Caryophylla-4,8-dien-5-one (betulenone) was synthesized from α-betulenol in 96% yield. Thioacetates and sulfides with aromatic and heterocyclic fragments were newly synthesized from it in yields up to 95% and diastereomeric excesses (de) up to 80% via addition of thioacetic acid and thiols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sesquiterpenoids are natural compounds with broad spectra of biological activity. Thus, derivatives of caryophylleneand humulene-type sesquiterpenes possessed hepatoprotective, antidepressant, immunosuppressive, and antiproliferative properties [1,2,3,4,5,6,7]. Introducing an S atom into the sesquiterpenoid structure can enhance already existing biological activity and cause new activities to appear. The literature on selective syntheses of S-containing sesquiterpenoids and studies of their biological properties is scant [4]. Therefore, functionalization of sesquiterpenoids with S-containing reagents is a critical problem.
The starting terpenoid was caryophyllene oxide, the most studied and available sesquiterpenoid that is susceptible to various rearrangements with alteration [4, 8] and retention [9] of the caryophyllene skeleton.
The goal of the research was to synthesize new S-containing caryophyllene derivatives from betulenone.
Thioacids and thiols underwent Michael addition to betulenone at the double bond activated by the oxo group [10, 11].
Caryophylla-4,8-dien-5-one (1, betulenone) occurs in trace quantities in essential oils from the family Lamiaceae [12] and is also formed as a side product from rearrangement of caryophyllene oxide [13]. Herein, 1 was synthesized intentionally via oxidation of α-betulenol (2), which was prepared from 3 [9], using [bis(acetoxy)iodo]benzene–tetramethylpiperidine-1-oxyl (BAIB–TEMPO) (Scheme 1), which is highly effective for oxidizing allylic alcohols [14].

Scheme 1
The IR spectrum of 1 showed a C=O absorption band at 1678 cm–1 and was missing the OH absorption band at 3354 cm–1 of starting 2. The C-5 resonance in the 13C NMR spectrum of 1 was shifted to weak field (205.20 ppm) as compared with the C-5 resonance of 2 (75.24 ppm).
Addition of thioacetic acid to 1 synthesized diastereomeric thioacetates 4 (Scheme 2).

Scheme 2
The synthesis conditions were varied in order to optimize the yields of the target products (Table 1).
Benzene as the solvent (condition e) gave rather high diastereoselectivity and the greatest yield of target products that could not be isolated pure because the diastereomers had similar chromatographic mobilities.
Michael addition of thiols produced corresponding thio-derivatives 5–11 (Scheme 2, Table 2) as mixtures of diastereomers that could not be separated by chromatography.
Sulfides were synthesized using Et3N and a modified method [15] in the presence of catalytic amounts of Cs2CO3–TBAI (tetrabutylammonium iodide). However, the reaction using Et3N proceeded only for sulfides with a benzyl moiety.
13C NMR spectra of thioacetates 4 and compounds 5–11 showed strong-field shifts (29–48 ppm) of the methylene C-14 resonances from 122.36 and 150.89 ppm in starting 1. 1H–1H NOESY NMR spectra of the dominant thio-derivative isomers showed coupling between the H-4 and H-9 protons, which defined the terpene C-4 configuration for diastereomers 4a–11a.
Addition of 3-mercapto-4-methyl-1,2,4-triazole (11) to 1 formed N-derivatives 11a and 11b instead of the expected sulfide. Formation of the thioamide derivative could be explained by the existence of 11 as tautomeric thiol–thione species with the latter dominating [16].

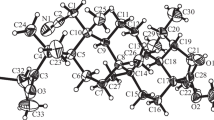
Crystals were obtained from mixtures of thio-derivatives 7a,b and 11a,b and were used to establish the structures by X-ray crystal structure analyses (XSA) (Fig. 1).
Analysis of the NMR spectra and XSA data together revealed that the latter belonged to major diastereomers with the R- and S-configurations of the C-4 atoms, respectively.
Thus, new caryophyllene sesquiterpene thio-derivatives with aromatic and heterocyclic moieties in addition to mixtures of thioacetate diastereomers were synthesized.
