Ethyl 5-chloro-4-(2-nitroethenyl)pyrrole-3-carboxylates, obtained by the condensation of ethyl 5-chloro-4-formylpyrrole-3-carboxylates with nitromethane, react with N-methylazomethine ylide with the formation of ethyl 5-chloro-4-(4-nitropyrrolidin-3-yl)pyrrole-3-carboxylates. Their hydrolysis yielded the corresponding acids, while reductive cyclization led to the synthesis of hexahydrodipyrrolo[3,4-b:3',4'-d]pyridin-5(1Н)-one via intermediate ethyl 4-(4-aminopyrrolidin-3-yl)-5-chloropyrrole-3-carboxylate derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Present drug development programs are largely aimed at the creation of heterocyclic structures capable of providing effective interaction with a variety of biological targets. Substituted pyrrolidines, which are found in the structure of compounds of natural origin,1 drugs,2 bioactive substances,3 and are also widely used as building blocks for their synthesis, can be confidently considered such structures.4 Nitro derivatives occupy a special place among functionalized pyrrolidines. They have found application as substrates for the design of pharmacologically promising compounds,5 including analogs of the alkaloid cephalotaxine.6
Most of 4-nitropyrrolidines currently known incorporate aryl substituents in position 3 and were accessed by [3+2] cycloaddition of unstabilized azomethine ylides to the corresponding β-nitrostyrenes.5,6,7 At the same time, data on their 3-heteryl-substituted analogs are limited by the examples of compounds with furyl, quinolinyl, benzopyranyl,8 thienyl,8,9 pyridinyl,10 and imidazolyl11 fragments. It seemed advantageous to us to introduce a pyrrole ring additionally functionalized with chlorine atoms and an ethoxycarbonyl group into the 4-nitropyrrolidine backbone. It could be expected that the presence of chlorine atoms, similar to pyrrolomycins,12 would affect the antimicrobial activity of the obtained compounds,13 while the ethoxycarbonyl group is very convenient for subsequent structural modification, including that accompanied by intramolecular cyclization.
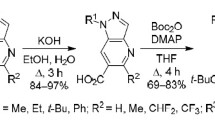
The proposed variation of the synthesis of this type of pyrrolidinylpyrrole compounds is based on the two-step transformation of 5-chloro-4-formylpyrrole-3-carboxylates 1a–f described earlier.14 In the first step, heating them under reflux in MeNO2 in the presence of AcONH4 afforded 5-chloro-4-(2-nitroethenyl)pyrrole-3-carboxylates 2a–f in high yields, which according to 1H NMR spectra exist in the form of E-isomers. Their subsequent [3+2] cycloaddition to N-methylazomethine ylide 3 generated in the reaction of sarcosine with paraformaldehyde in PhMe under reflux leads to ethyl 5-chloro-4-(4-nitropyrrolidin-3-yl)pyrrole-3-carboxylates 4а–f in 79–85% yields (Scheme 1). The presence in their 1H NMR spectra of triplets of the 3-CH (3.10–3.21 ppm) and 4-CH (5.32–5.44 ppm) protons of the pyrrolidine ring with SSCCs of 8.4 and 6.8 Hz indicates the trans arrangement of the nitro group and pyrrole fragment.8b,11

Scheme 1
The presence of several reactive centers within the structure of the obtained pyrrolidinylpyrroles 4a–f creates favorable conditions for accessing on their basis promising synthetic blocks. Thus, only the ester group undergoes transformation upon heating under reflux for 3 h in an aqueous dioxane solution of KOH which leads to the corresponding acids 5a–f. In turn, the reduction of the nitro group of compounds 4a–f with tin dichloride in EtOAc was successfully used to synthesize 4-aminopyrrolidine derivatives 6a–f in high yields. The possibility of annulation of the pyridone ring via the intramolecular reaction of ethyl 4-(2-aminoethyl)pyrrole-3-carboxylates by the action of LiOH under relatively severe conditions was shown previously.15 We have found that amino esters 6a–f are also susceptible to intramolecular cyclocondensation and, in EtOH under reflux in the presence of EtONa as a base, form derivatives of the novel heterocyclic system hexahydrodipyrrolo[3,4-b:3'4'-d]pyridine 7a–f (Scheme 2).

