N-Boc-4-aminopyrazole-5-carbaldehydes react with methyl 3,3-dimethoxypropanoate or β-keto esters in acetic acid under reflux to form methyl(ethyl) pyrazolo[4,3-b]pyridine-6-carboxylates, which were converted to the corresponding tert-butyl carboxylates via intermediate carboxylic acids. Their subsequent hydrogenation on a 10% Pd/C catalyst at 100°C and 25 atm afforded tert-butyl 4,5,6,7-tetrahydropyrazolo[4,3-b]pyridine-6-carboxylates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nipecotic (piperidine-3-carboxylic) acid (I) and its various N-substituted derivatives are effective inhibitors of the γ-aminobutyric acid (GABA) transporter of the GAT-1 subtype.1,2,3,4,5,6,7,8 Their pronounced pharmacological action resulted in the development and introduction into the therapeutic practice of the antiepileptic drug tiagabine (II)9 (Fig. 1).
Recently, nipecotic acid derivatives in which the piperidine ring is annulated with aromatic10,11,12,13,14,15 and heteroaromatic11,13,16,17,18 rings began to attract the attention of experts in the design of bioactive compounds. These studies revealed inhibitors of myeloid cell leukemia among 3-carboxy-substituted 1,2,3,4-tetrahydroquinolines12 and potential agents for the treatment of autoimmune diseases among 4,5,6,7-tetrahydropyrazolo[4,3-b]pyridine-6-carboxylic acid amides.17,18 It should be noted that in the latter case, the method for accessing the key compounds pyrazolo-[4,3-b]pyridine-6-carboxylic acids involves 9 steps and is based on annulation of the pyrazole ring to the polysubstituted pyridine backbone.
We have proposed a novel approach to the synthesis of derivatives of pyrazolo[4,3-b]pyridine-6-carboxylic acid based on the annulation of the pyridine ring to the pyrazole ring. The method for the synthesis of functionalized pyrazolo[4,3-b]pyridines assumes the use as substrates of previously synthesized by us N-Boc-4-aminopyrazole-5-carbaldehydes 1a–d which have shown their efficiency as bicenter components in cyclocondensations with ketones,19 β-diketones,20 as well as derivatives of malonic21 and cyanoacetic22 acids.
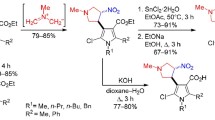
It was found that, in addition to the aforementioned active methylene compounds, amino aldehydes 1a–d in the presence of an equimolar amount of pyrrolidine in acetic acid under reflux react with methyl 3,3-dimethoxypropanoate 2a to form 5-unsubstituted pyrazolo[4,3-b]-pyridine-6-carboxylates 3a–d or with β-keto esters 2b–d to form 5-substituted (including with fluoromethyl groups) pyrazolo[4,3-b]pyridine-6-carboxylates 3e–k (Scheme 1). A feature of the reaction of 1-tert-butyl-substituted amino aldehyde 1c is that, along with carboxylate 3f (24% yield), the product of its hydrolysis and subsequent decarboxylation, compound 4, was isolated in 42% yield (Scheme 1).

Scheme 1
The IR spectra of compounds 3а–k contain absorption bands of ester carbonyl groups in the range of 1706–1713 cm–1. In the 1H NMR spectra of compounds 3a–d, the signals of protons H-5 appear as singlets in the 8.98–9.12 ppm range, while the signals of protons H-7 of all compounds 3а–k are manifest as singlets in the range of 8.55–8.81 ppm.
Usually, for pressure hydrogenation of the pyridine ring of pyrazolo[4,3-b]pyridine structures, depending on the presence and position of substituents, Pd/C (for nonfunctionalized derivatives),17 20% Pd(OH)2/C (for 5-carboxy(cyano) derivatives),23 and PtO2 (for 6-aminocarbonyl derivatives) catalysts are employed. Taking into account the availability and low cost of Pd/C catalysts, we thought it expedient to investigate their application for the reduction of the pyridine fragment of alkyl carboxylates 3а–k. Using model compounds 3a,j it was shown by LCMS that their hydrogenation with 10% Pd/C (at 60°C in MeOH solution for compound 3a or at 80°C in EtOH solution for compound 3j) under 25 atm pressure for 24 h leads to the formation of tetrahydro derivatives 5a,b (Scheme 2, the method is similar to that used in the preparation of compounds 9а–k) with a content in the reaction mixture of 12 and 15%, respectively. These products were registered by LC-MS but could not be isolated. It is possible that this result is a consequence of side condensations involving the alkoxycarbonyl group of the reduced products 5a,b. It could be assumed that a decrease in the electrophilicity of these groups by substitution with a more bulky tert-butyl group would prevent the occurrence of side processes and would allow obtaining the target reduction products in preparative yields.

Scheme 2
In this context, in the first step, alkyl carboxylates 3а–k were converted into carboxylic acids 6а–k by boiling in an aqueous ethanolic KOH (Scheme 2). A specially performed experiment of catalytic hydrogenation of acid 6j under the conditions described above for ester 3j showed that during the reduction of the pyridine ring, decarboxylation and the formation of pyrazolopiperidine 7 also take place. This result indicates that acids 6а–k cannot be used as substrates for hydrogenation with preservation of the carboxyl functionality. For this reason, tert-butyl pyrazolo[4,3-b]-pyridine-6-carboxylates 8а–k were synthesized in high yields by alkylation of the acids with di-tert-butyl carbonate (Boc2O) in the presence of 4-dimethylaminopyridine (DMAP) in THF under reflux (Scheme 2). They have proven to be suitable reagents for catalytic reduction and preparation of the desired tetrahydropyridine ring. It was found that, under rather harsh conditions in a t-BuOH solution at 100°С and an H2 pressure of 25 atm, they are converted in 52–92% yields into hydrogenated derivatives 9а–k with retention of the ester functionality (Scheme 2).
Spectral study of compounds 9а–k, including IR, 1Н, 13С NMR spectroscopy, and mass spectrometry, confirms the presence of the tetrahydropyridine ring in the structures of these compounds, but does not allow to unambiguously determine the stereochemistry of substituents R2 and CO2t-Bu at C-5 and C-6 atoms of compounds 9e–k. This problem was successfully solved by X-ray structural analysis of acid hydrochloride 10, obtained by mild acid hydrolysis of ester 9h, as an example (Fig. 2). It was found that the difluoromethyl and tert-butoxycarbonyl groups are in the axial-equatorial position; therefore, the same configuration of substituents in the tetrahydropyridine ring is accepted for the entire series of compounds 9e–k.
The general view of the molecule of compound 10 (atoms represented as thermal vibration ellipsoids with 50% probability) and its major geometrical parameters: bond lengths N(1)–N(2) 1.346(3), N(1)–C(3) 1.350(3), N(2)–C(1) 1.335(3), C(1)–C(2) 1.388(3), C(2)C(3) 1.393(3) Å; angles N(2)–N(1)–C(3) 108.6(2), C(1)–N(2)–N(1) 109.5(2), N(2)–C(1)–C(2) 108.0(2), C(1)–C(2)–C(3) 106.2(2), N(1)–C(3)–C(2) 107.7(2), C(3)–C(2)–N(3) 122.6(2), C(2)–N(3)–C(6) 114.5(2)°.
In compound 10, the pyrazole ring is planar; the mean square deviation of atoms from the plane equals 0.004 Å. The six-membered ring has the twist conformation with the N(3)–C(2)–C(3)–C(4) atoms lying in one plane (the mean square deviation of these atoms from the plane is 0.0067 Å), whereas the C(5) and C(6) atoms leave this plane in opposite directions by 0.506 and –0.232 Å. The pyrazole ring has the usual distribution of bond lengths and bond angles and indicates the delocalization of the electron density which leads to values of bond length intermediate between those of single and double bonds. Bonds N(3)–C(2) and N(3)–C(6) (1.403(3) and 1.458(3) Å, respectively) are nonequivalent; the first one is shorter in comparison with the standard length of a single N–C bond (1.45–1.47 Å) due to the conjugation of the LEP of the N(3) atom with the π-system of the pyrazole ring, while the length of the second bond, like that of the N(1)–C(7) bond at 1.465 (3) Å, is in the typical range for single C–N bonds.