Experimental
IR spectra were recorded from thin layers or KBr pellets on an IR Prestige 21 FTIR spectrometer (Shimadzu). PMR and 13C NMR spectra were taken from CDCl3 solutions using solvent resonances as internal standards on an Avance-300 spectrometer (300.17 MHz for 1H and 75.48 MHz for 13C) (Bruker). 13C NMR spectra were obtained in JMOD mode. Proton and 13C resonances were assigned using 2D homo- (1H–1H COSY, 1H–1H NOESY) and heteronuclear experiments (1H–13C HSQC, 1H–13C HMBC). The diastereomeric excess was calculated from PMR and 13C NMR zgig30 spectral data [17]. NMR spectra of diastereomeric mixtures were described together. The minor diastereomer was marked with a prime (′). ass spectra were recorded on a GCMS-QP 2010 Plus instrument (Shimadzu). The temperature program used 80°C/min to 250°C. The ion-source temperature was 200°C. Masses were scanned in the range m/z 2–800 using electron-impact ionization at 70 eV. Optical rotation angles were measured on a PolAAr3003 automated digital polarimeter (Shimadzu). TLC used Sorbfil plates, CHCl3 eluent, and KMnO4, vanillin, and I2 detectors. Solvents were used without additional purification. Column chromatography used silica gel (0.06–0.2 mm, Alfa Aesar) and 3HCl3–Et2O (2:1), petroleum ether–Et2O (5:1), and petroleum ether–EtOAc (10:1–1:10) solvent systems.
XSA used colorless prismatic crystals of size 0.25 × 0.2 × 0.15 mm.
Cell constants and an experimental dataset of reflections were measured at 295(2) K on an Xcalibur 3 automated four-circle diffractometer (Agilent Technologies) using the standard procedure [18] of ω-2θ-scanning in 1° steps and monochromatic Mo Kα-radiation.
Structures were solved and refined using Olex2 software [19]. Structures were solved by direct methods and refined by full-matrix anisotropic least-squares methods over F2 for all non-hydrogen atoms. H atoms were placed at the geometrically calculated position and refined isotropically. All calculations used the SHELX97 program suite [20].
Results of the X-ray experiments were deposited in the Cambridge Crystallographic Data Centre under numbers CCDC 1513955-1513956. The data are available for free and can be requested at the address www.ccdc.cam.ac.uk/data_request/cif.
α-Betulenol was synthesized from commercial (–)-8-methylidene-4,11,11-trimethyl-4,5-oxabicyclo[7.2.0]undecane (caryophyllene oxide), [α] 20D –70° (c 2.0, CHCl3) (Sigma-Aldrich).
(1 R ,5 S ,9 S )-11,11-Dimethyl-4,8-methylidenebicyclo[7.2.0]undecan-5-ol (2) was prepared by the literature method [9]. Spectral data agreed with those published. Yield 90%, colorless oily liquid, [α] 24D +5.33° (c 0.30, CHCl3); R f 0.41 (C6H6–EtOAc, 8:1).
(1 R ,9 S )-11,11-Dimethyl-4,8-methylidenebicyclo[7.2.0]undecan-5-one (1) was prepared in 95% yield via oxidation of 2 by the literature method [12]. The literature on 1 [10, 11] gave only GC-MS data. Therefore, more detailed characteristics are given herein. Colorless oil liquid; [α] 24D +17.00°(c 0.30, CHCl3); R f 0.36 (petroleum ether–Et2O, 5:1). IR spectrum (KBr, ν, cm–1): 1678 (C=O). 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.94 (12H, d, J = 3.4, CH3-12, 13), 1.36–1.51 (2H, m, H-1, 2β), 1.52–1.72 (3H, m, H-2α, 7α, 7β), 2.13–2.49 (4H, m, H-6α, 6β, 3α, 9), 2.50–2.62 (1H, m, H-3β), 2.68 (1H, td, J = 11.1, 4.8, H-10), 2.77–2.89 (1H, m, H-10α), 4.99 (2H, d, J = 18.8, H-15′, 15), 5.57 (2H, s, J = 17.4, H-14α, 14β). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.87 (C-13), 30.87 (C-2), 31.55 (C-6), 33.24 (C-11), 34.53 (C-3), 39.15 (C-7), 41.69 (C-10), 46.41 (C-9), 52.10 (C-1), 11.28 (C-15), 122.30 (C-14), 150.83 (C-4), 151.83 (C-8), 206.14 (C-5).