Scheme 2
To establish the structure and composition of the target products 5, 7 a–f and intermediates 6a–f, a complex physicochemical study was performed. In particular, it was found that the IR spectra of dipyrrolopyridine derivatives 7a–f contain strong absorption bands of C=O groups in the range of 1657–1664 cm–1 and weak bands of NH groups in the range of 3213–3220 cm–1. In their 1H NMR spectra the 8b-CH protons appear in the form of a triplet in a narrow range of 3.18–3.19 ppm with an SSCC of 9.6 Hz, while the remaining protons of the pyrrolidine ring annulated with the pyridone ring show as multiplets. The pyridone protons of the NH groups appear as singlets at 7.58–7.73 ppm whereas the signal of the carbonyl group bonded to it is observed in the 164.3–168.1 ppm region in the 13C NMR spectra. However, the obtained data do not allow us to reliably establish the stereochemistry of the synthesized hydrogenated dipyrrolopyridines 7а–f.
An unambiguous answer about their spatial structure indicating the transoid configuration of the 3a-CH and 8b-CH protons in the pyrrolidinopyridone fragment of the tricyclic system was obtained as a result of the X-ray structural analysis of compound 7a, the general view of the molecule of which and its main geometric parameters are depicted in Figure 1. The molecule of the compound in the solid state contains one H2O solvate molecule which also participates in the formation of hydrogen bonds. The pyrrole ring N(3)–C(8)–C(5)–C(6)–C(9) is planar, the standard deviation of its atoms from the plane of the ring is only 0.0022 Å. The five-membered ring N(1)–C(1)–C(4)–C(3)–C(2) is not planar and has the “envelope” conformation, whereas the atoms C(1), N(1), C(2), C(3) and C(1), C(4), C(3) lie in planes forming a dihedral angle of 44.2(2)° with each other. The six-membered ring N(2)–C(3)–C(4)–C(5)–C(6)–C(7) is also nonplanar and has a twisted half chair conformation. Within it, the N(2), C(3), C(4) and N(2), C(4), C(5), C(6) atoms (the maximum root-mean-square deviation of atoms from the plane reaches 0.068(2) Å) form two planes with a dihedral angle of 47.5(3)° between them.
The lengths of the C–N bonds with the nitrogen atom N(1) are in the range of 1.455–1.486(4) Å which is typical for the values of the C–N single bond (1.45–1.47 Å) in organic compounds. The bond N(2)–C(3) (1.437(3) Å) is also close to this range, whereas the N(2)–C(7) bond is significantly shortened to 1.361(4) Å which is due to conjugation of the LEP of the N(2) atom with the π-system of the carbonyl group C(7)=O(1). The formation of different types of hydrogen bonds between molecule 7a and the solvate H2O molecule with the following parameters is observed in the solid state: O(2)–H(1)O···N(1), length of bond O(2)–H(1)O – 0.80(4) Å, of bond O(2)···N(1) – 2.860(4) Å, angle O(2)–H(1)O–N(1) – 165(4)°; O(2)–H(2)O···O(1)*, length of bond O(2)–H(2)O – 0.77(4) Å, of bond O(2)···O(1) – 2.768(3) Å, angle O(2)–H(2)O–O(1) – 159(4)°; N(2)–H(2)N· O(2)**, length of bond N(2)–H(2)N – 0.84(3) Å, of bond N(2)···O(2) – 2.814(3) Å, angle N(2)–H(2)N–O(2) – 166(3)°. The symbols * and ** mark the atoms associated with the basic symmetry operations 0.5 – x, y + 0.5, z and x + 0.5, 0.5 – y, 1 – z, respectively.
Within the context of further investigation of the obtained dipyrrolopyridone derivatives as potential scaffolds for the search for bioactive compounds, it is reasonable to note that they are analogs of tetrahydropyrrolo[3,4-c]isoquinoline modulators of serotonin receptors which regulate physiological processes associated with the course of various metabolic diseases.16
To conclude, a method was developed as a result of this work for the preparation of previously undescribed polyfunctional pyrroles containing the synthetically and biologically promising 4-nitro-3-pyrrolidinyl moiety. The possibility of efficacious use of structures of this type for the construction of representatives of a novel heterocyclic system, hexahydrodipyrrolo[3,4-b:3'4'-d]pyridines, which are analogs of compounds with a pronounced pharmacological profile, was demonstrated.
Experimental
IR spectra were registered on a Bruker Vertex 70 spectrometer in KBr pellets (compounds 2, 5, 7 a–f) and in CН2Cl2 (compounds 4, 6 a–f). 1H NMR spectra were acquired in pulse Fourier transform mode on a Varian VXR-400 spectrometer (400 MHz) in DMSO-d6 (compounds 2, 5, 7 a–f) or CDCl3 (compounds 4, 6 a–f), while 13C NMR spectra of all compounds were recorded on a Bruker Avance DRX-500 spectrometer (125 MHz) in DMSO-d6. The solvent signal (DMSO-d6: 2.49 ppm for 1Н nuclei, 39.5 ppm for 13С nuclei; CDCl3: 7.26 ppm for 1Н nuclei) served as internal standard. Mass spectra were recorded on an Agilent LC/MSD SL mass spectrometer; column: Zorbax SB-C18, 4.6 × 15 mm, 1.8 μm (PN 82 (c)75-932); DMSO solvent, atmospheric pressure electrospray ionization. Elemental analysis was performed on a Perkin Elmer 2400 CHN-analyzer. Melting points were determined on a Kofler bench and are uncorrected.
