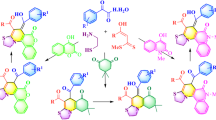
In this work, we developed a straightforward and convenient method for the synthesis of substituted pyrazoloquinolines – highly desirable compounds for many high-tech and biomedical applications. On the basis of intramolecular cyclization of 5-(N-aryl)aminopyrazoles in the presence of DMF diethyl acetal and POCl3, a series of differently substituted pyrazoloquinolines were synthesized. The synthetic procedure was thoroughly optimized taking into account key factors such as the type of formylating agent (DMF vs. DMF diethyl diacetal), reaction temperature, and stoichiometry of the reagents used. Because of its versatility, simplicity, and ease in purification of the obtained products, this synthetic approach provides a valuable guideline in the rational design of pyrazoloquinoline-based functional materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pyrazolo[3,4-b]quinolines are nitrogen-containing heterocycles that have high potential for many applications. However, since the synthesis of the first representatives of this group, almost 40 years have passed to the beginning of their practical use.1,2
In the 1950s and 1960s, pyrazoloquinolines were mainly tested as optical brighteners.3 Later, numerous studies have been aimed at their biological properties using them as potential antimalarial, interferon-inducing, anticancer, or antiviral agents.4,5,6,7
Moreover, many pyrazoloquinolines exhibit intense blue or bluish green emission in both the solution and the solid state. This feature was noticed a long time ago; however, more detailed studies in this field were performed only in the late 90s.8,9,10 In particular, pyrazoloquinolines substituted with azacrown or N,N-diethylaminoethanol moieties, linked to fluorophore via carboxylic group or nitrogen atom were widely used as fluorescent sensors.11,12,13 Another important application of 1H-pyrazolo[3,4-b]quinolines is closely related to the electroluminescence phenomenon, making them perfect candidates as main components in the construction of organic light-emitting diodes. These materials have been applied in various forms, including vacuum-deposited layers, dopants in poly-N-vinylcarbazole matrices, and premodified polymers.14,15,16
Pyrazoloquinolines can be synthesized according to many different procedures. Originally, in 1911, Michaelis synthesized the first series of these fluorophores, namely 4-aryl-substituted derivatives. However, he attributed the obtained compounds to a different structure1 (Scheme 1) and his results were corrected later.17 Michaelis used N-arylpyrazol- 5-amines 1 as the starting materials and heated them with aromatic aldehydes 2 in the presence of anhydrous zinc chloride. He attributed the resulted compounds to benzylidene structure 3. After the results of this research were reinvestigated, the structures of these products were finally confirmed to be pyrazoloquinoline derivatives 4.
The original synthesis of 1H-pyrazolo[3,4-b]quinoline performed by Michaelis in 19111
Another straightforward method applied for the synthesis of the pyrazoloquinoline core is the Friedländer condensation of anthranilaldehyde with corresponding pyrazol-3-ones. This approach, along with the original protocol by Michaelis, is also considered to be one of the earliest methods accessing a variety of pyrazoloquinolines.2,18 However, because of the restricted availability of differently substituted o-aminobenzaldehydes and tedious synthetic procedures, this method is limited only to derivatives unsubstituted at the quinoline site of the chromophore.
Khalafy et al. used 5-amino-3-(arylamino)-1H-pyrazole- 3-carbonitriles and 1,3-diketones in the presence of tin(II) chloride in neat conditions to synthesize the series of pyrazolo[3,4-b]quinolines.19 They also presented a threecomponent reaction of 5-aminopyrazole, arylglyoxals, and 1,3-dimethylbarbituric acid that led to 4-aryloyl-1Hpyrazolo[ 3,4-b]quinolines.20 Furthermore, the reaction between 5-aminopyrazoles, dimedone, and arylglyoxals in the presence of TBAB afforded the formation of 1H-pyrazolo[3,4-b]quinolines unsubstituted at the pyridine ring.21 In 2021, a paper on three-component pyrazoloquinoline synthesis was published.22 This approach based on the reaction between aromatic amines, pyrazolones, and DMSO in the presence of trifluoroacetic acid. However, this method resulted in the formation of the products unsubstituted at position 4 of the main core. Most recently, a review summarizing over a hundred years of research on the synthesis of 1H-pyrazolo[3,4-b]quinolines and their photophysical and biological properties has been published.23
Our research presented in this work is focused on the intramolecular cyclization of N-arylpyrazol-5-amines 1 that lead to 1H-pyrazolo[3,4-b]quinolines 4 diversified by substitution at positions 1, 3, and 6 of the main core. In 1976, Purnaprajna and Seshadri reported the two-step cyclization of 3-(N-aryl)pyrazolin-5-one 5 that led to the formation of the corresponding 2H-pyrazolo[3,4-b]- quinoline 6 (Scheme 2).24 However, this method was demonstrated with only one example of this class of compounds. Therefore, following our previous work,25 we decided to apply 1,3-disubstituted N-aryl-1H-pyrazol- 5-amines 1 as direct precursors for the synthesis of 1H-pyrazolo[3,4-b]quinoline derivatives 4.