({(1 R ,4 R ,9 S )-11,11-Dimethyl-8-methylidene-5-oxobicyclo[7.2.0]undec-4-yl}methyl)ethanethioate (4a); ({(1 R ,4 S ,9 S )-11,11-dimethyl-8-methylidene-5-oxobicyclo[7.2.0]undec-4-yl}methyl)ethanethioate (4b). Betulenone (1, 150 mg, 0.69 mmol) was dissolved in benzene (3 mL), cooled to 0°C, treated slowly dropwise with a solution of thioacetic acid (105 mg, 1.38 mmol) in benzene (1 mL), and stirred constantly at 0°C. The course of the reaction was monitored by TLC (CHCl3, alcoholic vanillin detector). When the reaction was finished, the product mixture was extracted with EtOAc, which was washed with saturated Na2CO3 solution and dried over Na2SO4. The solvent was vacuum distilled. Mixtures of thioacetate diastereomers were isolated by column chromatography over silica gel with elution by CH2Cl2. Yield 96% (100% conversion). Colorless oily liquid, R f 0.20 (CH2Cl2). IR spectrum (KBr, ν, cm–1): 1697 (C=O), 626. 567 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 294.20 (70) [M]+. Calcd for C17H26O2S, M = 294.45. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.93 (6H, d, J = 3.7, CH3-12, 13), 0.96 (6H, s, CH3-12′, 13′), 1.19–1.33 (4H, m, H-2α, 3α, 2α′, 3α′), 1.34–1.45 (1H, m, H-1), 1.45–1.73 (9H, m, H-1α, 6β, 6, 6α′, 6β′, 3β′, 2β′, 2, 3α), 1.86–1.99 (1H, m, H-9), 2.30 (3H, s, CH3-17), 2.26–2.70 (7H, m, H-9, 7α′, 7β′, 7α, 7β, 10α, 10β, 10α′, 10β′, 4, 4′), 2.85 (2H, dd, J = 13.2, 6.3, H-14α, 14α′), 3.03 (2H, dd, J = 14.5, 7.9, β-14, 14β), 4.87 (2H, dd, J = 3.5, H-15α, 15β), 4.94 (2H, s, H-15α′, 15β′). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.73 (C-12), 21.92 (C-12′), 26.68 (C-2), 29.08 (C-3), 29.61 (C-13), 29.68 (C-13′), 29.81 (C-14), 30.52 (C-17), 31.48 (C-7), 34.24 (C-11), 38.30 (C-6), 43.47 (C-9), 43.63 (C-10), 51.04 (C-1), 52.84 (C-4), 24.38, 28.17, 34.30, 39.53, 42.13, 42.54, 52.42, 55.60 (C-2′, 3′, 11′, 6′, 9′, 10′, 1′, 4′), 111.25 (C-15′), 111.60 (C-15), 152.02 (C-8), 153.60 (C-8′), 195.54 (C-16), 213.14 (C-5′), 214.32 (C-5).
General Method for Synthesizing Sulfides. Betulenone (1, 150 mg, 0.69 mmol) was dissolved in THF (7 mL) or DMF (10, 11), treated with Cs2CO3 (225 mg, 0.69 mmol) and TBAI (215 mg, 0.69 mmol) [or Et3N (0.15 mg, 20 mol%)], stirred for 5 min, and treated with a solution of thiol (1.3 mmol) in THF or DMF. The synthesis was carried out with constant stirring at room temperature or with refluxing for 8–24 h and was monitored by TLC. When the reaction was finished, the solvent was distilled off. The residue was extracted with EtOAc, which was washed with saturated KHCO3 solution. The combined organic fractions were dried over Na2SO4. Target sulfides were isolated by column chromatography over silica gel.
