One new natural product, cadinane-type sesquiterpenoid, (±)-(2S*,3S*)-dihydroxy-α-corocalene (1), has been isolated from the endophytic fungus Curvularia intermedia, derived from the plant Oryza sativa. The structure was determined using spectroscopic methods, mainly 1D and 2D NMR, and comparison of the spectroscopic data with those reported for structurally related compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endophytic fungi or bacterial microorganisms living in the intracellular spaces of plants are thought to possess different biological properties, and are expected to produce novel and bioactive substances [1, 2]. As they live in a special ecoenvironment, endophytic fungi seem to be especially fruitful producer of biologically active secondary metabolites with which they probably regulate their interactions with their hosts [3,4,5,6,7,8]. Many endophytic fungi have proven to be a rich source of bioactive secondary metabolites, which are of interest for proper medicinal or agrochemical applications. An endophytic fungal strain, collected from Nan-tou County, Taiwan, was determined to be Curvularia intermedia (Pleosporaceae) based on the cultural and anamorphic data. In the course of screening for biologically and chemically novel agents from Formosan fungal materials, Curvularia intermedia was chosen for further phytochemical investigation. To our knowledge this is also being studied and published for the first time. The culture broth of the fungus was subjected to solvent partitioning and chromatographic separation to one racemate cadinane compound while being isolated from a natural source for the first time. The structure was determined using spectroscopic methods, mainly 1D and 2D NMR.
Compound 1 was isolated as a brown gum. The molecular formula was assigned as C15H20O2 according to its 13C NMR spectroscopic data and the HR-ESI-MS ion at m/z 214.1358 [M – H2O]+ (calcd for C15H18O, 214.1350). The UV spectrum of 1 showed characteristic absorptions at 222 (4.89), 258 (2.97), 280 (3.79) nm, which are typical for cadinane derivatives [9]. The 1H NMR data of 1 (Table 1) showed the resonances of a typical moiety of isopropyl attached to the benzene ring [δ 1.19, 1.22 (each 3H, d, J = 6.8 Hz, H-12, 13), 3.26 (1H, sept., J = 6.8 Hz, H-11)], an aromatic Me singlet at δ 2.42 (1H, s, Me-14), a methyl singlet attached to a double bond at δ 2.08 (3H, s, Me-15), and two o-proton signals of tetrasubstituted benzene ring [δ 7.01 (1H, d, J = 8.0 Hz, H-9) and 7.14 (1H, d, J = 8.0 Hz, H-8)].
According to the carbon spectrum (13C NMR) and DEPT, in addition to the isopropyl group and the two methyl groups, there are two carbons at δ 69.2 (C-2) and δ 71.9 (C-3) as tertiary carbons with oxygen. Calculating the degree of unsaturation, subtracting a double bond and a benzene ring, there is one left; thus, it can be determined that there is still one ring. Based on the above spectral data, it is speculated that 1 might belong to α-corocalene analog [9].
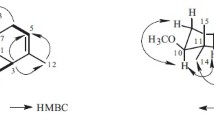
Using the 1H NMR and 13C NMR of the known compound α-corocalene to compare them with those of 1, two more oxygen-containing tertiary carbons can be found. In order to better determine the structural relevance and relative stereo orientation of 1, two-dimensional heteronuclear correlation spectroscopy (heteronuclear single quantum coherence [HSQC], heteronuclear multiple bond correlation [HMBC]) and nuclear Overhauser effect spectroscopy (NOESY) were performed for analysis. In the HMBC experiment, the cross-peaks from H-5 (δ 6.66)/CH3-15 (δ 2.08) to C-3 (δ 71.9), and from the δ 4.90 (H-2) to C-3, C-6, and C-10 placed the OH group at C-2 (69.2), and another hydroxy group at C-3, respectively. According to the signals from NOESY: (1) H-2 is related to CH3-14; (2) H-3 is related to CH3-15. With the coupling constant (J = 2.1 Hz), it is known that both H-2 and H-3 are located in the equatorial orientation. The planar structure of 1 was elucidated as 2,3-dihydroxy-α-corocalene. The relative configuration of 1 was assigned as (2β,3α) from the NOESY experiment.
Compound 1 showed zero optical activity with [α]25D ±0° (c 0.09, CHCl3). The 1H and 13C NMR chemical shifts are given in Table 1, and key HMBC and NOESY correlations are shown in Fig. 1. This is the first isolation of the racemic 1 from a natural source, although the (–)-(2β,3α)-α-corocalene-2,3-diol had been reported [10]. Accordingly, the structure of 1 was determined as (±)-(2S,3S)-7-isopropyl-4,10-dimethyl-2,3-dihydronaphthalene-2,3-diol and designated as (±)-(2S*,3S*)- dihydroxy-α-corocalene.