Molecules in the solid state are connected via hydrogen bonds O(1)–H(10)···Cl(1), N(2)–H(2N)···Cl(1) and N(3)–H(3N)···O(2) (Table 1).
To conclude, an efficient approach to the synthesis of methyl(ethyl) pyrazolo[4,3-b]pyridine-6-carboxylates – basic structures for the synthesis of their tert-butyl analogs through intermediate carboxylic acids – has been developed based on cyclocondensation of N-Boc-4-aminopyrazole-5-carbaldehydes with methyl 3,3-dimethoxypropanoate or β-keto esters. A new variant of the catalytic (Pd/C) hydrogenation of tert-butyl pyrazolo[4,3-b] pyridine-6-carboxylates was employed to obtain nipecotic acid derivatives annulated with the pyrazole ring.
Experimental
IR spectra were registered on a Bruker Vertex 70 spectrometer in KBr pellets. 1H and 13C NMR spectra were acquired on a Bruker Avance 500 spectrometer (500 and 126 MHz, respectively ) in pulse Fourier transform mode in DMSO-d6 with TMS as internal standard. 19F NMR spectra were recorded on a Varian Mercury-400 spectrometer (377 MHz) in DMSO-d6 with CF3Cl as internal standard. Elemental analysis was performed on a Perkin Elmer Series II 2400 CHN-analyzer. Mass spectra (atmospheric pressure electrospray ionization) were registered on an Agilent LC/MSD 1100 system; Zorbax SB-C18, 4.6 × 15 mm, 1.8 μm, mobile phase H2O–MeCN. Chromatographic separation of compounds 3f and 4 was carried out on a Teledyn Isco Combiflash Companion preparative chromatograph (eluent CHCl3–EtOAc, 4:1). Melting points were determined on a Kofler bench and are uncorrected.
Compounds 1а,с,d,19 and 1b21 used in the study are described previously.
Synthesis of alkyl pyrazolo[4,3- b ]pyridine-6-carboxylates 3a–k (General method). 3,3-Dimethoxypropanoate 2a (8.88 g, 0.06 mol) or β-keto ester 2b–d (0.06 mol) and pyrrolidine (5 ml, 0.06 mol) were successively added to N-Boc-4-aminopyrazole-5-carbaldehyde 1a–d (0.05 mol) in glacial AcOH (150 ml), and the mixture was heated under reflux for 8 h. The solvent was distilled off under reduced pressure, H2O (200 ml) was added to the formed oily residue, and the resulting mixture was extracted with EtOAc (3×150 ml). The combined organic layers were dried over anhydrous Na2SO4 and evaporated under reduced pressure. In case of obtaining products 3f and 4, they were isolated by chromatography. In other cases, MeCN (150 ml) was added to the resulting oily product, heated to dissolution, the solution was cooled to room temperature and left overnight at –18°C. The formed precipitate was filtered off and air-dried.
Methyl 1-methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3a). Yield 5.92 g (62%), light-yellow powder, mp 144–145°C. IR spectrum, ν, cm–1: 1712 (С=О). 1H NMR spectrum, δ, ppm: 3.92 (3H, s, ОCH3); 4.14 (3H, s, CH3); 8.37 (1H, s, H-3); 8.74 (1H, s, H-7); 8.98 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 36.7; 53.0; 121.2; 121.4; 122.5; 132.2; 133.0; 145.2; 165.8. Mass spectrum, m/z (Irel, %): 192 [M+Н]+ (100). Found, %: C 56.71; H 4.80; N 21.91. C9H9N3O2. Calculated, %: C 56.54; H 4.74; N 21.98.
Methyl 1-ethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3b). Yield 6.15 g (60%), yellow powder, mp 138–139°C. IR spectrum, ν, cm–1: 1707 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.42 (3H, t, J = 7.2, CH3); 3.95 (3H, s, ОCH3); 4.58 (2H, q, J = 7.2, CH2); 8.42 (1H, s, H-3); 8.80 (1H, s, H-7); 9.02 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 15.4; 44.3; 52.9; 120.3; 122.4; 131.1; 133.6; 143.5; 145.6; 166.0. Mass spectrum, m/z (Irel, %): 206 [M+Н]+ (100). Found, %: C 58.34; H 5.49; N 20.66. C10H11N3O2. Calculated, %: C 58.53; H 5.40; N 20.48.
Methyl 1- tert -butyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3c). Yield 7.10 g (61%), light-brown powder, mp 99–100°C. IR spectrum, ν, cm–1: 1709 (С=О). 1H NMR spectrum, δ, ppm: 1.74 (9H, s, 3CH3); 3.94 (3H, s, ОCH3); 8.39 (1H, s, H-3); 8.71 (1H, s, H-7); 8.99 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 29.8; 53.0; 61.5; 121.9; 122.0; 129.9; 132.6; 144.8; 145.2; 166.0. Mass spectrum, m/z (Irel, %): 234 [M+Н]+ (100). Found, %: C 61.96; H 6.43; N 18.11. C12H15N3O2. Calculated, %: C 61.79; H 6.48; N 18.01.
Methyl 1-phenyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3d). Yield 4.17 g (33%), brown powder, mp 175– 177°C. IR spectrum, ν, cm–1: 1706 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 3.92 (3H, s, ОCH3); 7.50 (1H, t, 3J = 6.8, Н Ph); 7.66 (2H, t, 3J = 8.0, Н Ph); 7.81 (2H, d, 3J = 8.0, Н Ph); 8.64 (1H, s, H-3); 8.76 (1H, s, H-7); 9.12 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 53.1; 120.7; 122.8; 123.5; 128.1; 130.4; 130.7; 136.3; 139.1; 144.8; 146.5; 165.7. Mass spectrum, m/z (Irel, %): 254 [M+Н]+ (100). Found, %: C 66.21; H 4.45; N 16.42. C14H11N3O2. Calculated, %: C 66.40; H 4.38; N 16.59.
Ethyl 1,5-dimethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3e). Yield 6.82 g (62%), light-yellow powder, mp 136–137°C. IR spectrum, ν, cm–1: 1711 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.37 (3H, t, 3J = 6.8, CH2CH3); 2.76 (3H, s, CH3); 4.12 (3H, s, CH3); 4.36 (2H, q, 3J = 7.2, CH2CH3); 8.22 (1H, s, H-3); 8.55 (1H, s, H-7). 13C NMR spectrum, δ, ppm: 14.5; 25.1; 36.6; 61.7; 121.2; 123.4; 130.9; 132.5; 141.9; 153.1; 167.0. Mass spectrum, m/z (Irel, %): 220 [M+Н]+ (100). Found, %: C 60.09; H 5.83; N 19.02. C11H13N3O2. Calculated, %: C 60.26; H 5.98; N 19.17.
Ethyl 1- tert -butyl-5-methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (3f). Yield 3.13 g (24%), brown powder, mp 132–133°C. IR spectrum, ν, cm–1: 1707 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.36 (3H, t, 3J = 7.0, CH2CH3); 1.72 (9H, s, 3CH3); 2.75 (3H, s, CH3); 4.36 (2H, q, 3J = 7.2, CH2CH3); 8.22 (1H, s, H-3); 8.59 (1H, s, H-7). 13C NMR spectrum, δ, ppm: 14.5; 24.9; 29.8; 61.1; 61.7; 122.8; 123.0; 129.0; 131.8; 143.2; 152.6; 167.1. Mass spectrum, m/z (Irel, %): 262 [M+Н]+ (100). Found, %: C 64.13; H 7.39; N 16.16. C14H19N3O2. Calculated, %: C 64.35; H 7.33; N 16.08.