Synthesis of 2H-pyrazolo[3,4-b]quinolines developed by Purnaprajna and Seshadri (1976)24
In the first step of our current study, we focused on the choice of the most efficient formylating agent. The classical approach based on the DMF–POCl3 formylation system was compared with the use of the diethyl acetal of DMF (DMF–DEA) as an alternative. As a model reaction, the formation of 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline (4a) from N,1,3-triphenyl-1H-pyrazol-5-amine (1a) was selected. The results obtained are presented in Table 1.
In comparison, a higher conversion was observed with the use of DMF–DEA, rather than DMF as a formylating agent (Table 1, entries 1–4). To rationalize the choice of the more expensive acetal instead of easily accessible DMF, the attempt with less reactive N-(4-nitrophenyl)- 1,3-diphenyl-1H-pyrazol-5-amine (1b) was also conducted (entries 3 and 4). The results were consistent with those obtained for the model reaction: significantly higher yield of 38% was obtained in the reaction with DMF–DEA, compared to 24% with the use of DMF. Moreover, the chromatographic purification of the obtained product was much easier for the run employing DMF–DEA, compared to DMF. In the latter case, the reaction often led to a hardly separable mixture of byproducts, making the isolation of the desired compound being far from trivial. The main difference between DMF and DMF–DEA agents would correspond to their relative reactivity and the rate of formation of formylating complex with POCl3. DMF itself is known as quite reactive solvent, which often promotes uncontrolled decomposition of organic species during reactions and therefore resulting in complex (often inseparable) mixtures of side products. In contrast, diacetal form of this reagent significantly weakens the reactivity of formylating system. As a result, the desired products are obtained more efficiently in much milder conditions. On the basis of these preliminary results, the DMF–DEA/POCl3 system was selected as a formylating agent for further studies.
In the next step of this optimization study, the impact of the molar quantity of DMF–DEA and POCl3 relative to the starting material on the reaction yield was tested. The results presented in Table 1 (entries 2 and 5) show that the decrease in both the amount of DMF–DEA and POCl3 from 2 to 1 equivalent considerably reduces the reaction yield. In the final step, the influence of temperature on the reaction yield was investigated (Table 1, entries 5–8). Noticeably, the lowest conversion, being of 88%, was observed while heating the reaction mixture at 50°C (Table 1, entry 6). With further increase of the reaction temperature there were no significant differences between yields spanning from 60 to 80°C. For the exploration of the reaction scope, however, 80°C was selected, mainly due to lower reactivity of the starting materials containing deactivating nitro groups.
Taking into account all these results, optimized reaction conditions for the intramolecular cyclization of substituted aminopyrazoles 1 to 1H-pyrazolo[3,4-b]quinolines 4a–v were established as follows: DMF–DEA (2 equiv), POCl3 (2 equiv), toluene as reaction solvent, temperature 80°C, and reaction time 24 h (Table 2).
In most cases the products were obtained with very good to excellent yields ranging from 60 to ca. 100%. However, much lower conversions were achieved for compounds 4i,p,t with reaction yields of 27–38%. Because of the presence of strongly deactivating nitro groups, the formation of those compounds was mechanistically unfavorable. Interestingly, compared to other 6-substituted analogs with donor substituents, 6-OMe derivatives 4c,l,o,s were obtained with noticeably lower yields. Furthermore, it can be observed that all 1,3-dimethylpyrazoloquinolines were obtained with much lower yields than the counterparts substituted with at least one phenyl group. This observation could be attributed to a lower chemical stability of the corresponding precursors.
In conclusion, an efficient synthetic method was developed for the preparation of 1,3,6-trisubstituted 1H-pyrazolo- [3,4-b]quinoline derivatives. Intramolecular cyclization of 1,3-disubstituted N-arylpyrazol-5-amines with the DMF–DEA and POCl3 as reagent system yielded the target products with moderate to high yields, depending on their substitution pattern. In most cases, the reaction proceeded smoothly and without the formation of any undesired side products. In particular, in terms of purification of the final compounds, this procedure proved to be more convenient and time-saving compared to the up-to-date reported protocols.