5-Chloro-4-formylpyrrole-3-carboxylates 1a–f were obtained following a previously described method.14
Synthesis of compounds 2a–f (General method). AcONH4 (0.58 g, 7.5 mmol) was added to a solution of aldehyde 1а–f (15 mmol) in MeNO2 (5 ml), and the resulting mixture was heated under reflux for 4 h. The excess MeNO2 was distilled off under reduced pressure, and the residue was recrystallized from 70% aqueous EtOH.
Ethyl 5-chloro-1,2-dimethyl-4-(( E )-2-nitroethenyl)-1 H -pyrrole-3-carboxylate (2а). Yield 3.48 g (85%), yellow powder, mp 131–132°С. IR spectrum, ν, cm–1: 1621 (С=С), 1703 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.29 (3H, t, J = 7.2, ОСН2СН3); 2.48 (3H, s, 2-СН3); 3.52 (3H, s, NСН3); 4.21 (2H, q, J = 7.2, OCH2СН3); 7.85 (1Н, d, J = 14.2, НС=); 8.42 (1Н, d, J = 14.2, НС=). 13C NMR spectrum, δ, ppm: 11.9; 14.0; 31.4; 60.0; 109.6; 121.2; 130.6; 135.1; 135.4; 138.7; 163.3. Mass spectrum, m/z (Irel, %): 273 [М+Н]+ (100). Found, %: C 48.62; H 4.90; N 10.11. С11H13ClN2O4. Calculated, %: C 48.45; H 4.81; N 10.27.
Ethyl 5-chloro-2-methyl-4-(( E )-2-nitroethenyl)-1-propyl-1 H -pyrrole-3-carboxylate (2b). Yield 3.70 g (82%), yellow powder, mp 115–116°С. IR spectrum, ν, cm–1: 1619 (С=С), 1700 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.89 (3H, t, J = 7.2, СН2СН2СН3); 1.31 (3H, t, J = 7.2, ОСН2СН3); 1.62–1.67 (2H, m, СН2СН2СН3); 2.48 (3H, s, 2-СН3); 3.98 (2H, t, J = 7.2, NСН2); 4.26 (2H, q, J = 7.2, OCH2СН3); 7.92 (1Н, d, J = 13.6, НС=); 8.47 (1Н, d, J = 13.6, НС=). 13C NMR spectrum, δ, ppm: 10.6; 11.8; 14.0; 22.5; 45.7; 60.0; 109.9; 110.6; 120.8; 130.5; 135.4; 138.2; 163.3. Mass spectrum, m/z (Irel, %): 301 [М+Н]+ (100). Found, %: C 52.17; H 5.60; N 9.43. С13H17СlN2O4. Calculated, %: C 51.92; H 5.70; N 9.32.
Ethyl 1-butyl-5-chloro-2-methyl-4-(( E )-2-nitroethenyl)-1 H -pyrrole-3-carboxylate (2с). Yield 3.92 g (83%), yellow powder, mp 84–85°С. IR spectrum, ν, cm–1: 1616 (С=С), 1702 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.90 (3Н, t, J = 7.2, СН2СН2СН2СН3); 1.28–1.32 (5Н, m, ОСН2СН3, СН2СН2СН2СН3); 1.55–1.59 (2Н, m, СН2СН2СН2СН3); 3.28 (3H, s, 2-СН3); 3.98 (2Н, t, J = 7.2, NCH2); 4.23 (2Н, q, J = 6.8, OCH2СН3); 7.89 (1Н, d, J = 14.0, HС=); 8.44 (1Н, d, J = 14.0, HС=). 13C NMR spectrum, δ, ppm: 11.8; 13.4; 14.0; 19.2; 31.1; 44.2; 60.0; 109.9; 110.7; 120.8; 130.6; 135.5; 138.1; 163.4. Mass spectrum, m/z (Irel, %): 315 [М+Н]+ (100). Found, %: C 53.20; H 5.97; N 9.03. С14H19СlN2O4. Calculated, %: C 53.42; H 6.08; N 8.90.