Ethyl 5-(difluoromethyl)-1-methyl-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylate (3g). Yield 9.81 g (77%), orange powder, mp 151–153°C. IR spectrum, ν, cm–1: 1709 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.40 (3H, t, 3J = 6.4, CH2CH3); 4.20 (3H, s, CH3); 4.44 (2H, q, 3J = 7.6, CH2CH3); 7.47 (1H, d, 2JHF = 54.4, CHF2); 8.50 (1H, s, H-3); 8.74 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 14.4; 36.9; 62.4; 112.0 (t, 1JСF = 237.3); 122.2; 123.1; 131.9; 134.2; 141.3; 145.9 (t, 2JCF = 22.6); 165.6. 19F NMR spectrum, δ, ppm (J, Hz): –115.15 (d, 2JFH = 51.8, CHF2). Mass spectrum, m/z (Irel, %): 256 [M+Н]+ (100). Found, %: C 51.93; H 4.18; N 14.68. C11H11F2N3O2. Calculated, %: C 51.77; H 4.34; N 14.89.
Ethyl 5-(difluoromethyl)-1-ethyl-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylate (3h). Yield 11.57 g (86%), lightbrown powder, mp 130–131°C. IR spectrum, ν, cm–1: 1713 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.35–1.46 (6H, m, 2CH2CH3); 4.40 (2H, q, 3J = 7.2, CH2CH3); 4.61 (2H, q, 3J = 7.2, CH2CH3); 7.49 (1H, d, 2JHF =54.1, CHF2); 8.55 (1H, s, H-3); 8.81 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 14.3; 15.2; 44.5; 62.4; 112.0 (t, 1JCF = 238.2); 121.9; 123.0; 131.1; 134.3; 141.3; 146.0 (t, 2JCF = 25.0); 165.6. 19F NMR spectrum, δ, ppm (J, Hz): –115.09 (d, 2JFH = 51.7, CHF2). Mass spectrum, m/z (Irel, %): 270 [M+Н]+ (100). Found, %: C 53.66; H 4.91; N 15.68. C12H13F2N3O2. Calculated, %: C 53.53; H 4.87; N 15.61.
Ethyl 1- tert -butyl-5-(difluoromethyl)-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (3i). Yield 11.88 g (80%), brown powder, mp 161–163°C. IR spectrum, ν, cm–1: 1709 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.35 (3H, t, 3J = 7.0, CH2CH3); 1.74 (9H, s, 3CH3); 4.39 (2H, q, 3J = 7.2, CH2CH3); 7.44 (1H, d, 2JHF = 54.5, CHF2); 8.52 (1H, s, H-3); 8.75 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 14.3; 28.3; 29.8; 62.4; 112.1 (t, 1JCF = 240.0); 122.8; 123.5; 129.9; 133.4; 142.4; 145.5 (t, 2JCF = 25.4); 165.7. 19F NMR spectrum, δ, ppm (J, Hz): –115.21 (d, 2JFH = 51.8, CHF2). Mass spectrum, m/z (Irel, %): 298 [M+Н]+ (100). Found, %: C 56.63; H 5.80; N 14.20. C14H17F2N3O2. Calculated, %: C 56.56; H 5.76; N 14.13.
Ethyl 1-methyl-5-(trifluoromethyl)-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylate (3j). Yield 11.05 g (81%), orange powder, mp 146–148°C. IR spectrum, ν, cm–1: 1708 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.35 (3H, t, 3J = 7.2, CH2CH3); 4.19 (3H, s, CH3); 4.41 (2H, q, 3J = 7.2, CH2CH3); 8.60 (1H, s, H-3); 8.77 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 13.7; 36.4; 62.7; 121.3; 121.9 (q, 1JCF = 273.7); 123.8; 131.7; 134.1; 138.6 (q, 2JCF = 33.8); 139.9; 165.4. 19F NMR spectrum, δ, ppm (J, Hz): –62.56 (s, CF3). Mass spectrum, m/z (Irel, %): 274 [M+Н]+ (100). Found, %: C 48.27; H 3.74; N 15.31. C11H10F3N3O2. Calculated, %: C 48.36; H 3.69; N 15.38.
Ethyl 1-ethyl-5-(trifluoromethyl)-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylate (3k). Yield 11.48 g (80%), yellow powder, mp 118–119°C. IR spectrum, ν, cm–1: 1710 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.33–1.45 (6H, m, 2CH2CH3); 4.41 (2H, q, 3J = 7.2, CH2CH3); 4.59 (2H, q, 3J = 7.0, CH2CH3); 8.62 (1H, s, H-3); 8.81 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 14.1; 15.2; 44.7; 62.7; 120.5 (q, 1JCF = 273.8); 121.5; 124.3; 131.4; 134.7; 139.1 (q, 2JCF = 33.7); 140.3; 165.9. 19F NMR spectrum, δ, ppm: –61.48 (s, CF3). Mass spectrum, m/z (Irel, %): 288 [M+Н]+ (100). Found, %: C 50.25; H 4.17; N 14.69. C12H12F3N3O2. Calculated, %: C 50.18; H 4.21; N 14.63.
1- tert -Butyl-5-methyl-1 Н -pyrazolo[4,3- b ]pyridine (4). Yield 3.97 g (42%), brownish liquid. 1H NMR spectrum, δ, ppm (J, Hz): 1.68 (9H, s, 3CH3); 2.56 (3H, s, CH3); 7.20 (1H, d, 3J = 8.8, H Ar); 8.07 (1H, s, H-3); 8.21 (1H, 3J = 8.8, H Ar). 13C NMR spectrum, δ, ppm: 24.3; 29.7; 60.3; 120.8; 120.9; 129.8; 131.4; 142.4; 153.3. Mass spectrum, m/z (Irel, %): 190 [M+Н]+ (100). Found, %: C 69.97; H 8.06; N 22.12. C11H15N3. Calculated, %: C 69.81; H 7.99; N 22.20.
Synthesis of N-substituted pyrazolo[4,3-b]pyridine-6-carboxylic acids 6a–k (General method). 2 M Aqueous KOH (100 ml) was added with stirring to a solution of pyrazolo[4,3-b]pyridine-6-carboxylate 3a–k (0.025 mol) in EtOH (50 ml), and the resulting mixture was heated under reflux for 3 h. The solvent was distilled off under reduced pressure, H2O (100 ml) was added to the residue, and the resulting mixture was acidified with 1 M HCl to pH 3. The formed precipitate was stirred for 10–15 min, filtered off, air-dried, and recrystallized from MeOH.
1-Methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6а). Yield 4.29 g (97%), white powder, mp 206–208°C. IR spectrum, ν, cm–1: 1704 (С=О), 2408–2613 (COOH dimer). 1H NMR spectrum, δ, ppm: 4.16 (3H, s, CH3); 8.36 (1H, s, H-3); 8.69 (1H, s, H-7); 9.01 (1H, s, H-5); 13.62 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 36.6; 120.5; 123.5; 132.1; 133.3; 143.4; 146.0; 167.0. Mass spectrum, m/z (Irel, %): 178 [M+Н]+ (100). Found, %: C 54.08; H 4.04; N 23.56. C8H7N3O2. Calculated, %: C 54.24; H 3.98; N 23.72.
1-Ethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6b). Yield 4.01 g (84%), beige powder, mp 215–217°C. IR spectrum, ν, cm–1: 1709 (С=О), 2388–2603 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 1.41 (3H, t, 3J = 7.2, CH2CH3); 4.58 (2H, q, 3J = 7.2, CH2CH3); 8.40 (1H, s, H-3); 8.74 (1H, s, H-7); 9.00 (1H, s, H-5); 13.36 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 15.4; 44.3; 120.3; 123.5; 131.3; 133.5; 143.4; 146.0; 167.1. Mass spectrum, m/z (Irel, %): 192 [M+Н]+ (100). Found, %: C 56.32; H 4.80; N 21.83. C9H9N3O2. Calculated, %: C 56.54; H 4.74; N 21.98.