Experimental
1H and 13C NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer (400 and 101 MHz, respectively, for compounds 4a–g,i–w) and on a Bruker Avance III 600 MHz spectrometer (600 and 151 MHz, respectively, for compound 4h) in deuterated solvents using CDCl3 (δ 1H 7.26 ppm, δ 13C 77.2 ppm) and DMSO-d6 (δ 1H 2.50 ppm, δ 13C 39.5 ppm) for compounds 4l,q, residual signals or TMS as internal standard. Elemental analyzes were performed using a vario MICRO cube analyzer combined with an electronic microbalance. Melting points were determined on a MEL-TEMP II apparatus. TLC was performed on 0.2 mm precoated plates of silica gel on TLC Al foils with fluorescence indicator 254 nm purchased from Sigma Aldrich. Visualization of the TLC spots was performed with a UV lamp Spectroline model ENF-260C/FE (254 and 356 nm). Column chromatography was carried out using 70–230 mesh ASTM silica gel purchased in Merck KGaA or 70–230 mesh ASTM 90 active neutral Al2O3 (activity stage I) purchased from Merck KGaA.
Synthesis of 1,3,6-trisubstituted 1 H -pyrazolo[3,4- b ]- quinoline 4a–v (General method). The appropriate 1,3-disubstituted N-aryl-1H-pyrazol-5-amine 1 (1 equiv) and DMF–DEA (2 equiv) were dissolved in PhMe (5 ml, for 0.4–1.7 mmol of compound 1). The mixture was cooled to 0°C, and POCl3 (2 equiv) was added in small portions. The mixture was allowed to reach room temperature and stirred at 80°C for 24 h. Afterward, the reaction was quenched with H2O (5–10 ml). The mixture was neutralized with the solid NaHCO3 (to pH 7) and stirred for 1 h. The organic phase was separated, and the aqueous phase was extracted with PhMe (3×10 ml). The combined organic layers were dried over anhydrous MgSO4, filtered, and the solvent was removed under reduced pressure. The residue was purified by column chromatography. In some cases the precipitate was washed with MeOH or the product was obtained without any further purification.
1,3-Diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4a) was purified by column chromatography on SiO2 (eluent PhMe). Yield 238 mg (95%), bright-yellow solid, mp 163–165°C (mp 163–164°C26). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.95 (1H, s, H Ar); 8.65 (2H, dd, J = 8.7, J = 1.1, H Ar); 8.26–8.16 (3H, m, H Ar); 8.07–8.01 (1H, m, H Ar); 7.86–7.79 (1H, m, H Ar); 7.68–7.57 (4H, m, H Ar); 7.58–7.49 (2H m, H Ar); 7.40–7.32 (1H, m, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.9; 148.2; 144.5; 140.0; 132.6; 131.0; 130.9; 129.2; 129.1 (3 partially overlapped signals); 128.9; 127.5; 125.4; 124.8; 124.4; 120.7; 116.5. Found, %: C 82.17; H 4.72; N 13.11. C22H15N3. Calculated, %: C 82.22; H 4.70; N 13.08.
6-Methyl-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4b) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 136 mg (88%), bright-yellow solid, mp 169– 171°C (mp 175–177°C22). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.83 (1H, s, H Ar); 8.64 (2H, d, J = 7.6, H Ar); 8.21–8.15 (2H, m, H Ar); 8.11 (1H, d, J = 8.8, H Ar); 7.77 (1H, s, H Ar); 7.67–7.57 (5H, m, H Ar); 7.56–7.49 (1H, m, H Ar); 7.34 (1H, t, J = 7.4, H Ar); 2.59 (3H, s, CH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.7; 146.9; 144.4; 140.0; 134.1; 133.5; 132.8; 129.9; 129.1; 128.9; 128.6; 127.6; 127.5; 125.3; 124.9; 120.6; 116.5; 21.5. Found, %: C 82.40; H 5.14; N 12.46. C23H17N3. Calculated, %: C 82.36; H 5.11; N 12.53.
6-Methoxy-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4c) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 165 mg (60%), bright-yellow solid, mp 187–188°C (mp 138°C27). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.80 (1H, s, H Ar); 8.63 (2H, d, J = 7.7, H Ar); 8.18 (2H, d, J = 7.2, H Ar); 8.10 (1H, d, J = 9.4, H Ar); 7.65–7.57 (4H, m, H Ar); 7.56–7.46 (2H, m, H Ar); 7.34 (1H, t, J = 7.4, H Ar); 7.24 (1H, d, J = 2.8, H Ar); 3.98 (3H, s, OCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 156.2; 150.1; 144.6; 143.9; 140.1; 132.8; 130.2; 129.0 (2 partially overlapped signals); 128.9; 128.8; 127.5; 125.5; 125.2; 125.0; 120.5; 116.5; 105.1; 55.5. Found, %: C 78.68; H 4.93; N 11.90. C23H17N3O. Calculated, %: C 78.61; H 4.88; N 11.96.
