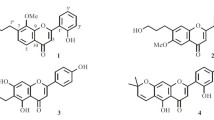
Two new flavones, 8,4′-dimethoxy-6-(2-oxopropyl)-flavone (1) and 8,4′-dimethoxy-6-(3-hydroxypropyl)- flavone (2), were isolated from the seeds of Arctium lappa. Their structures were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. Compounds 1 and 2 were evaluated for their anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity. The results showed that compounds 1 and 2 exhibited weak active with diameter of inhibition zone (IZD) 10.8 ± 1.0 and 9.3 ± 0.8 mm, respectively. Compounds 1 and 2 were also tested for antioxidant activity, and they show good antioxidant activity with IC50 values of 3.85 and 3.47 μg/mL, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Burdock (Arctium lappa) is a biennial herb plant belonging to the genus Arctium. It is also called niubang in Chinese and gobo in Japanese [1, 2]. This plant has been cultivated in Eastern Asia (particularly in China, Japan, and Korea) as a root vegetable or traditional medicinal plant for centuries and remains popular [3]. Its seeds (known as niubangzi) have been widely used in traditional Chinese medicine as a diuretic, diaphoretic, and blood purifying agent [4].
Previous studies have shown the presence of lignans [5,6,7], terpenoids [8, 9], phenolic acids [10,11,12], flavonoids [13, 14], and the like in this plant. Flavones are a class of flavonoids based on the backbone of 2-phenylchromen-4-one mainly found in cereals, herbs, and microorganisms [15]. The capacity of flavones to act as antioxidants and their role in the prevention of coronary heart diseases are the most important actions of flavones [16]. The other actions are those against bacteria, fungal ulcers, viruses, inflammation, arthritis, osteoporosis, and diarrhea [17].
In our continuing efforts to identify bioactive natural products from traditional Chinese medicine, we now investigate the chemical constituents of the seeds of Arctium lappa. This leads to the isolation of two new flavones (1 and 2). The structures of 1 and 2 were elucidated by spectroscopic methods, including extensive 1D and 2D NMR techniques. The structure elucidation of 1 and 2 and biological evaluation of their anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity and antioxidant activity are described in this paper.
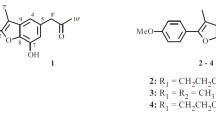
The seeds of Arctium lappa were extracted with 95% methanol followed by repeated column chromatography on silica gel, Sephadex LH-20, and RP-18 silica gel. Final purification by semipreparative RP-HPLC afforded two new flavones, 8,4′-dimethoxy-6-(2-oxopropyl)-flavone (1) and 8,4′-dimethoxy-6-(3-hydroxypropyl)-flavone (2). The structures of 1 and 2 are shown in Fig. 1, and the 1H and 13C NMR data of 1 and 2 are given in Table 1.
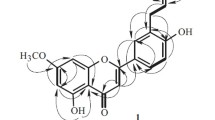
Compound 1 was obtained as a yellow gum. It has the molecular formula C20H18O5 from HR-ESI-MS (m/z 361.1058 [M + Na]+, calcd 361.1052). Its IR spectral data showed the presence of carbonyl (1728 and 1662 cm–1) and phenyl groups (1610, 1532, and 1441 cm–1). The UV absorptions at 362, 265, and 210 nm also showed the existence of a substituted aromatic ring. The 1H and 13C NMR spectrum of 1 (Table 1) along with analysis of the DEPT spectra displayed 20 carbon signals and 18 proton signals, respectively, corresponding to a 1,2,3,5-tetrasubstituted aromatic ring (C-5–C-10; H-5 and H-7), a 1,4-disubstituted aromatic ring (C-1′–C-6′; H2-2′, 6′, and H2-3′, 5′), an α,β-unsaturated ketone (C-2 and C-3; H-3), a 2-oxopropyl group (CH3-CO-CH2-, C-1′′–C-3′′, H2-1′′, and H3-3′′) [18], and two methoxy groups (δC 56.2 q and 56.0 q, δH 3.83 s and 3.80 s). The typical NMR signal of two aromatic rings and the α,β-unsaturated ketone should form a flavone nucleus [19]. This was also supported by the HMBC correlations (Fig. 1) from H-3 to