(1 S ,6 R ,9 R )-10,10-Dimethyl-6-[(benzylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (5a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[(benzylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (5b). Yield 45% (49% conversion). Yellowish liquid, R f 0.12 (petroleum ether–Et2O, 5:1). IR spectrum (KBr, ν, cm–1): 1705 (C=O), 1633, 1600, 1452 (C6H5), 646 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 342.15 (48) [M]+. Calcd for C22H30OS, M = 342.54. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.93 (6H, d, J = 4.4, CH3-12, 13), 0.98 (6H, s, CH3-12′, 13′), 1.11–1.44 (7H, m, H-1, 1′, 3α′, 3β′, 6α′, 6β′, 6β), 1.47–1.73 (6H, m, H-6α, 3α, 2α, 2, 2α′, 2β′), 1.74–1.90 (1H, m, H-3), 2.17–2.27 (1H, m, H-9′), 2.28–2.74 (15H, m, H-10α′, 10β′, 4, 4′, 14α′, 14β′, 14α, 14β, 7α′, 7β′, 7α, 7β, 10α, 10β, 9), 3.68 (2H, s, h-16β, 16), 4.89 (2H, d, J = 3.9, H-15α, 15β), 4.96 (2H, d, J = 3.9, H-16α′, 16β′), 7.19–7.41 (10H, m, 2C6H5). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.74 (C-13), 22.40 (C-13′), 25.11, 28.00, 32.33, 33.78, 36.42, 39.41, 42.54 (C-2′, 3′, 11′, 10′, 7′, 6′, 16′), 27.21 (C-6), 29.43 (C-13), 29.55 (C-12), 29.67 (C-12′), 31.54 (C-10), 32.76 (C-14), 34.00 (C-11), 36.60 (C-16), 38.21 (C-2), 43.60 (C-7), 43.66 (C-9), 51.88 (C-1), 52.52 (C-4), 42.40, 52.06, 54.10 (C-9′, 1′, 4′), 106.96 (C-15′), 111.28 (C-15), 126.94 (C-20, 20′), 128.39 (C-19, 21, 19′, 21′), 128.74 (C-18, 18′, 22, 22′), 138.04 (C-17), 152.11 (C-8), 153.47 (C-8′), 214.40 (C-5), 214.24 (C-5′).
(1 S ,6 R ,9 R )-10,10-Dimethyl-6-[(phenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (6a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[(phenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (6b). Yield 52% (100% conversion). Yellowish liquid, R f 0.30 (petroleum ether–EtOAc, 10:1). IR spectrum (KBr, ν, cm–1): 1705 (C=H), 1635, 1444 (C6H5), 623 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 328.20 (60) [M]+. Calcd for C21H28OS, M = 328.51. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm): 0.94 (6H, s, CH3-12, 13), 0.97 (6H, s, CH3-12′, 13′), 1.17–1.34 (2H, m, H-2α, 2α′), 1.34–1.46 (3H, m, H-2β, 2β′, 1′), 1.47–1.80 (6H, m, H-10α, 10β, 10β′, 10α, 3α, 3α′), 1.82–2.02 (2H, m, H-3β, 3β′), 2.14–2.27 (1H, m, H-9′), 2.28–2.74 (11H, m, H-9, 6α, 6β, 6α′, 6β′, 7α, 7β, 4, 4′, 7α′, 7β′), 2.75–2.91 (2H, H-14α, 14β), 3.08–3.24
(2H, H-14α′, 14β′), 7.49–7.91 (4H, H-15β, 15, 15α′, 15β′), 7.15–7.28 (10H, m, 2C6H5). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.77 (C-12), 21.88 (C-12′), 24.82, 28.00, 34.93, 39.48, 42.77, 51.50 (C-2′, 3′, 14′, 10′, 7′, 4′), 26.97 (C-2), 29.13 (C-13), 29.74 (C-13′), 31.47 (C-6), 35.10 (C-14), 38.37 (C-10), 41.31 (C-9′), 43.67 (C-9), 43.82 (C-7), 51.50 (C-1), 52.27 (C-4), 55.01 (C-1′), 111.28 (C-15′), 111.54 (C-15), 126.32 (C-19, 19′), 128.68 (C-17, 18, 20, 21), 135.88 (C-16), 152.08 (C-8), 153.88 (C-8′), 214.53 (C-5), 214.41 (C-5′).
