospitalizations, it failed to present any association with increased levels of inflammatory biomarkers (CRP, ESR, albumin). This is likely explained by the fact that we included median levels of the above biomarkers in our analysis, measured during both disease flare and disease remission.
The reported increased risk of cardiovascular events in IBD along with lower cholesterol levels and chronic inflammation reflect a complicated and yet poorly understood pathogenic mechanism. This is described by the term “lipid paradox.” Better understanding of dyslipidemia in IBD will help to prevent and manage atherosclerosis and cardiovascular events, a common cause of morbidity and mortality in IBD.
This study has potential limitations. Firstly, our center is a tertiary referral center treating complex IBD cases which may predispose to a selection bias to our study. Second, lipid profile measurements were taken based on the discretion of the treating physicians. Therefore, not all of our IBD patients have their serum lipid levels measured, which may pose further biases. The lack of control group (without IBD) from the same institution is also an important limitation. On the other hand, the present study has numerous strengths. These include the large cohort size of IBD patients and the investigation of long-term lipid profiles, instead of point measurements, showing more prominent alterations over time. Furthermore, all patients in our cohort were prospectively enrolled, well phenotyped, and followed over a multiyear time period.
In conclusion, the present study is the largest study to date showing that IBD patients have long-term lower HDL cholesterol and elevated triglyceride levels when compared to healthy controls. This pattern of serum lipids is more prominent in CD than in UC patients and is a potential contributing factor to the increased cardiovascular risk observed in IBD. Finally, low total cholesterol levels and high triglyceride levels are independently associated with more severe disease as reflected by higher number of IBD surgeries and hospitalizations. Further studies are needed to investigate the role of this dyslipidemia and its relationship with cardiovascular complications in IBD patients.
References
Buchwald H, Rudser KD, Williams SE, et al. Overall mortality, incremental life expectancy, and cause of death at 25 years in the program on the surgical control of the hyperlipidemias. Ann Surg. 2010;251:1034–1040.
Adhyaru BB, Jacobson TA. New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines with the 2014 National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia. Cardiol Clin. 2015;33:181–196.
Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation. 1965;31:321–327.
Robertson J, Peters MJ, McInnes IB, et al. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513–523.
Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults, 1988–2010. Jama. 2012;308:1545–1554.
Borba EF, Carvalho JF, Bonfa E. Mechanisms of dyslipoproteinemias in systemic lupus erythematosus. Clin Dev Immunol. 2006;13:203–208.
Singh S, Kullo IJ, Pardi DS, et al. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26–35.
Levy E, Rizwan Y, Thibault L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–815.
Ripolles Piquer B, Nazih H, Bourreille A, et al. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism. 2006;55:980–988.
Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med. 1990;323:946–955.
Becker SA, McClave SA. Lipid profiles in Crohn’s disease patients with and without ileal resection. Am J Gastroenterol. 1996;91:2452.
Sappati Biyyani RS, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol. 2010;4:478–482.
Koutroubakis IE, Oustamanolakis P, Malliaraki N, et al. Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:283–288.
Koutroubakis IE, Ramos-Rivers C, Regueiro M, et al. Persistent or recurrent anemia is associated with severe and disabling inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:1760–1766.
Koutroubakis IE, Ramos-Rivers C, Regueiro M, et al. The influence of anti-tumor necrosis factor agents on hemoglobin levels of patients with inflammatory Bowel disease. Inflamm Bowel Dis. 2015;21:1587–1593.
Ramos-Rivers C, Regueiro M, Vargas EJ, et al. Association between telephone activity and features of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:986–94.e1.
Carroll MD, Kit BK, Lacher DA. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009–2010. NCHS Data Brief. 2012;1–8.
Carroll M, Kit B, Lacher D. Trends in elevated triglyceride in adults: United States, 2001–2012. NCHS Data Brief. 2015;198.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5a–36a.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514.
Kozarek RA, Patterson DJ, Gelfand MD, et al. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356.
Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96:2921–2928.
Miranda-Bautista J, de Gracia-Fernández C, López-Ibáñez M, et al. Lipid profile in inflammatory bowel disease patients on anti-TNFα therapy. Dig Dis Sci. 2015;60:2130–2135.
Crockett SD, Hansen RA, Sturmer T, et al. Statins are associated with reduced use of steroids in inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2012;18:1048–1056.
Romanato G, Scarpa M, Ruffolo C, et al. Lipid and phospholipid profile after bowel resection for Crohn’s disease. Int J Colorectal Dis.. 2008;23:931–938.
Kohan AB. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2015;22:119–125.
Romanato G, Scarpa M, Angriman I, et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment Pharmacol Ther. 2009;29:298–307.
Acknowledgments
Michael A. Dunn and David G. Binion were supported by a Grant [W81XWH-11-2-0133] from the US Army Medical Research and Material Command. Ioannis Koutroubakis was supported by a sabbatical salary of Medical Faculty University of Crete Greece.
Author contributions
Efstratios Koutroumpakis acquired, analyzed, and interpreted the data and drafted the manuscript. Claudia Ramos Rivers acquired and analyzed the data, critically revised the manuscript, and provided administrative and technical support. Miguel Regueiro, Jana G. Hashash, Arthur Barrie, III, Jason Swoger, Leonard Baidoo, Marc Swartz, Michael Dunn, and Ioannis E. Koutroubakis acquired the data, critically revised the manuscript, and supervised the study. David G. Binion was a mentor and contributed to study concept, interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest regarding this manuscript.
Rights and permissions
About this article
Cite this article
Koutroumpakis, E., Ramos-Rivers, C., Regueiro, M. et al. Association Between Long-Term Lipid Profiles and Disease Severity in a Large Cohort of Patients with Inflammatory Bowel Disease. Dig Dis Sci 61, 865–871 (2016). https://doi.org/10.1007/s10620-015-3932-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3932-1