C-2, C-4, C-10, and C-1′, from H-5 to C-4, C-9, C-10, from H-7 to C-9, and from H-2′, 6′ to C-2. The nucleus of the compound had been determined, and the additional signals of the 2-oxopropyl group and two methoxy groups accounted for the remaining substituents. The HMBC correlations of two methoxy protons (δ 3.83, 3.80) with C-8 (δ 155.7) and C-4' (δ 161.4) suggested the attachment position of the two methoxy groups at C-8 and C-4′, respectively. The 2-oxopropyl group located at C-6 was supported by the HMBC correlations from H2-1′′ (δ 3.53) to C-5 (δ 122.2), C-6 (δ 130.5), and C-7 (δ 118.3) and from H-5 (δ 7.16) and H-7 (δ 6.78) to C-1′′ (δ 48.7). Furthermore, the typical proton signals (H-5, H-7, H2-2′, 6′, and H2-3′, 5′) also supported a 6,8-disubstitution for ring A and a 4′-monosubstitution for ring B. The structure of 8,4′-dimethoxy-6-(2-oxopropyl)-flavone (1) was therefore established as shown in Fig. 1.
The 1H and 13C NMR spectra of 8,4′-dimethoxy-6-(3-hydroxypropyl)-flavone (2) were similar to those of 1. The marked differences between them were due to the absence of a 2-oxopropyl group signal and the appearance of a 3-hydroxypropyl group signal (-CH2-CH2-CH2-OH, C-1′′–C-3′′, H2-1′′–H2-3′′) [20] in compound 2. This change indicated that the 2-oxopropyl group in 1 was replaced by a 3-hydroxypropyl group in compound 2. The HMBC correlations of H-1′′ (δ 2.73) with C-5 (δ 122.0), C-6 (δ 132.8), and C-7 (δ 117.5), of H-2′′ (δ 1.90) with C-6 (δ 132.8), and of H-5 (δ 7.19) and H-7 (δ 6.82) with C-1′′ (δ 28.7) supported this 3-hydroxypropyl group's location at C-6. In addition, the other substituent positions were also determined by further analysis of its HMBC correlations. Thus, the structure of 2 was determined as shown.
Compounds 1 and 2 were screened for anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) activity [21] according to an arbitrary criterion with diameter of inhibition zone (IZD) as follows: very weak inhibition (IZD 6–8 mm), weak inhibition (IZD 8–12 mm), good inhibition (IZD 12–16 mm), and strong inhibition (IZD > 16 mm). The IZD of the positive control was 32 mm and that of the negative control was zero. The results revealed that compounds 1 and 2 showed weak inhibition with IZD 10.8 ± 1.0 and 9.3 ± 0.8 mm, respectively.
Compounds 1 and 2 were also tested for antioxidant activity by detection of the oxidative products with the 2′,7′-dichlorofluorescein diacetate (DCFH) method reported previously [22]. The results revealed that compounds 1 and 2 show good antioxidant activity with IC50 values 3.85 and 3.47 μg/mL, respectively.
Experimental
General. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer. A Tenor 27 spectrophotometer was used for scanning IR spectroscopy with KBr pellets. 1D and 2D spectra were run on Bruker DRX-500 spectrometers with TMS as internal standard. Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. ESI-MS spectrum was tested on a Bruker HCT or Esquire spectrometer. HR-EI-MS spectrum was acquired on a Waters AutoSpec Premier P776 spectrometer. An Ez instrument was used for middle-performance liquid chromatography (MPLC) performed on a Lisui EZ Purifier III System including pump manager P03, detector modules P02, and fraction collector P01 (Shanghai Li Sui Chemical Engineering Co., Ltd., China) and columns packed with MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Japan). Semipreparative HPLC was performed on an Agilent 1200 liquid chromatograph with a ZorbaxSB-C18 (5 μm, 9.6 . 250 mm) column. Column chromatography (CC) was performed on Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, P. R. China) and Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd.). Fractions were monitored by TLC (GF 254, Qingdao Haiyang Chemical Co., Ltd., Qingdao, P. R. China), and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.