6-Phenoxy-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4d) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 159 mg (96%), bright-yellow solid, mp 139–140°C (mp 145–146°C28). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.78 (1H, s, H Ar); 8.63 (2H, d, J = 7.6, H Ar); 8.22 (1H, d, J = 9.3, H Ar); 8.15 (2H, d, J = 7.1, H Ar); 7.67–7.56 (5H, m, H Ar); 7.54– 7.40 (4H, m, H Ar); 7.35 (1H, t, J = 7.4, H Ar); 7.23 (1H, t, J = 7.4, H Ar); 7.19–7.16 (2H, m, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 156.7; 154.1; 150.5; 145.2; 144.1; 140.0; 132.6; 130.8; 130.0; 129.6; 129.1 (2 signals); 129.0; 128.2; 127.5; 125.5; 125.4; 125.2; 124.0; 120.6; 119.6; 116.7; 113.3. Found, %: C 81.38; H 4.70; N 10.12. C28H19N3O. Calculated, %: C 81.34; H 4.63; N 10.16.
N , N -Dimethyl-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinolin- 6-amine (4e) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 146 mg (72%), orange solid, mp 203–204°C (mp 200–201°C29). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.72 (1H, s, H Ar); 8.64 (2H, d, J = 7.6, H Ar); 8.19 (2H, d, J = 7.1, H Ar); 8.08 (1H, d, J = 9.5, H Ar); 7.64–7.55 (5H, m, H Ar); 7.51 (1H, t, J = 7.4, H Ar); 7.32 (1H, t, J = 7.4, H Ar); 7.00 (1H, d, J = 2.8, H Ar); 3.11 (6H, s, N(CH3)2). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 149.6; 147.2; 143.8; 143.1; 140.2; 133.1; 129.3; 129.0 (2 partially overlapped signals); 128.7; 127.8; 127.4; 126.4; 125.0; 122.7; 120.4; 116.7; 105.6; 41.0. Found, %: C 79.08; H 5.50; N 15.42. C24H20N4. Calculated, %: C 79.10; H 5.53; N 15.37.
N , N ,1,3-Tetraphenyl-1 H -pyrazolo[3,4- b ]quinolin-6-amine (4f) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 195 mg (95%), deep-yellow solid, mp 207–208°C (mp 214–218°C30). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.68 (1H, s, H Ar); 8.63 (2H, d, J = 7.6, H Ar); 8.14 (2H, d, J = 7.1, H Ar); 8.09 (1H, d, J = 9.3, H Ar); 7.68 (1H, dd, J = 9.3, J = 2.6, H Ar); 7.63– 7.53 (5H, m, H Ar); 7.49 (1H, t, J = 7.4, H Ar); 7.38–7.31 (5H, m, H Ar); 7.24–7.17 (4H, m, H Ar); 7.13 (2H, t, J = 7.3, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.4; 147.5; 145.4; 144.2 (2 partially overlapped signals); 140.0; 132.7; 129.7; 129.6; 129.5; 129.2; 129.1; 129.0; 128.9; 127.4; 125.8; 125.3; 124.7; 123.5; 120.5; 119.3; 116.7. Found, %: N 83.66; H 4.99; N 11.35. C34H24N4. Calculated, %: C 83.58; H 4.95; N 11.47.
1,3,6-Triphenyl-1 H -pyrazolo[3,4- b ]quinoline (4g) was precipitated with MeOH, the suspension was sonicated and filtered. Yield 195 mg (94%), bright-yellow solid, mp 208– 209°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.99 (1H, s, H Ar); 8.65 (2H, d, J = 7.7, H Ar); 8.28 (1H, d, J = 9.0, H Ar); 8.24–8.18 (3H, m, H Ar); 8.10 (1H, dd, J = 9.0, J = 2.1, H Ar); 7.82–7.75 (2H, m, H Ar); 7.66–7.60 (4H, m, H Ar); 7.55 (3H, t, J = 7.6, H Ar); 7.44 (1H, t, J = 7.4, H Ar); 7.36 (1H, t, J = 7.4, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.9; 147.6; 144.5; 140.2; 139.9; 137.1; 132.6; 131.1; 130.8; 129.3; 129.1 (2 partially overlapped signals); 129.0; 127.6; 127.5; 127.3; 126.6; 125.4; 125.0; 120.7; 116.9. Found, %: C 84.82; H 4.77; N 10.41. C28H19N3. Calculated, %: C 84.61; H 4.82; N 10.57.
6- tert -Butyl-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4h) was purified by column chromatography on Al2O3 (eluent PhMe). Yield 165 mg (95%), bright-yellow powder, mp 154–157°C. 1H NMR spectrum (600 MHz, CDCl3), δ, ppm (J, Hz): 8.91 (1H, s, H Ar); 8.65–8.61 (2H, m, H Ar); 8.18 (2H, d, J = 7.0, H Ar); 8.15 (1H, d, J = 8.8, H Ar); 7.96–7.87 (2H, m, H Ar); 7.62–7.58 (4H, m, H Ar); 7.51 (1H, t, J = 7.4, H Ar); 7.32 (1H, t, J = 7.4, H Ar); 1.48 (9H, s, C(CH3)3). 13C NMR spectrum (151 MHz, CDCl3), δ, ppm: 150.9; 147.1; 147.0; 144.4; 140.1; 132.8; 130.7; 130.3; 129.1; 129.0; 128.5; 127.5; 125.3; 124.6; 123.7; 120.6; 116.5; 34.9; 31.2. Found, %: C 82.69; H 6.09; N 11.22. C26H23N3. Calculated, %: C 82.73; H 6.14; N 11.13.