1- tert -Butyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6c). Yield 4.60 g (84%), brown powder, mp 203–205°C. IR spectrum, ν, cm–1: 1703 (С=О), 2399–2614 (COOH dimer). 1H NMR spectrum, δ, ppm: 1.72 (9H, s, 3CH3); 8.36 (1H, s, H-3); 8.68 (1H, s, H-7); 8.98 (1H, s, H-5); 13.91 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 29.8; 61.5; 122.0; 123.0; 130.1; 132.6; 144.6; 145.6; 167.0. Mass spectrum, m/z (Irel, %): 220 [M+Н]+ (100). Found, %: C 60.47; H 6.13; N 19.28. C11H13N3O2. Calculated, %: C 60.26; H 5.98; N 19.17.
1-Phenyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6d). Yield 5.38 g (90%), beige powder, mp 233–235°C. IR spectrum, ν, cm–1: 1709 (С=О), 2418–2607 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 7.48 (1H, t, 3J = 7.2, Н Ph); 7.65 (2H, t, 3J = 7.6, Н Ph); 7.83 (2H, d, 3J = 8.0; Н Ph); 8.62 (1H, s, H-3); 8.73 (1H, s, H-7); 9.10 (1H, s, H-5); 14.06 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 120.7; 122.8; 124.7; 128.9; 130.4; 130.9; 136.3; 139.2; 144.9; 147.0; 165.5. Mass spectrum, m/z (Irel, %): 240 [M+Н]+ (100). Found, %: C 65.05; H 3.91; N 17.72. C13H9N3O2. Calculated, %: C 65.27; H 3.85; N 17.56.
1,5-Dimethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6е). Yield 4.44 g (93%), white powder, mp 242–244°C. IR spectrum, ν, cm–1: 1705 (С=О), 2425–2594 (COOH dimer). 1H NMR spectrum, δ, ppm: 2.80 (3H, s, CH3); 4.11 (3H, s, CH3); 8.22 (1H, s, H-3); 8.59 (1H, s, H-7); 13.25 (1H, br. s, СО2Н). 13C NMR spectrum, δ, ppm: 25.3; 36.5; 121.3; 124.1; 131.1; 132.4; 141.8; 153.5; 168.6. Mass spectrum, m/z (Irel, %): 192 [M+Н]+ (100). Found, %: C 56.71; H 4.85; N 21.91. C9H9N3O2. Calculated, %: C 56.54; H 4.79; N 21.98.
1- tert -Butyl-5-methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6f). Yield 5.07 g (87%), yellow powder, mp 195–197°C. IR spectrum, ν, cm–1: 1708 (С=О), 2415–2599 (COOH dimer). 1H NMR spectrum, δ, ppm: 1.72 (9H, s, 3CH3); 2.81 (3H, s, CH3); 8.25 (1H, s, H-3); 8.69 (1H, s, H-7); 13.69 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 24.5; 29.8; 61.4; 123.8; 124.1; 129.6; 131.0; 143.5; 153.0; 168.1. Mass spectrum, m/z (Irel, %): 234 [M+Н]+ (100). Found, %: C 61.63; H 6.55; N 18.18. C12H15N3O2. Calculated, %: C 61.79; H 6.48; N 18.01.
5-(Difluoromethyl)-1-methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6g). Yield 5.39 g (95%), lightyellow powder, mp 205–207°C. IR spectrum, ν, cm–1: 1710 (С=О), 2418–2603 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 4.19 (3H, s, CH3); 7.60 (1H, d, 2JHF = 54.8, CHF2); 8.51 (1H, s, H-3); 8.79 (1H, s, H-7); 13.54 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm (J, Hz): 36.4; 111.3 (t, 1JCF = 237.1); 121.9; 123.4; 131.7; 133.7; 140.9; 145.8 (t, 2JCF = 25.0); 166.7. 19F NMR spectrum, δ, ppm (J, Hz): –115.37 (d, 2JFH = 56.5, CHF2). Mass spectrum, m/z (Irel, %): 228 [M+Н]+ (100). Found, %: C 47.36; H 3.26; N 18.42. C9H7F2N3O2. Calculated, %: C 47.58; H 3.11; N 18.50.
5-(Difluoromethyl)-1-ethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6h). Yield 5.72 g (95%), beige powder, mp 210–212°C. IR spectrum, ν, cm–1: 1707 (С=О), 2410–2593 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 1.41 (3H, t, 3J = 7.0, CH2CH3); 4.58 (2H, q, 3J = 7.4, CH2CH3); 7.59 (1H, d,2JHF = 54.8, CHF2); 8.50 (1H, s, H-3); 8.80 (1H, s, H-7); 13.75 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm (J, Hz): 15.4; 44.6; 111.7 (t, 1JCF = 234.0); 122.1; 123.8; 131.3; 134.3; 141.4; 146.5 (t, 2JCF = 25.6); 167.2. 19F NMR spectrum, δ, ppm (J, Hz): –115.40 (d, 2JFH = 56.4, CHF2). Mass spectrum, m/z (Irel, %): 242 [M+Н]+ (100). Found, %: C 49.92; H 3.81; N 15.68. C10H9F2N3O2. Calculated, %: C 49.80; H 3.76; N 15.75.
1- tert -Butyl-5-(difluoromethyl)-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylic acid (6i). Yield 6.12 g (91%), lightbrown powder, mp 222–224°C. IR spectrum, ν, cm–1: 1708 (С=О), 2417–2598 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 1.73 (9H, s, 3CH3); 7.55 (1H, d, 2JHF = 54.0, CHF2); 8.51 (1H, s, H-3); 8.75 (1H, s, H-7); 13.51 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm (J, Hz): 29.7; 61.8; 111.6 (t, 1JCF = 237.5); 123.3; 123.7; 130.1; 133.4; 142.5; 146.0 (t, 2JCF = 23.0); 167.1. 19F NMR spectrum, δ, ppm (J, Hz): –115.68 (d, 2JFH = 56.4, CHF2). Mass spectrum, m/z (Irel, %): 270 [M+Н]+ (100). Found, %: C 53.45; H 4.81; N 15.84. C12H13F2N3O2. Calculated, %: C 53.53; H 4.87; N 15.61.
1-Methyl-5-(trifluoromethyl)-1 Н -pyrazolo[4,3- b ]pyridine- 6-carboxylic acid (6j). Yield 5.69 g (93%), brown powder, mp 199–201°C. IR spectrum, ν, cm–1: 1704 (С=О), 2412–2608 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 4.18 (3H, s, CH3); 8.56 (1H, s, H-3); 8.73 (1H, s, H-7); 13.70 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm (J, Hz): 36.4; 121.0; 121.7 (q, 1JCF = 272.5); 125.2; 131.9; 134.0; 138.7 (q, 2JCF = 33.8); 139.6; 166.9. 19F NMR spectrum, δ, ppm (J, Hz): –61.37 (s, CF3). Mass spectrum, m/z (Irel, %): 246 [M+Н]+(100). Found, %: C 44.27; H 2.58; N 17.01. C9H6F3N3O2. Calculated, %: C 44.09; H 2.47; N 17.14.
1-Ethyl-5-(trifluoromethyl)-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylic acid (6k). Yield 6.02 g (93%), light-yellow powder, mp 236–238°C. IR spectrum, ν, cm–1: 1707 (С=О), 2409–2601 (COOH dimer). 1H NMR spectrum, δ, ppm (J, Hz): 1.42 (3H, t, 3J = 7.2, CH2CH3); 4.58 (2H, q, 3J = 7.2, CH2CH3); 8.58 (1H, s, H-3); 8.77 (1H, s, H-7); 13.89 (1H, br. s, CO2H). 13C NMR spectrum, δ, ppm: 14.8; 44.2; 120.5 (q, 1JCF = 272.5); 120.6; 125.4; 131.1; 134.0; 138.8 (q, 2JCF = 33.7); 139.7; 165.8. 19F NMR spectrum, δ, ppm: –61.38 (s, CF3). Mass spectrum, m/z (Irel, %): 260 [M+Н]+ (100). Found, %: C 46.16; H 3.04; N 16.38. C10H8F3N3O2. Calculated, %: C 46.34; H 3.11; N 16.21.