Plant Material. The plant material was purchased from the Juhuacun Chinese Traditional Medicine Market, Kunming, Yunnan Province, P. R. China, in February 2016 and identified by Prof. Xiao Cheng, Kunming Institute of Botany, Chinese Academy of Sciences. A voucher specimen (No. KIB-21702) has been deposited in Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and Isolation. The samples (2.2 kg) were crushed to 30 mesh, and the powders were extracted with 95% aqueous MeOH (4 . 4 L) at room temperature and filtered. The filtrate was evaporated under reduced pressure, and the crude extract (136 g) was applied to a silica gel (150–200 mesh) column eluted with chloroform–methanol (CHCl3–MeOH) gradients (20:1, 9:1, 8:2, 7:3, 6:4, 5:5) to afford six fractions (Frs. A–F). Further separation of Fr. C (8:2, 10.6 g) by silica gel column chromatography, eluted with chloroform–acetone (9:1–1:2), yielded subfractions C1–C6. Subfraction C3 (7:3, 2.87 g) was loaded onto another silica gel column using petroleum ether–ethyl acetate elution, followed by semipreparative HPLC (52% MeOH–50%H2O, flow rate 3.0 mL/min) to afford 1 (12.6 mg) and 2 (16.4 mg).
8,4′-Dimethoxy-6-(2-oxopropyl)-flavone (1). Obtained as yellow gum. UV (MeOH, λmax, nm) (log ε): 210 (4.10), 265 (3.85), 362 (3.81). IR (KBr, νmax, cm–1): 3122, 2942, 2869, 1728, 1662, 1610, 1532, 1441, 1357, 1152, 1058, 863, 784. For 1H and 13C NMR data (500 and 125 MHz, CDCl3), see Table 1. ESI-MS m/z 361 [M + Na]+; HR-ESI-MS m/z 361.1058 [M + Na]+ (calcd for C20H18NaO5, 361.1052).
8,4′-Dimethoxy-6-(3-hydroxypropyl)-flavone (2). UV (MeOH, λmax, nm) (log ε): 356 (3.75), 254 (3.88), 210 (4.21). IR (KBr, νmax, cm–1): 3425, 3097, 2948, 2854, 1660, 1612, 1547, 1460, 1342, 1150, 1060, 884, 763. For 1H and 13C NMR data (500 and 125 MHz), see Table 1. ESI-MS m/z 363; HR-ESI-MS m/z 363.1202 [M + Na]+ (calcd for C20H20NaO5, 363.1208).
Anti-MRSA Agar Disc Diffusion Assay. The MRSA strain ZR11 was clinically isolated from infectious samples of critically ill patients in the Clinical Laboratory of the First People′s Hospital of Yunnan Province and confirmed by standard cefoxitin disk diffusion test following CLSI standard procedures [21]. The anti-MRSA activity of the compounds was evaluated via the disc diffusion method. The ZR11strain was inoculated in Mueller Hinton Broth and incubated at 37°C for 24 h. The turbidity of bacterial suspension was adjusted to 0.5 McFarland standard, which equals 1.5 × 108 colony-forming units (CFU)/mL. Sterile filter paper discs (6 mm) were impregnated with 20 mL (50 mg) of each compound and placed on inoculated Mueller Hinton agar containing bacterial suspension adjusted to 0.5 McFarland standard. Commercially available discs containing 30 mg vancomycin were used as positive control, whereas discs without samples (5% DMSO) served as negative control. The inhibition zones including the diameter of the disc (mm) were measured and compared after incubation at 37°C for 24 h. The tests were carried out in triplicate for each sample.
Antioxidant Activity Assay. Antioxidant activity was determined by detection of the oxidative products with the 2′,7′-dichlorofluorescein diacetate (DCFH) method reported previously [22].