Experimental
General Experimental Procedures. Optical rotation was measured on a Jasco P-1020 digital polarimeter, UV spectra were obtained on a Jasco UV-240 spectrophotometer in MeOH, and IR spectra (KBr or neat) were taken on a Perkin-Elmer System 2000 FT-IR spectrometer. 1D (1H, 13C, DEPT) and 2D (COSY, NOESY, HSQC, HMBC) NMR spectra using CDCl3 as a solvent were recorded on a Varian VNMRS-600 (600 MHz for 1H NMR, 150 MHz for 13C NMR) spectrometer. Chemical shifts were internally referenced to the solvent signals in CDCl3 (1H, δ 7.26; 13C, δ 77.0) with TMS as the internal standard. Low-resolution ESI-MS spectra were obtained on an API 3000 (Applied Biosystems); and high-resolution ESI-MS spectra, on a Bruker Daltonics APEX II 30e spectrometer. Low-resolution EI-MS spectra were recorded on a Quattro GC/MS spectrometer with a direct inlet system. Silica gel (70–230, 230–400 mesh) (Merck) was used for column chromatography, and silica gel 60 F-254 (Merck) was used for TLC and preparative TLC.
Fungus Material. Curvularia intermedia (Pleosporaceae) was used throughout this study, and specimens deposited at the Bioresource Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (FIRDI).
Extraction and Isolation. The research material was maintained on potato dextrose agar (PDA) and the strain was cultured on PDA slants at 25°C for 7 days and then the spores were harvested by sterile water. The spores (5 × 105) were seeded into 300-mL shake flasks containing 50 mL RGY medium (3% rice starch, 7% glycerol, 1.1% polypeptone, 3% soybean powder, 0.1% MgSO4, 0.2% NaNO3), and cultivated with shaking (150 rpm) at 25°C for 7 days. The culture was filtered to separate broth and mycelia. The culture broth was extracted with n-BuOH (6 × 10 L) twice. The combined organic layer was concentrated under vacuum to afford 1.2 g of residue. The crude extract was separated into three fractions by column chromatography on RP-18 silica gel, eluted by methanol–H2O (0:100, 50:50, and 100:0). Fraction 3 (650 mg) was subjected to silica gel CC (step gradient, elution with 0–10% MeOH in CH2Cl2) to afford 1 (0.9 mg).
(±)-(2S*,3S*)-Dihydroxy-α-corocalene (1), gum, [α]25D ±0° (c 0.09, CHCl3). UV (λmax, nm) (log ε): 222 (4.89), 258 (2.97), 280 (3.79). IR (neat, νmax, cm–1): 3376 (OH), 1654, 1450 (aromatic). EI-MS (70 eV) (m/z, Irel., %): 232 (9), 214 [M – H2O]+ (50), 200 (81), 191 (84), 91 (100), 57 (75). HR-EI-MS m/z 214.1358 ([M – H2O]+ (calcd for C15H18O, 214.1350). 1H NMR (600 MHz, CDCl3, δ, ppm, J/Hz) and 13C NMR (150 MHz, CDCl3, δ, ppm), see Table 1.
References
G. A. Strobel and D. M. Long, ASM News, 64, 263 (1998).
G. A. Strobel, Crit. Rev. Biotechnol., 22, 315 (2002).
G. Strobel, B. Daisy, U. Castillo, and J. Harper, J. Nat. Prod., 67, 257 (2004).
L. P. Bush, H. H. Wilkinson, and C. L. Schardl, Plant Physiol., 114, 1 (1997).
R. X. Tan and W. X. Zou, Nat. Prod. Rep., 18, 448 (2001).
F. B. Sean, M. B. Shana, and C. Jon, J. Am. Chem. Soc., 123, 9900 (2001).
C. Lu and Y. Shen, J. Antibiot., 56, 415 (2003).
R. X. Tan and W. X. Zou, Nat. Prod. Rep., 18, 448 (2001).
K. S. Ngo, K. K. Cheung, and G. D. Brown, J. Chem. Res., 1998, 80 (1998).
C. F. Chyu, M. R. Ko, Y. S. Chang, S. C. Chang, and Y. H. Kuo, Helv. Chim. Acta, 90, 1514 (2007).
Acknowledgment
This work was kindly supported by the Food Industry Research and Development Institute (FIRDI) and supported by Ministry of Science and Technology, R.O.C. (MOST-108-2320-B-080-002-). The authors also thank Senior Technician Mrs. Chyi Jia Wang of Center for Resources, Research and Development (CRRD) of Kaohsiung Medical University for measuring the 2D NMR data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 4, July–August, 2022, pp. 556–557.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, MJ., Wu, MD., Hsieh, SY. et al. Isolation of One Sesquiterpenoid from the Endophytic Fungus Curvularia intermedia. Chem Nat Compd 58, 653–655 (2022). https://doi.org/10.1007/s10600-022-03763-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03763-1