(1 S ,6 R ,9 R )-10,10-Dimethyl-6-[(4-methylphenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (7a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[(4-methylphenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (7b). Yield 95% (100% conversion). White powder, Rf 0.47 (petroleum ether–Et2O, 5:1). IR spectrum (KBr, ν, cm–1): 1703 (C=H), 1633, 1448, 1492 (C6H5), 632 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 342.65 (46) [M]+. Calcd for C22H30OS, M = 342.54. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.94 (6H, s, CH3-12, 13), 0.97 (6H, s, CH3-12′, 13′), 1.19–1.33 (2H, m, H-2α, 2α′), 1.33–1.47 (3H, m, H-4, 2β′, 4′), 1.47–1.77 (7H, m, 2β, 3α, 10α, 10β, 10α′, 10β′, 3α′), 1.82–2.00 (2H, m, H-3β, 3β′), 2.33 (3H, s, CH3-22), 2.29–2.71 (12H, m, H-9, 9α, 6α′, 6β′, 6β, 6, 1, 1′, 7α, 7β, 7α′, 7β′), 2.80 (2H, dd, J = 12.9, 6.8, H-14α, 14α′), 3.09 (2H, dd, J = 12.9, 7.4, H-14, 14 β′), 4.86 (2H, d, J = 4.0, H-15α, 15β), 4.89 (2H, s, 15α′, 15β′), 7.11 (4H, d, J = 8.1, H-18, 20, 18′, 20′), 7.25 (4H, d, J = 8.1, H-17, 21, 17′, 21′). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.00 (C-22), 21.80 (C-12, 12′), 24.89, 27.84, 34.00, 35.72, 37.17, 39.50 (C-2′, 3′, 11′, 14′, 7′, 10′), 27.00 (C-2), 29.11 (C-3), 29.64 (C-13), 29.74 (C-13′), 31.53 (C-7), 34.19 (C-11), 35.89 (C-14), 38.37 (C-10), 43.69 (C-6), 43.61 (C-9), 51.69 (C-4), 52.39 (C-1), 42.35, 42.66, 51.88, 54.84 (C-9′, 1′, 4′, 6′), 111.24 (C-15′), 111.46 (C-15), 129.75 (C-18, 20, 18′, 20′), 130.54 (C-17, 21, 17′, 21′), 131.99 (C-16), 136.58 (C-19), 152.14 (C-8), 153.43 (C-8′), 214.00 (C-5′), 214.51 (C-5).
1 S ,6 R ,9 R )-10,10-Dimethyl-6-[(4-methoxyphenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (8a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[(4-methoxyphenylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (8b). Yield 67% (91% conversion). Light-yellow liquid, R f 0.95 (petroleum ether–Et2O, 5:1). IR spectrum (KBr, ν, cm–1): 1705 (C=H), 1633, 1593, 1492 (C6H4), 630 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 358.20 (70) [M]+. Calcd for C22H30O2S, M = 358.54. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.93 (6H, s, CH3-12, 13), 0.96 (6H, s, CH3-12′, 13′), 1.18–1.33 (2H, m, H-3α, 3α′), 1.34–1.74 (11H, m, H-3β, 3β′, 4, 7′, 10α, 10β, 10α′, 10β′, 2α, 2α′,7′ (4′)), 1.82–1.97 (2H, m, H-2β, 2β′), 2.14–2.27 (1H, m, H-9′), 2.29–2.79 (13H, m, H-14α, 14β, 14α′, 14β′, 9, 6α′, 7β′, 7α, 6β′, 6α, 6β′, 1′ (4′),1), 2.96–3.13 (2H, m, H-7α, 7α′). 3.80 (6H, s, CH3-22, 22′), 4.85 (1H, d, J = 3.7, H-5), 4.88 (1H, s, H-5′), 6.84 (4H, d, J = 8.8, H-18, 20, 18′, 20′), 7.33 (4H, d, J = 8.8, H-17, 21, 17′, 21′). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.83 (C-12, 12′), 25.11, 28.00, 32.33, 33.78, 36.42, 39.41, 42.54 (C-2′, 3′, 11′, 10′, 7′, 6′, 16′), 27.03 (C-3), 29.07 (C-2), 29.64 (C-13), 29.74 (C-13′), 31.63 (C-14), 33.99 (C-11), 37.30 (C-7), 38.40 (C-10), 43.48 (C-6), 43.56 (C-9), 51.92 (C-4), 52.59 (C-1), 56.32 (C-22), 42.44, 52.05, 54.53 (C-9′, 1′, 4′), 111.28 (C-15′), 111.43 (C-15), 114.63 (C-18, 20, 18′, 20′), 113.61 (C-17, 21, 17′, 21′), 152.23 (C-16), 153.43 (C-8), 159.14 (C-19), 214.13 (C-5′), 214.53 (C-5).