6-Nitro-1,3-diphenyl-1 H -pyrazolo[3,4- b ]quinoline (4i) precipitated from the reaction mixture, and the solid was washed with hot MeOH and filtered off. Yield 58 mg (38%), orange solid, mp 275°C (decomp.). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 9.18 (1H, s, H Ar); 9.10 (1H, d, J = 2.5, H Ar); 8.62–8.53 (3H, m, H Ar); 8.33 (1H, d, J = 9.4, H Ar); 8.22–8.15 (2H, m, H Ar); 7.70–7.56 (5H, m, H Ar); 7.41 (1H, t, J = 7.4, H Ar). 13C NMR was not recorded because of low solubility of compound 4i. Found, %: C 72.16; H 3.83; N 15.32. C22H14N4O2. Calculated, %: C 72.12; H 3.85; N 15.29; O 8.73.
1,3-Dimethyl-1 H -pyrazolo[3,4- b ]quinoline (4j) was purified by column chromatography on Al2O3 (eluent CHCl3) and dried under vacuum. Yield 127 mg (64%), light-yellow solid, mp 59–60°C (mp 68°C26). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.51 (1H, s, H Ar); 8.11 (1H, d, J = 8.7, H Ar); 7.97 (1H, d, J = 8.3, H Ar); 7.76 (1H, t, J = 7.0, H Ar); 7.44 (1H, t, J = 8.0, H Ar); 4.19 (3H, s, NCH3); 2.70 (3H, s, CCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 151.2; 148.4; 141.0; 130.5; 129.7; 129.3; 128.1; 123.7; 123.3; 116.8; 33.6; 12.7. Found, %: C 73.09; H 5.65; N 21.26. C12H11N3. Calculated, %: C 73.07; H 5.62; N 21.30.
1,3,6-Trimethyl-1 H -pyrazolo[3,4- b ]quinoline (4k) was prepurified by column chromatography on Al2O3 (eluent CHCl3), next purified by column chromatography on SiO2 (eluent PhMe) and dried under vacuum. Yield 50 mg (48%), yellow waxy solid. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.40 (1H, s, H Ar); 7.99 (1H, d, J = 8.8, H Ar); 7.69 (1H, s, H Ar); 7.60–7.56 (1H, m, H Ar); 4.17 (3H, s, NCH3); 2.67 (3H, s, CCH3); 2.54 (3H, s, CCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 151.0; 147.1; 140.8; 133.2; 129.9; 128.7; 127.8; 127.6; 123.8; 116.7; 33.6; 21.4; 12.7. Found, %: C 73.85; H 6.23; N 19.92. C13H13N3. Calculated, %: C 73.91; H 6.20; N 19.89.
6-Methoxy-1,3-dimethyl-1 H -pyrazolo[3,4- b ]quinoline (4l) was obtained by general method without further purification. Yield 110 mg (29%), yellow waxy solid. 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 8.75 (1H, s, H Ar); 7.98–7.85 (1H, m, H Ar); 7.51–7.42 (2H, m, H Ar); 4.02 (3H, s, NCH3); 3.90 (3H, s, OCH3); 2.61 (3H, s, CCH3). 13C NMR spectrum (101 MHz, DMSO-d6), δ, ppm: 155.2; 150.3; 144.5; 140.2; 129.4; 128.8; 124.8; 124.6; 116.6; 106.4; 55.8; 33.7; 12.8. Found, %: C 68.77; H 5.84; N 18.44. C13H13N3O. Calculated, %: C 68.70; H 5.77; N 18.49.
3-Methyl-1-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4m) was purified by column chromatography on SiO2 (eluent PhMe). Yield 84 mg (82%), yellow solid, mp 94–96°C (mp 97–98°C26). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.58–8.49 (3H, m, H Ar); 8.20 (1H, d, J = 8.8, H Ar); 8.04–7.96 (1H, m, H Ar); 7.82–7.77 (1H, m, H Ar); 7.63–7.53 (2H, m, H Ar); 7.52–7.48 (1H, m, H Ar); 7.33–7.27 (1H, m, H Ar); 2.79 (3H, s, CH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.5; 148.4; 143.4; 140.0; 130.6; 129.6; 129.1; 129.0; 128.9; 124.7; 124.2; 124.1; 120.2; 118.4; 12.8. Found, %: C 78.62; H 5.12; N 16.24. C17H13N3. Calculated, %: C 78.74; H 5.05; N 16.20.