1-Methyl-5-(trifluoromethyl)-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine (7). EtOH (100 ml), acid 6j (2.45 g, 10 mmol), 10% Pd/С catalyst (0.2 g, 0.188 mmol) were charged into a 250-ml pressure vessel and heated under a hydrogen pressure of 25 atm at 80°C for 24 h. The reaction mixture was cooled, the catalyst was filtered off, the filtrate was evaporated under reduced pressure, and the residue was recrystallized from MTBE. Yield 1.81 g (88%), white powder, mp 82–83°C. IR spectrum, ν, cm–1: 3305 (NH). 1H NMR spectrum, δ, ppm: 1.91–2.15 (2H, m, CH2); 2.63–2.88 (2H, m, CH2); 3.79 (3H, s, CH3); 3.85–3.97 (1H, m, CH); 4.41 (1H, br. s, NH); 7.37 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 17.1; 20.3; 35.7; 52.1 (q, 2JCF = 30.0); 121.6; 125.5; 126.4 (q, 1JCF = 280.4); 129.2. 19F NMR spectrum, δ, ppm: –75.0 (s, CF3). Mass spectrum, m/z (Irel, %): 206 [M+Н]+ (100). Found, %: C 46.90; H 4.86; N 20.41. C8H10F3N3. Calculated, %: C 46.83; H 4.91; N 20.48.
Synthesis of tert-butyl pyrazolo[4,3-b]pyridine-6-carboxylates 8a–k (General method). DMAP (1.1 g, 0.009 mol) and di-tert-butyl dicarbonate (5.5 g, 0.025 mol) were successively added to a solution of acid 6a–k (0.018 mol) in THF (150 ml), and the resulting mixture was heated with stirring under reflux for 4 h. The solvent was distilled off under reduced pressure, 1 M aqueous NaHSO4 (200 ml) was added to the formed oily residue, and the resulting mixture was extracted with EtOAc (3×150 ml). The combined organic layers were dried over anhydrous Na2SO4 and evaporated under reduced pressure. The residue was recrystallized from MTBE.
tert -Butyl 1-methyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8а). Yield 3.43 g (82%), yellow powder, mp 82–83°C. IR spectrum, ν, cm–1: 1712 (С=О). 1H NMR spectrum, δ, ppm: 1.60 (9H, s, 3CH3); 4.16 (3H, s, CH3); 8.36 (1H, s, H-3); 8.69 (1H, s, H-7); 9.01 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 28.3; 39.9; 80.6; 120.1; 124.0; 131.9; 133.2; 143.3; 145.5; 164.6. Mass spectrum, m/z (Irel, %): 234 [M+Н]+ (100). Found, %: C 61.60; H 6.56; N 17.92. C12H15N3O2. Calculated, %: C 61.79; H 6.48; N 18.01.
tert -Butyl 1-ethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8b). Yield 3.15 g (71%), beige powder, mp 113–114°C. IR spectrum, ν, cm–1: 1714 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.42 (3H, t, 3J = 7.2, CH2CH3); 1.60 (9H, s, 3CH3); 4.60 (2H, q, 3J = 7.2, CH2CH3); 8.40 (1H, s, H-3); 8.67 (1H, s, H-7); 8.97 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 15.4; 28.2; 44.2; 82.2; 120.0; 124.1; 131.2; 133.5; 143.4; 145.7; 164.7. Mass spectrum, m/z (Irel, %): 248 [M+Н]+ (100). Found, %: C 63.31; H 6.79; N 17.14. C13H17N3O2. Calculated, %: C 63.14; H 6.93; N 16.99.
tert -Butyl 1- tert -butyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8c). Yield 3.81 g (77%), brown powder, mp 103–104°C. IR spectrum, ν, cm–1: 1709 (С=О). 1H NMR spectrum, δ, ppm: 1.60 (9H, s, 3CH3); 1.72 (9H, s, 3CH3); 8.39 (1H, s, H-3); 8.65 (1H, s, H-7); 8.97 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 27.8; 29.8; 61.5; 81.9; 121.4; 123.1; 129.4; 132.2; 144.2; 144.9; 164.3. Mass spectrum, m/z (Irel, %): 276 [M+Н]+ (100). Found, %: C 65.27; H 7.63; N 15.35. C15H21N3O2. Calculated, %: C 65.43; H 7.69; N 15.26.
tert -Butyl 1-phenyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8d). Yield 4.40 g (83%), brown powder, mp 163–165°C. IR spectrum, ν, cm–1: 1714 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.59 (9H, s, 3CH3); 7.50 (1H, t, 3J = 7.6, Н Ph); 7.66 (2H, t, 3J = 7.6, Н Ph); 7.83 (2H, d, 3J = 8.0, Н Ph); 8.58 (1H, s, H-3); 8.74 (1H, s, H-7); 9.08 (1H, s, H-5). 13C NMR spectrum, δ, ppm: 28.2; 82.6; 120.5; 122.9; 125.1; 128.0; 130.4; 130.9; 136.4; 139.2; 144.9; 146.6; 165.4. Mass spectrum, m/z (Irel, %): 296 [M+Н]+ (100). Found, %: C 69.28; H 5.74; N 14.07. C17H17N3O2. Calculated, %: C 69.14; H 5.80; N 14.23.
tert -Butyl 1,5-dimethyl-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8е). Yield 3.29 g (74%), yellow powder, mp 206–208°C. IR spectrum, ν, cm–1: 1713 (С=О). 1H NMR spectrum, δ, ppm: 1.59 (9H, s, 3CH3); 2.73 (3H, s, CH3); 4.11 (3H, s, CH3); 8.20 (1H, s, H-3); 8.45 (1H, s, H-7). 13C NMR spectrum, δ, ppm: 24.6; 27.8; 36.1; 81.9; 120.2; 124.8; 130.6; 132.1; 141.2; 152.1; 166.2. Mass spectrum, m/z (Irel, %): 248 [M+Н]+ (100). Found, %: C 63.29; H 6.89; N 16.82. C13H17N3O2. Calculated, %: C 63.14; H 6.93; N 16.99.
tert -Butyl 1- tert -butyl-5-methyl-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylate (8f). Yield 3.69 g (71%), yellow powder, mp 139–140°C. IR spectrum, ν, cm–1: 1711 (С=О). 1H NMR spectrum, δ, ppm: 1.59 (9H, s, 3CH3); 1.72 (9H, s, 3CH3); 2.74 (3H, s, CH3); 8.23 (1H, s, H-3); 8.55 (1H, s, H-7). 13C NMR spectrum, δ, ppm: 28.1; 29.8; 61.3; 62.4; 82.5; 123.2; 124.8; 129.3; 131.3; 142.0; 152.2; 166.2. Mass spectrum, m/z (Irel, %): 290 [M+Н]+ (100). Found, %: C 66.58; H 7.96; N 14.35. C16H23N3O2. Calculated, %: C 66.41; H 8.01; N 14.52.
tert -Butyl 5-(difluoromethyl)-1-methyl-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (8g). Yield 3.77 g (75%), yellow powder, mp 122–123°C. IR spectrum, ν, cm–1: 1714 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.60 (9H, s, 3CH3); 4.19 (3H, s, CH3); 7.11 (1H, d, 2JHF = 54.6, CHF2); 8.51 (1H, s, H-3); 8.69 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 27.9; 36.6; 83.3; 112.6 (t, 1JCF = 228.7); 121.6; 124.8; 131.9; 134.0; 141.0; 145.6 (t, 2JCF = 21.4); 165.0. 19F NMR spectrum, δ, ppm (J, Hz): –114.77 (d, 2JFH = 55.1, CHF2). Mass spectrum, m/z (Irel, %): 284 [M+Н]+ (100). Found, %: C 55.33; H 5.30; N 14.72. C13H15F2N3O2. Calculated, %: C 55.12; H 5.34; N 14.83.