(1 S ,6 R ,9 R )-10,10-Dimethyl-6-[(pyridin-2-ylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (9a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[(pyridin-2-ylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (9b). Yield 51% (69% conversion). Yellowish liquid, R f 0.19 (petroleum ether–Et2O, 5:1). IR spectrum (KBr, ν, cm–1): 1705 (C=H). Mass spectrum (EI, 70 eV), m/z (I rel, %): 329.08 (38) [M]+. Calcd for C20H27NOS, M = 329.49. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.93 (6H, s, CH3-12, 13), 0.96 (3H, s, CH3-12′), 1.16–1.80 (9H, m, H-2α, 2β, 1, 10α, 10β, 3α, 5H′, 1′, 4′), 1.87–2.03 (3H, m, H-3, 3′), 2.13–2.27 (1H, m, H-9′), 2.27–2.57 (5H, m, H-9, 6α, 6β, 7β, 5H′), 2.59–2.74 (m, H-7α), 2.77–2.91 (1H, m, H-4), 2.91–3.00 (1H, m, H-4′), 3.06–3.20 (2H, m, H-14, 14′), 3.27–3.45 (2H, m, H-14α, 14α′), 4.86 (2H, s, H-15α, 15β), 4.89 (2H, s, H-15α′, 15β′), 6.94 (2H, td, J = 7.8, 4.4, 1.3, H-20, 20′), 7.4 (1H, d, J = 7.8, H-18), 7.43 (2H, td, J = 7.8, 1.3, H-19, 19′), 7.38 (2H, d, J = 4.4, H-21, 21′). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.71 (C-12 (13)), 21.79 (C-12′, (13′)), 26.85 (C-2′), 27.16 (C-2), 28.20 (C-3′), 29.26 (C-3), 29.57 (C-13 (12)), 29.63 (C-13′, (12′)), 30.58 (C-14′), 30.87 (C-14), 31.40 (C-6), 34.15 (C-11), 38.16 (C-10), 39.47 (C-10′), 42.32 (C-9′), 42.59 (C-7′), 43.76 (C-9), 43.85 (C-7), 51.36 (C-1), 51.98 (C-4′), 52.40 (C-4), 55.07 (C-1′), 111.09 (C-15′), 111.37 (C-15), 119.29 (C-19, 19′), 122.27 (C-21, 21′), 135.73 (C-20, 20′), 149.28 (C-18, 18′), 151.95 (C-8), 153.35 (C-8′), 158.30 (C-16, 16′), 214.03 (C-5′), 214.75 (C-5).