3,6-Dimethyl-1-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4n) was purified by column chromatography on SiO2 (eluent PhMe). Yield 85 mg (83%), yellow solid, mp 121–123°C (mp 129°C31). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.55–8.50 (2H, m, H Ar); 8.41 (1H, s, H Ar); 8.08 (1H, d, J = 8.8, H Ar); 7.70 (1H, s, H Ar); 7.64–7.51 (3H, m, H Ar); 7.33–7.22 (1H, m, H Ar); 2.76 (3H, s, CH3); 2.57 (3H, s, CH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.2; 147.0; 143.2; 140.1; 133.7; 133.2; 129.0; 128.6; 128.5; 127.4; 124.7; 124.2; 119.9; 118.3; 21.5; 12.8. Found, %: C 79.20; H 5.50; N 15.30. C18H15N3. Calculated, %: C 79.10; H 5.53; N 15.37.
6-Methoxy-3-methyl-1-phenyl-1 H -pyrazolo[3,4- b ]- quinoline (4o) was purified by column chromatography on SiO2 (eluent PhMe). Yield 158 mg (77%), bright-yellow powder, mp 180–182°C (mp 158–160°C32). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.51 (2H, d, J = 7.6, H Ar); 8.40 (1H, s, H Ar); 8.08 (1H, d, J = 9.3, H Ar); 7.59– 7.54 (2H, m, H Ar); 7.50–7.45 (1H, m, H Ar); 7.31–7.25 (1H, m, H Ar); 7.19 (1H, d, J = 2.8, H Ar); 3.96 (3H, s, OCH3); 2.75 (3H, s, CCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 155.9; 149.7; 144.7; 142.7; 140.1; 130.2; 129.0; 127.6; 124.8; 124.7; 124.5; 119.8; 118.3; 105.2; 55.5; 12.8. Found, %: C 74.68; H 5.27; N 14.59. C18H15N3O. Calculated, %: C 74.72; H 5.23; N 14.52.
3-Methyl-6-nitro-1-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4p) precipitated from the reaction mixture, and the solid was washed with hot MeOH and filtered off. Yield 43 mg (31%), deep-yellow powder, mp 262–264°C, (mp 258– 259°C26). 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm (J, Hz): 9.39 (1H, s, H Ar); 9.23 (1H, d, J = 2.4, H Ar); 8.50 (1H, dd, J = 9.5, J = 2.5, H Ar); 8.42 (2H, d, J = 8.4, H Ar); 8.25 (1H, d, J = 9.5, H Ar); 7.61 (2H, t, J = 8.0, H Ar); 7.36 (1H, t, J = 7.4, H Ar); 2.79 (3H, s, CCH3). 13C NMR spectrum (101 MHz, DMSO), δ, ppm: 151.7; 149.9; 145.2; 143.8; 139.6; 134.9; 130.2; 129.6; 127.5; 126.0; 124.2; 122.7; 120.6; 119.9; 12.8. Found, %: C 67.15; H 4.02; N 18.35. C17H12N4O2. Calculated, %: C 67.10; H 3.97; N 18.41.
1-Methyl-3-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4q) was purified by column chromatography on SiO2 (eluent PhMe). Yield 96 mg (77%), yellow solid, mp 142–144°C (mp 146–147°C26). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.89 (1H, s, H Ar); 8.15 (1H, d, J = 8.8, H Ar); 8.09 (2H, d, J = 8.3, H Ar); 8.01 (1H, d, J = 8.4, H Ar); 7.79 (1H, m, H Ar); 7.59 (2H, t, J = 7.6, H Ar); 7.52– 7.44 (2H, m, H Ar); 4.32 (3H, s, NCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 151.3; 148.2; 142.8; 133.0; 131.0; 129.4; 129.1; 128.9; 128.6; 128.5; 128.2; 127.1; 124.5; 114.9; 34.0. Found, %: C 78.67; H 5.07; N 16.25. C17H13N3. Calculated, %: C 78.84; H 5.05; N 16.20.
1,6-Dimethyl-3-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4r) was purified by column chromatography on SiO2 (eluent gradient mixture of PhMe–EtOAc, from 10:1 to 3:1) and dried under vacuum. Yield 112 mg (85%), light-yellow solid, mp 141–144°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm: 8.79 (1H, s, H Ar); 8.12–8.02 (3H, m, H Ar); 7.76 (1H, s, H Ar); 7.65–7.54 (3H, m, H Ar); 7.50– 7.45 (1H, m, H Ar); 4.31 (3H, s, NCH3); 2.58 (3H, s, CCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 151.2; 147.0; 142.5; 133.5; 133.2 (2 signals); 130.0; 129.0; 128.4; 127.8; 127.7; 127.1; 124.6; 114.9; 34.0; 21.5. Found, %: C 79.46; H 5.61 N 14.93. C18H15N3. Calculated, %: C 79.10; H 5.53 N 15.37.