tert -Butyl 5-(difluoromethyl)-1-ethyl-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (8h). Yield 3.69 g (69%), brown powder, mp 131–132°C. IR spectrum, ν, cm–1: 1714 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.44 (3H, t, 3J = 7.2, CH2CH3); 1.60 (9H, s, 3CH3); 4.60 (2H, q, 3J = 7.2, CH2CH3); 7.40 (1H, d, 2JHF = 54.4, CHF2); 8.53 (1H, s, H-3); 8.72 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 15.3; 28.0; 44.5; 83.5; 112.4 (t, 1JCF = 229.0); 121.6; 124.8; 131.2; 134.3; 141.0; 145.7 (t, 2JCF = 23.2); 165.0. 19F NMR spectrum, δ, ppm (J, Hz): –114.76 (d, 2JFH = 52.7, CHF2). Mass spectrum, m/z (Irel, %): 298 [M+Н]+ (100). Found, %: C 56.73; H 5.81; N 14.20. C14H17F2N3O2. Calculated, %: C 56.56; H 5.76; N 14.13.
tert -Butyl 1- tert -butyl-5-(difluoromethyl)-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (8i). Yield 4.09 g (70%), brown powder, mp 88–89°C. IR spectrum, ν, cm–1: 1713 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.60 (9H, s, 3CH3); 1.73 (9H, s, 3CH3); 7.40 (1H, d, 2JHF = 54.4, CHF2); 8.52 (1H, s, H-3); 8.69 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 28.0; 29.8; 61.8; 83.6; 112.5 (t, 1JCF = 232.0); 123.5; 124.3; 130.1; 133.4; 142.3; 145.3 (t, 2JCF = 23.0); 164.9. 19F NMR spectrum, δ, ppm (J, Hz): –115.11 (d, 2JHF = 56.4, CHF2). Mass spectrum, m/z (Irel, %): 326 [M+Н]+ (100). Found, %: C 59.25; H 6.55; N 12.79. C16H21F2N3O2. Calculated, %: C 59.07; H 6.51; N 12.92.
tert -Butyl 1-methyl-5-(trifluoromethyl)-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (8j). Yield 3.84 g (71%), orange powder, mp 82–83°C. IR spectrum, ν, cm–1: 1710 (С=О). 1H NMR spectrum, δ, ppm: 1.57 (9H, s, 3CH3); 4.18 (3H, s, CH3); 8.56 (1H, s, H-3); 8.68 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 27.8; 36.9; 83.8; 120.9 (q, 1JCF = 273.7); 121.2; 125.4; 131.8; 134.0; 138.4 (q, 2JCF = 33.6); 139.7; 164.8. 19F NMR spectrum, δ, ppm: –61.12 (s, CF3). Mass spectrum, m/z (Irel, %): 302 [M+Н]+ (100). Found, %: C 51.62; H 4.73; N 13.78. C13H14F3N3O2. Calculated, %: C 51.83; H 4.68; N 13.95.
tert -Butyl 1-ethyl-5-(trifluoromethyl)-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (8k). Yield 4.36 g (77%), brown powder, mp 70–71°C. IR spectrum, ν, cm–1: 1708 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.42 (3H, t, 3J = 7.2, CH2CH3); 1.56 (9H, s, 3CH3); 4.58 (2H, q, 3J = 7.2, CH2CH3); 8.59 (1H, s, H-3); 8.74 (1H, s, H-7). 13C NMR spectrum, δ, ppm (J, Hz): 15.2; 27.7; 44.6; 83.7; 120.7 (q, 1JCF = 273.9); 121.1; 125.9; 131.5; 134.6; 138.9 (q, 2JCF = 33.7); 140.0; 165.2. 19F NMR spectrum, δ, ppm: –61.17 (s, CF3). Mass spectrum, m/z (Irel, %): 316 [M+Н]+ (100). Found, %: C 53.22; H 5.23; N 13.41. C14H16F3N3O2. Calculated, %: C 53.33; H 5.11; N 13.33.
Synthesis of 4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]-pyridine-6-carboxylates 9a–k (General method). t-BuOH (100 ml), ester 8a–k (10 mmol), 10% Pd/С catalyst (0.2 g, 0.188 mmol) were charged into a 250-ml pressure vessel and heated under a hydrogen pressure of 25 atm at 100°C for 24 h (72 h for ester 8j). The reaction mixture was cooled, the catalyst was filtered off, the filtrate was evaporated under reduced pressure, and the residue was recrystallized from a 1:9 heptane–MTBE mixture.
tert -Butyl 1-methyl-4,5,6,7-tetrahydro-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (9а). Yield 2.08 g (88%), white powder, mp 112–113°C. IR spectrum, ν, cm–1: 1722 (С=О), 3385 (N–H). 1H NMR spectrum, δ, ppm: 1.41 (9H, s, 3CH3); 2.55–2.96 (4H, m, 2CH2); 3.20–3.38 (2H, m, NH, CH); 3.61 (3H, s, CH3); 6.84 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 22.5; 28.2; 31.9; 36.1; 45.7; 80.5; 125.2; 126.7; 128.6; 172.9. Mass spectrum, m/z (Irel, %): 238 [M+Н]+ (100). Found, %: C 60.95; H 8.00; N 17.56. C12H19N3O2. Calculated, %: C 60.74; H 8.07; N 17.71.
tert -Butyl 1-ethyl-4,5,6,7-tetrahydro-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (9b). Yield 2.15 g (86%), light-beige powder, mp 89–90°C. IR spectrum, ν, cm–1: 1724 (С=О), 3381 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 1.24 (3H, t, 3J = 6.8, CH2CH3); 1.41 (9H, s, 3CH3); 2.59–2.87 (5Н, m, NH, 2СН2); 3.18–3.26 (1H, m, СН); 3.92 (2H, q, 3J = 6.8, CH2CH3); 6.85 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 15.3; 22.0; 27.7; 31.2; 43.2; 45.3; 80.1; 123.9; 126.4; 128.2; 172.6. Mass spectrum, m/z (Irel, %): 252 [M+Н]+ (100). Found, %: C 62.31; H 8.36; N 16.56. C13H21N3O2. Calculated, %: C 62.13; H 8.42; N 16.72.
tert -Butyl 1- tert -butyl-4,5,6,7-tetrahydro-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (9с). Yield 2.48 g (89%), beige powder, mp 125–126°C. IR spectrum, ν, cm–1: 1720 (С=О), 3388 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 1.38 (9H, s, 3CH3); 1.47 (9H, s, 3CH3); 2.61–2.68 (1H, m, CH); 2.90–3.01 (4H, m, 2CH2); 3.16–3.19 (1H, m, CH); 6.81 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 25.2; 28.1; 29.8; 38.6; 43.9; 60.6; 81.6; 120.6; 124.8; 128.5; 170.4. Mass spectrum, m/z (Irel, %): 280 [M+Н]+ (100). Found, %: C 64.68; H 9.09; N 14.88. C15H25N3O2. Calculated, %: C 64.49; H 9.02; N 15.04.
tert -Butyl 1-phenyl-4,5,6,7-tetrahydro-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (9d). Yield 2.12 g (71%), yellow powder, mp 148–150°C. IR spectrum, ν, cm–1: 1719 (С=О), 3385 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 1.39 (9H, s, 3CH3); 2.88–3.08 (4H, m, 2СH2); 3.33–3.38 (1H, m, СH); 4.54 (1H, br. s, NH); 7.21 (1H, s, H-3); 7.32 (1H, t, 3J = 7.2, Н Ph); 7.44–7.56 (4H, m, Н Ph). 13C NMR spectrum, δ, ppm: 24.9; 27.9; 45.1; 57.0; 80.6; 121.6; 123.6; 126.3; 129.5; 130.0; 131.0; 140.3; 172.7. Mass spectrum, m/z (Irel, %): 300 [M+Н]+ (100). Found, %: C 68.39; H 7.12; N 13.88. C17H21N3O2. Calculated, %: C 68.20; H 7.07; N 14.04.