References
R. Lin and Z. Shi, Flora of China, Vol. 78, Chinese Science Press, Beijing, 1987, p. 58.
T. Ozawa, J. Pharm. Soc. Jpn., 16, 551 (1952).
M. Abe, K. Ueno, Y. Ishiguro, T. Omori, S. Onodera, and N. Shiomi, J. Appl. Glycosci., 56, 239 (2009).
Y. S. Chan, L. N. Cheng, J. H. Wu, E. Chan, Y. W. Kwan, S. M. Lee, G. P. Leung, P. H. Yu, and S. C. Wan, Inflammopharmacology, 19, 245 (2011).
T. Matsumoto, K. Hosononishiyama, and H. Yamada, Planta Med., 72, 276 (2005).
Y. N. Yang, X. Y. Huang, Z. M. Feng, J. S. Jiang, and P. C. Zhang, J. Agric. Food Chem., 63, 7958 (2015).
C. Lou, Z. Zhu, Y. Zhao, R. Zhu, and H. Zhao, Oncol. Rep., 37, 179 (2017).
J. M. Yoo, J. H. Yang, H. J. Yang, W. K. Cho, and J. Y. Ma, Int. J. Mol. Med., 37, 501 (2016).
I. Iochkova, Dokl. Boly. Akac. Nauk., 42, 43 (1989).
X. W. Jiang, J. P. Bai, Q. Zhang, X. L. Hu, X. Tian, J. Zhu, J. Liu, W. H. Meng, and Q. C. Zhao, Phytochem. Lett., 15, 159 (2016).
B. Hou, W. Wang, H. Gao, S. Cai, and C. Wang, J. Int. Med. Res., 46, 158 (2018).
Z. Zheng, X. Wang, P. Liu, M. Li, H. Dong, and X. Qiao, Molecules, 23, E429 (2018).
A. R. C. Souza, A. R. Guedesa, J. M. F. Rodriguez, M. C. M. Bombardelli, and M. L. Corazza, J. Supercrit. Fluid., 140, 137 (2018).
Z. Lou, C. Li, X. Kou, F. Yu, H. Wang, G. M. Smith, and S. Zhu, J. Food. Prot., 79, 1404 (2016).
G. L. Hostetler, R. A. Ralston, and S. J. Schwartz, Adv. Nutr., 8 (3), 423 (2017).
V. D. Amelia, R. Aversano, P. Chiaiese, and D. Carputo, Phytochem. Rev., 17, 611 (2018).
T. Y. Wang, Q. Li, and K. S. Bi, Asian. J. Pharm. Sci., 13, 12 (2018).
Q. F. Hu, B. Zhou, X. M. Gao, L. Y. Yang, L. D. Shu, Y. Q. Shen, G. P. Li, C. T. Che, and G. Y. Yang, J. Nat. Prod., 75, 1909 (2012).
B. Zhou, Y. K. Li, X. X. Wu, M. Li, Y. Q. Ye, X. M. Gao, and Q. F. Hu, Chem. Nat. Compd., 51, 840 (2015).
M. Zhou, K. Zhou, P. He, K. M. Wang, R. Z. Zhu, Y. D. Wang, W. Dong, G. P. Li, H. Y. Yang, Y. Q. Ye, G. Du, X. M. Li, and Q. F. Hu, Planta Med., 82, 414 (2016).
Clinical and Laboratory Standards Institute, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard, Vol. 32, Clinical and Laboratory Standards Institute, Wayne, PA, USA, 9th edition (2012).
E. Tripoli, M. Guardia, S. Giammanco, D. Majo, and M. Giammanco, Food Chem., 104, 466 (2007).
Acknowledgment
This work was financially supported by the Joint Foundation of Yunnan Province-Kunming Medical University [2017FE468(-186)], the Research Foundation of China Tobacco Yunnan Industrial Co., Ltd (No. 2017JC05), and the National Natural Science Foundation of China (No. 81660717).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2019, pp. 886–889.
Rights and permissions
About this article
Cite this article
Liang, MJ., Deng, L., Zeng, WL. et al. Two New Flavones from the Seeds of Arctium lappa and Their Bioactivity. Chem Nat Compd 55, 1028–1031 (2019). https://doi.org/10.1007/s10600-019-02886-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-019-02886-2