6-Methoxy-1-methyl-3-phenyl-1 H -pyrazolo[3,4- b ]- quinoline (4s) was purified by column chromatography on SiO2 (eluent gradient mixture of PhMe–EtOAc, from 10:1 to 3:1) and dried under vacuum. Yield 90 mg (64%), lightyellow solid, mp 151–153°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 8.77 (1H, s, H Ar); 8.10–8.02 (3H, m, H Ar); 7.60–7.54 (2H, m, H Ar); 7.51–7.43 (2H, m, H Ar); 7.22 (1H, d, J = 2.7, H Ar); 4.29 (3H, s, NCH3); 3.97 (3H, s, OCH3). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 155.6; 150.6; 144.7; 142.0; 133.2; 129.5; 129.0; 128.9 (2 signals); 128.5; 127.0; 125.0; 114.9; 105.2; 55.5; 34.1. Found, %: C 74.58; H 5.15; N 14.40. C18H15N3O. Calculated, %: C 74.72; H 5.23; N 14.52.
1-Methyl-6-nitro-3-phenyl-1 H -pyrazolo[3,4- b ]quinoline (4t) was purified by column chromatography on SiO2 (eluent gradient mixture of PhMe–EtOAc, from 1:0 to 50:1) and dried under vacuum. Yield 55 mg (27%), deepyellow powder, mp >300°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 9.12 (1H, s, H Ar); 9.07 (1H, d, J = 2.5, H Ar); 8.54 (1H, dd, J = 9.5, J = 2.6, H Ar); 8.24 (1H, d, J = 9.5, H Ar); 8.12–8.05 (2H, m, H Ar); 7.62 (2H, t, J = 7.5, H Ar); 7.57–7.50 (1H, m, H Ar); 4.35 (3H, s, NCH3). 13C NMR spectrum was not recorded because of low solubility of compound 4u. Found, %: C 67.38; H 3.89; N 18.21. C17H12N4O2. Calculated, %: C 67.10; H 3.97; N 18.41.
8,10-Diphenyl-10 H -benzo[ h ]pyrazolo[3,4- b ]quinoline (4u) was purified by column chromatography on SiO2 (eluent PhMe – petroleum ether (bp 60–90°C), 1:1). Yield 135 mg (89%), light-yellow powder, mp 210–212°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 9.33 (1H, d, J = 7.8, H Ar); 8.78–8.69 (3H, m, H Ar); 8.23–8.14 (2H, m, H Ar); 7.91–7.82 (1H, m, H Ar); 7.80–7.70 (3H, m, H Ar); 7.69–7.59 (5H, m, H Ar); 7.57–7.50 (1H, m, H Ar); 7.43–7.35 (1H, m, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 150.0; 146.8; 144.3; 140.1; 134.4; 132.8; 131.2; 130.1; 129.0; 128.9; 128.9; 127.8; 127.5; 126.9; 126.2; 125.8; 125.4; 125.3; 122.7; 120.7; 115.7. Found, %: C 84.23; H 4.69; N 11.08. C26H17N3. Calculated, %: C 84.07; H 4.61; N 11.31.
8,10-Diphenyl-8 H -benzo[ f ]pyrazolo[3,4- b ]quinoline (4v) was purified by column chromatography on Al2O3 (eluent CHCl3). Yield 270 mg (80%), light-yellow solid, mp 244– 247°C. 1H NMR spectrum (400 MHz, CDCl3), δ, ppm (J, Hz): 9.69 (1H, s, H Ar); 8.79 (1H, d, J = 8.3, H Ar); 8.66 (2H, d, J = 7.6, H Ar); 8.24 (2H, d, J = 7.1, H Ar); 8.13– 8.03 (2H, m, H Ar); 7.98 (1H, d, J = 7.8, H Ar); 7.78 (1H t, J = 7.0, H Ar); 7.72–7.53 (6H, m, H Ar); 7.37 (1H, t, J = 7.4, H Ar). 13C NMR spectrum (101 MHz, CDCl3), δ, ppm: 148.6; 144.7; 139.9; 132.8; 132.7; 130.9; 130.6; 129.2; 129.1; 129.0; 128.9; 128.4; 127.7; 127.5; 126.9; 125.6; 125.1; 122.2; 121.8; 120.8; 115.5. Found, %: C 84.34; H 4.74; N 10.92. C26H17N3. Calculated, %: C 84.07; H 4.61; N 11.31.
Supplementary information file containing 1H and 13C NMR spectra of synthesized compounds is available at the journal website http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
Michaelis, A. Justus Liebigs Ann. Chem. 1911, 385, 1.
Musierowicz, A.; Niementowski, S.; Tomasik, Z. Rocz. Chem. 1928, 8, 325.
Wolfrum, G.; Putter, R.; Hanke, H.-G.; Menzel, K.-H. Patent US3234142.
Stein, R. G.; Biel, J. H.; Singh, T. J. Med. Chem. 1970, 13, 153.