tert -Butyl 1,5-dimethyl-4,5,6,7-tetrahydro-1 Н -pyrazolo-[4,3- b ]pyridine-6-carboxylate (9е). Yield 2.00 g (80%), beige powder, mp 72–73°C. IR spectrum, ν, cm–1: 1721 (С=О), 3388 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 0.92 (3H, d, 3J = 6.0, CH3) 1.42 (9H, s, 3CH3); 2.65–2.78 (3H, m, CH, CH2); 3.42 (1H, br. s, NH); 3.51–3.62 (4H, m, CH, CH3); 6.83 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 15.9; 19.1; 28.2; 36.1; 43.5; 48.8; 80.5; 124.4; 127.1; 132.7; 172.4. Mass spectrum, m/z (Irel, %): 252 [M+Н]+ (100). Found, %: C 62.29; H 8.48; N 16.61. C13H21N3O2. Calculated, %: C 62.13; H 8.42; N 16.72.
tert -Butyl 1- tert -butyl-5-methyl-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9f). Yield 2.22 g (76%), white powder, mp 81–82°C. IR spectrum, ν, cm–1: 1724 (С=О), 3391 (NH), 1H NMR spectrum, δ, ppm (J, Hz): 0.95 (3H, d, 3J = 6.0, CH3) 1.41 (9H, s, 3CH3); 1.50 (9H, s, 3CH3); 2.68 (1H, br. s, NH); 2.80–3.01 (3H, m, CH, CH2); 3.48–3.55 (1H, m, СH); 6.84 (1H, s, H-3). 13C NMR spectrum, δ, ppm: 15.6; 22.4; 27.7; 29.5; 43.4; 48.0; 58.7; 80.0; 122.4; 125.8; 127.8; 171.8. Mass spectrum, m/z (Irel, %): 294 [M+Н]+ (100). Found, %: C 66.22; H 7.96; N 14.35. C16H27N3O2. Calculated, %: C 66.41; H 8.01; N 14.52.
tert -Butyl 5-(difluoromethyl)-1-methyl-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9g). Yield 2.10 g (73%), gray powder, mp 96–97°C. IR spectrum, ν, cm–1: 3393 (NH), 1718 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.42 (9H, s, 3CH3); 2.72–2.91 (3H, m, NH, СН2); 3.62 (3H, s, CH3); 3.67–3.73 (1H, m, CH); 4.90–4.94 (1H, m, CH); 5.88 (1H, dd, 2JHF = 60.0, 3JHF = 6.4, CHF2); 6.88 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 19.4; 27.6; 35.7; 40.5; 55.1 (dd, 2JCF = 21.6, 2JCF = 20.1); 80.6; 115.5 (t, 1JCF = 252.5); 124.4; 126.0; 126.1; 170.2. 19F NMR spectrum, δ, ppm (J, Hz): –123.05 (ddd, 2JFH = 282.0, 3JFH = 51.7, 4JFH = 14.1, CHF2). Mass spectrum, m/z (Irel, %): 288 [M+Н]+ (100). Found, %: C 54.17; H 6.73; N 14.76. C13H19F2N3O2. Calculated, %: C 54.35; H 6.67; N 14.63.
tert -Butyl 5-(difluoromethyl)-1-ethyl-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9h). Yield 4.98 g (92%), white powder, mp 118–119°C. IR spectrum, ν, cm–1: 1722 (С=О), 3384 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.26 (3H, t, 3J = 6.8, CH2CH3) 1.41 (9H, s, 3CH3); 2.73–2.94 (3H, m, NH, СН2); 3.66–3.75 (1H, m, CH); 3.91 (2H, q, 3J = 7.2, CH2CH3); 4.90–4.94 (1H, m, CH); 5.90 (1H, dd, 2JHF = 56.0, 3JHF = 6.4, CHF2); 6.90 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 15.6; 19.4; 28.0; 40.3; 43.7; 55.6 (dd, 1JCF = 21.0, 2JCF = 20.4); 81.0; 116.0 (t, 1JCF = 241.5); 123.9; 126.4; 126.6; 170.6. 19F NMR spectrum, δ, ppm (J, Hz): –123.12 (ddd, 2JFH = 286.8, 3JFH = 56.4, 4JFH = 14.1, CHF2). Mass spectrum, m/z (Irel, %): 302 [M+Н]+ (100). Found, %: C 55.97; H 6.87; N 14.09. C14H21F2N3O2. Calculated, %: C 55.80; H 7.02; N 13.94.
tert -Butyl 1- tert -butyl-5-(difluoromethyl)-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9i). Yield 1.71 g (52%), white powder, mp 107–108°C. IR spectrum, ν, cm–1: 1725 (С=О), 3390 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.46–1.52 (18H, m, 6CH3); 2.79–2.87 (2H, m, СН2); 3.64–3.70 (1H, m, CH); 4.88–4.93 (1H, m, CH); 5.86 (1H, dd, 2JHF = 58.0, 2JHF = 6.2, CHF2); 7.06 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 22.6; 27.3; 29.5; 42.0; 49.2; 60.6 (dd, 1JCF = 21.1, 2JCF = 20.2); 72.1; 115.6 (t, 1JCF = 241.5); 122.4; 124.3; 131.1; 173.1. 19F NMR spectrum, δ, ppm (J, Hz): –121.52 (ddd, JFH = 291.4, 3JFH = 56.4, 4JFH = 14.1, CHF2). Mass spectrum, m/z (Irel, %): 330 [M+Н]+ (100). Found, %: C 55.67; H 6.97; N 13.79. C16H25F2N3O2. Calculated, %: C 55.80; H 7.02; N 13.94.
tert -Butyl 1-methyl-5-(trifluoromethyl)-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9j). Yield 2.13 g (70%), gray powder, mp 147–148°C. IR spectrum, ν, cm–1: 1727 (С=О), 3386 (NH). 1H NMR spectrum, δ, ppm: 1.44 (9H, s, 3CH3); 2.59–2.67 (1H, m, NH); 2.89–2.99 (2H, m, CH2); 3.63 (3H, s, CH3); 4.17– 4.22 (1H, m, CH); 5.16–5.21 (1H, m, CH); 6.92 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 19.2; 27.9; 36.0; 40.4; 54.2 (q, 2JCF = 27.5); 81.4; 124.5; 126.5; 126.6 (q, 1JCF = 287.5); 127.0; 170.1. 19F NMR spectrum, δ, ppm: –70.00 (s, CF3). Mass spectrum, m/z (Irel, %): 306 [M+Н]+ (100). Found, %: C 51.02; H 5.89; N 13.80. C13H18F3N3O2. Calculated, %: C 51.14; H 5.94; N 13.76.
tert -Butyl 1-ethyl-5-(trifluoromethyl)-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridine-6-carboxylate (9k). Yield 2.87 g (90%), white powder, mp 129–130°C. IR spectrum, ν, cm–1: 1725 (С=О) 3384 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.51 (3H, t, 3J = 7.2, CH2CH3); 1.68 (9H, s, 3CH3); 2.74–3.08 (3H, m, СН2, NН); 4.32 (2H, q, 2J = 7.2, CH2CH3); 4.47–4.52 (1H, m, CH); 4.62–4.69 (1H, m, CH); 6.94 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 15.2; 18.8; 27.5; 43.3; 53.7 (q, 2JCF = 27.8); 81.0; 123.2; 125.5; 126.0 (q, 1JCF = 283.7); 126.2; 126.3; 169.6. 19F NMR spectrum, δ, ppm: –70.51 (s, CF3). Mass spectrum, m/z (Irel, %): 320 [M+Н]+ (100). Found, %: C 52.83; H 6.35; N 13.02. C14H20F3N3O2. Calculated, %: C 52.66; H 6.31; N 13.16.