(1 S ,6 R ,9 R )-10,10-Dimethyl-6-[( N -methylimidazol-2-ylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (10a); (1 S ,6 S ,9 R )-10,10-dimethyl-6-[( N -methylimidazol-2-ylsulfanyl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (10b). Yield 69% (86% conversion). Yellow liquid, R f 0.34 (CHCl3–Et2O, 2:1). IR spectrum (KBr, ν, cm–1): 1600, 1519 (imidazole), 628 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 332.15 (36) [M]+. Calcd for C19H28N2OS, M = 332.50. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.95 (12H, dd, J = 6.7, 5.8, CH3-12, 13, 12′, 13′), 1.29–1.79 (14H, m, H-3α, 2α, 2β, 2α′, 1′, 4′, 3β′, 3α′, 6α, 6β, 6α′, 6β′,), 1.89–2.05 (1H, m, H-3α), 2.16 (1H, q, J = 9.9, 9.9, 9.4, H-4), 2.24–2.68 (9H, m, H-10α, 10β, 9, 7α, 7β, 7α′, 7β′, 10α′, 10β′), 3.30–3.38 (1H, m, H-1), 3.57 (1H, s, CH3-21, 21′), 3.97–4.06 (2H, m, H-14α′, 14β′), 4.06–4.17 (1H, m, H-14), 4.21–4.32 (1H, m, H-14α), 4.87 (2H, d, J = 4.6, H-15β, 15), 4.93 (2H, s, H-15α′, 15β′), 6.60 (1H, t, J = 2.9, 2.3, H-18), 6.74 (1H, t, J = 3.2, 2.3, H-19). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.73 (C-12), 22.12 (C-12′), 26.44 (C-2), 27.70 (C-3), 29.65 (C-13), 29.69 (C-13′), 31.18 (C-7), 33.32 (C-11′), 33.40 (C-11), 34.94 (C-21), 35.00 (C-21′), 38.08 (C-6), 41.36 (C-4), 43.28 (C-9), 44.23 (C-10), 48.90 (C-14), 50.70 (C-1), 111.22 (C-15′), 111.69 (C-15), 117.11 (C-18), 118.38 (C19), 151.34 (C-8), 153.60 (C-8′), 212.92 (C-5′), 215.04 (C-5), 23.56, 26.59, 34.27, 39.47, 42.82, 48.17, 50.88, 50.70, 56.83 (C-2′, 3′, 6′, 10′, 14′, 9′, C-7′, C-1′).
(1 S ,6 S ,9 R )-10,10-Dimethyl-6-[(4-methyl-5-thioxo-4,5-dihydro-1 H -1,2,4-triazol-1-yl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (11a); (1 S ,6 R ,9 R )-10,10-dimethyl-6-[(4-methyl-5-thioxo-4,5-dihydro-1 H -1,2,4-triazol-1-yl)methyl]-2-methylidenebicyclo[7.2.0]undecan-5-one (11b). Yield 55% (85% conversion). White powder, R f 0.35 (petroleum ether–EtOAc, 1:1). IR spectrum (KBr, ν, cm–1): 1701 (C=H), 675 (C–S). Mass spectrum (EI, 70 eV), m/z (I rel, %): 334 (56) [M]+. Calcd for C18H27N3OS, M = 333.49. 1H NMR spectrum (300 MHz, CDCl3, δ, ppm, J/Hz): 0.94 (6H, s, CH3-12′, 13′), 0.95 (6H, s, CH3-12, 13), 1.21–1.30 (1H, m, H-3), 1.70–1.76 (12H, m, H-1, 1′, 6α, 6β, 6α′, 6β′, 2α, 2β, 2α′, 2β′, 3α, 3α′), 1.77–1.97 (1H, m, H-3), 2.16–2.20 (1H, m, H-9′), 2.32–2.46 (3H, m, H-10, 10β′, 9), 2.46–2.73 (6H, m, H-10α, 10α′, 7α, 7β, 7α′, 7β′), 3.21–3.38 (2H, H-4, 4′), 3.57 (3H, s, CH3-21), 4.17–4.45 (4H, H-14α, 14β, 14α′, 14β′), 4.88 (2H, d, J = 4.3, H-15α, 15), 4.94 (2H, s, H-15α′, 15β′), 7.73 (1H, s, H-19). 13C NMR spectrum (75 MHz, CDCl3, δ, ppm): 21.80 (C-12), 21.98 (C-12′), 26.49 (C-6), 26.69 (C-3), 29.64 (C-13, 13′), 31.62 (C-10), 32.67 (C-21), 34.25 (C-11), 38.33 (C-2), 41.94 (C-9′), 43.17 (C-9), 43.31 (C-7), 49.74 (C-14), 24.53, 25.86, 33.52, 34.02, 39.48, 42.50, 49.56, 50.84 (C-6′, 3′, 11′, 10′, 2′, 7′, 14′, 4′), 51.06 (C-1), 51.35 (C-4), 56.29 (C-1′), 111.29 (C-15′), 111.57 (C-15), 139.30 (C-17), 152.14 (C-8), 153.60 (C-8′), 166.73 (C-16), 212.73 (C-5′), 213.68 (C-5).