Crenshaw, R. R.; Luke, G. M.; Siminoff, P. J. Med. Chem. 1976, 19, 262.
Rádl, S.; Zikán, V.; Šmejkal, F. Czesk. Farm. 1984, 33, 429.
Jitender, D. G.; Poornachandra, Y.; Reddy, K. R.; Kumar, N. R.; Ravikumar, N.; Swaroop, D. K.; Ranjithreddy, P.; Kumar, S. G.; Nanubolu, B. J.; Kumar, C. G.; Narsaiah, B. Eur. J. Med. Chem. 2017, 130, 223.
Brack, A. Liebigs Ann. Chem. 1965, 681, 105.
Parusel, A. B. J.; Rechthaler, K.; Piorun, D.; Danel, A.; Khatchatryan, K.; Rotkiewicz, K.; Köhler, G. J. Fluoresc. 1998, 8, 375.
Rechthaler, K.; Rotkiewicz, K.; Danel, A.; Tomasik, P.; Khatchatryan, K.; Kohler, G. J. Fluoresc. 1997, 7, 301.
Rurack, K.; Danel, A.; Rotkiewicz, K.; Grabka, D.; Spieles, M.; Rettig, W. Org. Lett. 2002, 4, 4647.
Mac, M.; Uchacz, T.; Danel, A.; Musiolik, H. J. Fluoresc. 2013, 23, 1207.
Danel, A.; Kolbus, A.; Grabka, D.; Kucharek, M.; Pokładko- Kowar, M. Dyes Pigm. 2021, 195, 109713.
Chen, C.-H.; Wu, F.-I.; Shu, C.-F.; Chien, C.-H; Tao, Y.-T. J. Mater. Chem. 2004, 14, 1585.
Wan, W.; Wang, H.; Lin, H.; Wang, J.; Jiang, Y.; Jiang, H.; Zhu, S.; Wang, Z.; Hao, J. Dyes Pigm. 2015, 121, 138.
He, Z.; Milburn, G. H. W.; Danel, A.; Puchała, A.; Tomasik, P.; Rasała, D. J. Mater. Chem. 1997, 7, 2323.
Danel, A. PhD Thesis; Krakow, 1996.
Tomasik, P.; Tomasik, D.; Abramovitch, R. J. Heterocycl. Chem. 1983, 20, 1539.
Marjani, A. P.; Khalafy, J.; Salami, F.; Mohammadlou, M. Synthesis 2015, 47, 1656.
Ezzati, M.; Khalafy, J.; Marjani, A. P.; Prager, R. H. Tetrahedron 2017, 73, 6587.
Marjani, A. P.; Khalafy, J.; Akbarzadeh, S. Green Process. Synth. 2019, 8, 533.
Yadav, P.; Awasthi, A.; Gokulnath, S.; Tiwari, D. K. J. Org. Chem. 2021, 86, 2658.
Danel, A.; Gondek, E.; Kucharek, M.; Szlachcic, P.; Gut, A. Molecules 2022, 27, 2775.
Purnaprajna, V.; Seshadri, S. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1976, 14B, 971.
Kucharek, M.; Danel, A. Chem. Heterocycl. Compd. 2021, 57, 633.
Szlachcic, P.; Kucharek, M.; Jarosz, B.; Danel, A.; Stadnicka, K. J. Heterocycl. Chem. 2017, 54, 1729.
Całus, S.; Gondek, E.; Pokladko, M.; Kulig, E.; Jarosz, B.; Kityk, A. V. Spectrochim. Acta, Part A 2007, 67, 1007.
Gondek, E.; Danel, A.; Nizioł, J.; Armatys, P.; Kityk, I. V.; Szlachcic, P.; Karelus, M.; Uchacz, T.; Chwast, J.; Lakshminarayana, G. J. Lumin. 2010, 130, 2093.
Mac, M.; Uchacz, T.; Andrzejak, M.; Danel, A.; Szlachcic, P. J. Photochem. Photobiol., A 2007, 187, 78.
Gondek, E.; Nosidlak, N.; Kulig, E.; Danel, A.; Al Zayed, N.; Kityk, I. V.; Karasinki, P. J. Mater. Sci.: Mater. Electron. 2013, 24, 613.
Brack, A. BE Patent 617180.
Shiba, S. A.; Harb, N. M. S.; Hassan, M. A.; El-Kassaby, M. A.; Abou-El-Regal, M. M. K. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1996, 35B, 426.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(11), 639–645
Supplementary Information
ESM 1
(PDF 3117 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kucharek, M., Danel, A. & Gut, A. A simple and efficient synthesis of 1,3,6-trisubstituted pyrazoloquinolines via intramolecular cyclization – optimization studies. Chem Heterocycl Comp 58, 639–645 (2022). https://doi.org/10.1007/s10593-022-03127-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03127-1