1-Ethyl-5-(difluoromethyl)-4,5,6,7-tetrahydro-1 Н -pyrazolo[4,3- b ]pyridinium-6-carboxylic acid hydrochloride (10). 20% HCl in 1,4-dioxane (20 ml) was added to a solution of compound 9h (0.9 g, 0.003 mol) in CHCl3 (40 ml), and the resulting mixture was stirred for 4 h. The formed precipitate was filtered off, washed with MTBE (25 ml), and dried under reduced pressure. Yield 0.67 g (80%), white crystals, mp 182–183°C. IR spectrum, ν, cm–1: 3408 (NH), 2495–2602 (COOH), 1697 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.30 (3H, t, 3J = 7.2, CH2CH3); 2.84–3.23 (3H, m, СН2, СН); 3.87–3.90 (1H, m, CH); 4.10 (2H, q, 3J = 7.2, CH2CH3); 6.09 (1H, dd, 2JFH = 52.0, 3JFH = 6.0, CHF2); 7.31 (1H, s, H-3). 13C NMR spectrum, δ, ppm (J, Hz): 15.2; 27.2; 37.9; 43.9; 54.6 (dd, 1JCF = 21.1, 2JCF = 20.4); 115.0 (t, 1JCF = 241.5); 120.1; 129.8; 133.8; 172.0. 19F NMR spectrum, δ, ppm (J, Hz): –123.61 (ddd, 2JFH = 286.6, 3JFH = 56.4, 4JFH = 14.1, CHF2). Mass spectrum, m/z (Irel, %): 246 [M–HCl]+ (100). Found, %: C 49.17; H 5.40; Cl 12.63; N 17.78. C10H14ClF2N3O2. Calculated, %: C 48.99; H 5.35; Cl 12.54; N 17.86.
X-ray structural analysis of a single crystal of compound 10 with linear dimensions 0.05 × 0.14 × 0.43 mm was performed at 173K on a Bruker Smart Apex II diffractometer (MoKα radiation, graphite monochromator, θmax 25.7°). The sample for X-ray structural analysis was obtained by crystallization from a MeCN–AcOH, 1:2 mixture. The crystals of compound 10 (C10H14ClFN3O2, M 281.69) are triclinic, space group P1; а 7.6005(5), b 9.3743(6), c 9.5781(6) Å; α 71.557(4), β 87.842(4), γ 70.630(4)°; V 609.01(7) Å3; Z 2; dcalc 1.536 g/cm3; μ 0.338 mm–1; F(000) 292. A total of 9284 reflections were collected, of which 2314 were independent (R factor 0.0521). The structure was solved by the direct method and refined by the least-squares method in the full-matrix anisotropic approximation using the Bruker SHELXTL software package.24 The positions of all hydrogen atoms (CH) were calculated geometrically and refined according to the rider model, while the positions of hydrogen atoms at heteroatoms were revealed from the difference Fourier synthesis of the electron density and refined isotropically. The final probability factors were R1(F) 0.0504, wR2(F2) 0.1085 over 1761 reflections with I > 2σ(I), R1(F) 0.0724, wR2(F2) 0.1183, GOF 1.056 over all independent reflections, 175 refinable parameters, the weighing scheme ω = 1/(σ2(Fo2) + (0.0546P)2 + 0.1359P), where Р = (Fo2 + + 2Fc2)/3 was used, the maximum (average) shift / error ratio in the last cycle 0.000(0.000). Residual electron density from the difference Fourier series after the last refinement cycle was 0.35 and –0.22 е/Å3. The full set of X-ray structural data was deposited at the Cambridge Crystallographic Data Center (deposit CCDC 2090694).
Supplementary information file containing 1H, 13C, and 19F NMR spectra of compounds 3a–k, 4, 6a–k, 7, 8a–k, 9a–k, and 10 is available at the journal website http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
Seth, A.; Sharma, P. A.; Tripathi, A.; Choubey, P. K.; Srivastava, P.; Tripathi, P. N.; Shrivastava, S. K. Med. Chem. 2018, 14, 409.
Lutz, T.; Wein, T.; Höfner, G.; Wanner, K. T. ChemMedChem 2017, 12, 362.
Hellenbrand, T.; Höfner, G.; Wein T.; Wanner, K. T. Bioorg. Med. Chem. 2016, 24, 2072.
Tóth, K.; Höfner, G.; Wanner, K. T. Bioorg. Med. Chem. 2018, 26, 3668.
Hauke, T. J.; Höfner, G.; Wanner, K. T. ChemMedChem 2019, 14, 583.
Tóth, K.; Höfner, G.; Wanner, K. T. Bioorg. Med. Chem. 2019, 27, 822.
Moldavan, M.; Cravetchi, O.; Allen, C. N. J. Neurophysiol. 2017, 118, 3092.
Schaarschmidt, M.; Höfner, G.; Wanner, K. T. ChemMedChem 2019, 14, 1135.
Madsen, K. K.; White, H. S.; Schousboe, A. Pharmacol. Ther. 2010, 125, 394.
Rabong, C.; Hametner, C.; Mereiter, K.; Kartsev, V. G.; Jordis, U. Heterocycles 2008, 75, 799.
Ryabukhin, S. V.; Plaskon, A. S.; Volochnyuk, D. M.; Pipko, S. E.; Tolmachev, A. A. Synth. Commun. 2008, 38, 3032.
Chen, L.; Wilder, P. T.; Drennen, B.; Tran, J.; Roth, B. M.; Chesko, K.; Shapiro, P.; Fletcher, S. Org. Biomol. Chem. 2016, 14, 5505.
Yu, J.-S.; Espinosa, M.; Noda, H.; Shibasaki, M. J. Am. Chem. Soc. 2009, 141, 10530.
Grenier, M. C.; Ding. S.; Vézina, D.; Chapleau, J.-P.; Tolbert, W. D.; Sherburn R.; Schön A.; Somisetti S.; Abrams, C. F.; Pazgier, M.; Finzi, A.; Smith, A. B., III ACS Med. Chem. Lett. 2020, 11, 371.
Anderson, D.; Beutel, B.; Cooper, C.; Dandliker, P.; David, C.; Gu, Y.-G.; Hinman, M.; Kalvin, D.; Lynch, L.; Ma, Z.; Motter, C.; Rosenberg, T.; Sanders, W.; Tufano, M.; Wagner, R.; Weitzberg, M.; Yong, H. US Patent 2004072817.
Sun, C.; Ewing, W. R.; Sulsky, R.; Huang, Y. US Patent 20060155126.
Betageri, R.; Cook, B. N.; Disalvo, D.; Harcken, C.; Kuzmich, D.; Liu, P.; Lord, J.; Mao, C.; Razavi, H. WO Patent 2012087782.
Lapointe, B. T.; Fuller, P. H.; Gunaydin, H.; Liu, K.; Sciammetta, N.; Trotter, B. W.; Zhang, H.; Barr, K.; Maclean, J. K. F.; Molinari, D. F.; Simov, V. US Patent 2018016239A1.
Yakovenko, G. G.; Lukianov, O. A.; Bol'but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 379. [Khim. Geterotsikl. Soedin. 2019, 55, 379.]
Yakovenko, G. G.; Yagodkina-Yakovenko, M. S.; Suykov, S. Yu.; Vovk, M. V. Chem. Heterocycl. Compd. 2021, 57, 199. [Khim. Geterotsikl. Soedin. 2021, 57, 199.]
Yakovenko, G. G.; Lukianov, O. А.; Bol'but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 1211. [Khim. Geterotsikl. Soedin. 2019, 55, 1211.]
Yakovenko, G. G.; Lukianov, O. А.; Yagodkina-Yakovenko, M. S.; Bol'but, A. V.; Vovk, M. V. Chem. Heterocycl. Compd. 2020, 56, 347. [Khim. Geterotsikl. Soedin. 2020, 56, 347.]
Chen, X.; Dragoli, D. R.; Fan, P.; Li, Y.; Powers, J. P.; Punna, S.; Tanaka, H.; Zhang, P. US Patent 2014057937.
Sheldrick, G. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(11), 1137–1145
Supplementary Information
ESM 1
(PDF 33845 kb)
Rights and permissions
About this article
Cite this article
Yakovenko, G.G., Saliyeva, L.N., Rusanov, E.B. et al. Synthesis of methyl(ethyl) pyrazolo[4,3-b]pyridine-6-carboxylates and their conversion to tert-butyl 4,5,6,7-tetrahydropyrazolo-[4,3-b]pyridine-6-carboxylates. Chem Heterocycl Comp 57, 1137–1145 (2021). https://doi.org/10.1007/s10593-021-03032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-03032-z