References
M. A. Getman and J. Gertsch, Pat. WO2013184036 A3, Dec. 12, 2013.
M. J. Chavan, P. S. Wakte, and D. B. Shinde, Phytomedicine, 17, 149 (2010).
W. Zhang, Z. Yao, Y. W. Zhang, X. X. Zhang, Y. Takaishi, and H. Q. Duan, Planta Med., 76, 1882 (2010).
L. Siaz-Urra, J. C. Racero, A. J. Macias-Sanchez, R. Hernandez-Galan, J. R. Hanson, M. Perez-Gonzalez, and I. G. Collado, J. Agric. Food Chem., 57, 2420 (2009).
X. Cheng, N. Harzdorf, Z. Khaing, D. Kang, A. M. Camelio, T. Shaw, C. E. Schmidt, and D. Siege, Org. Biomol. Chem., 10, 383 (2012).
M. Xian, K. Ito, T. Nakazato, T. Shimizu, C.-K. Chen, K. Yamato, A. Murakami, H. Ohigashi, Y. Ikeda, and M. Kizaki, Cancer Sci., 98, 118 (2007).
Q.-X. Wu, Y.-P. Shi, and L. Yang, Org. Lett., 6, 2313 (2004).
E. P. Romanenko and A. V. Tkachev, Chem. Sustainable Develop., 15, 571 (2007).
U. Vogt, U. Eggert, A. M. Z. Slawin, D. J. Williams, and H. M. R. Hoffmann, Angew. Chem., Int. Ed. Engl., 29 (12), 1456 (1990).
S. Sobhani and S. Rezazadeh, Phosphorus Sulfur Silicon Relat. Elem., 185, 2076 (2010).
D. P. Nair, S. Chatani, T. Gong, W. Xi, C. R. Fenoli, and C. N. Bowman, Chem. Mater., 26, 724 (2014).
A. V. Tkachev, Chem. Nat. Compd., 23, 393 (1987).
R. Kaiser and D. Lamparsky, Helv. Chim. Acta, 66 (6), 1843 (1983).
A. De Mico, R. Margarita, L. Parlanti, A. Vescovi, and G. Piancatelli, J. Org. Chem., 62, 6974 (1997).
R. N. Salvatore, R. A. Smith, A. K. Nischwitz, and T. Gavin, Tetrahedron Lett., 46, 8931 (2005).
J. K. Shneine and Y. H. Alaraji, Int. J. Sci. Res. (IJSR), 5 (3), 1411 (2016).
D. A. Otte, D. E. Borchmann, C. Lin, M. Weck, and K. A. Woerfel, Org. Lett., 16, 1566 (2014).
CrysAlisPro, Agilent Technologies, Version 1.171.36.28 (release 01-02-2013 CrysAlis171.NET).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. Howard, and H. Puschmann, J. Appl. Crystallogr., 42, 339 (2009).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 64, 112 (2008).
Acknowledgment
The work was supported financially by the RFBR (Project No. 16-03-01064) using equipment at the Khimiya Center for Collective Use (CCU), Inst. Chem., Komi SC, UB, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2017, pp. 56–60.
Rights and permissions
About this article
Cite this article
Gyrdymova, Y.V., Sudarikov, D.V., Rubtsova, S.A. et al. Synthesis of New Sesquiterpenoid Thio-Derivatives Based on Betulenone. Chem Nat Compd 53, 66–71 (2017). https://doi.org/10.1007/s10600-017-1913-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1913-7