Ethyl 1-benzyl-5-chloro-2-methyl-4-(( E )-2-nitroethenyl)-1 H -pyrrole-3-carboxylate (2d). Yield 4.39 g (84%), yellow powder, mp 128–129°С. IR spectrum, ν, cm–1: 1620 (С=С), 1704 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.32 (3Н, t, J = 7.0, ОСН2СН3); 3.27 (3H, s, 2-СН3); 4.28 (2Н, q, J = 7.0, OCH2СН3); 5.36 (2Н, s, CH2Ph); 7.05 (2Н, d, J = 7.2, H Ph); 7.32–7.37 (3Н, m, H Ph); 7.97 (1Н, d, J = 14.0, HС=); 8.52 (1Н, d, J = 14.0, HС=). 13C NMR spectrum, δ, ppm: 12.1; 14.0; 47.4; 60.2; 110.2; 111.2; 121.3; 125.9 (2C); 127.7; 128.9 (2C); 130.6; 135.4; 135.9; 138.6; 163.4. Mass spectrum, m/z (Irel, %): 349 [М+Н]+ (100). Found, %: C 58.79; H 5.01; N 7.93. С17H17СlN2O4. Calculated, %: C 58.54; H 4.91; N 8.03.
Ethyl 5-chloro-4-(( E )-2-nitroethenyl)-1-methyl-2-phenyl-1 H -pyrrole-3-carboxylate (2e). Yield 4.07 g (81%), yellow powder, mp 131–132°С. IR spectrum, ν, cm–1: 1621 (С=С), 1706 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.83 (3Н, t, J = 7.2, OCH2СН3); 3.38 (3Н, s, NCH3); 3.93 (2Н, q, J = 7.2, OCH2СН3); 7.33–7.38 (2Н, m, H Ph); 7.48–7.50 (3Н, m, H Ph); 8.02 (1Н, d, J = 13.6, HС=); 8.49 (1Н, d, J = 13.6, HС=). 13C NMR spectrum, δ, ppm: 13.3; 32.8; 39.4; 59.8; 110.2; 111.7; 122.8; 128.1 (2С); 129.1; 130.2; 130.4 (2С); 135.9; 140.4; 163.0. Mass spectrum, m/z (Irel, %): 335 [М+Н]+ (100). Found, %: C 57.19; H 4.63; N 8.53. С16H15СlN2O4. Calculated, %: C 57.41; H 4.52; N 8.37.
Ethyl 5-chloro-4-(( E )-2-nitroethenyl)-2-phenyl-1-propyl-1 H -pyrrole-3-carboxylate (2f). Yield 4.35 g (80%), yellow powder, mp 89–90°С. IR spectrum, ν, cm–1: 1622 (С=С), 1707 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.66 (3Н, t, J = 7.2, СН2СН2СН3); 0.80 (3Н, t, J = 7.2, OCH2СН3); 1.45–1.51 (2Н, m, СН2СН2СН3); 3.76 (2Н, t, J = 7.2, NСН2); 3.92 (2Н, q, J = 7.2, OCH2СН3); 7.33–7.38 (2Н, m, H Ph); 7.45–7.50 (3Н, m, H Ph); 8.04 (1Н, d, J = 14.0, HС=); 8.50 (1Н, d, J = 14.0, HС=). 13C NMR spectrum, δ, ppm: 10.6; 13.2; 22.6; 46.7; 59.7; 110.5; 112.2; 122.2; 128.1 (2C); 129.1; 130.1; 130.4 (2C); 130.5; 136.1; 140.3; 163.0. Mass spectrum, m/z (Irel, %): 363 [М+Н]+ (100). Found, %: C 59.78; H 5.19; N 7.83. С18H19СlN2O4. Calculated, %: C 59.59; H 5.28; N 7.72.
Synthesis of compounds 4a–f (General method). A mixture of 2-nitroethenylpyrrole 2а–f (10 mmol), sarcosine (2.22 g, 25 mmol), paraformaldehyde (1.80 g, 60 mmol) in PhMe (20 ml) was heated under reflux with continuous removal of the formed water. The reaction mixture was cooled, the formed precipitate was filtered off, and the filtrate was evaporated under reduced pressure. The residue was purified by chromatography on silica gel (eluent hexane–EtOAc, 3:1).
Ethyl 5-chloro-1,2-dimethyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylate (4а). Yield 2.80 g (85%), light-yellow oil. IR spectrum, ν, cm–1: 1690 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.35 (3Н, t, J = 7.2, ОСН2CH3); 2.43 (3Н, s, 2-CH3); 2.49 (3Н, s, NCH3); 2.59 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.04 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.10 (1Н, t, J = 8.4, CH pyrrolidine); 3.49 (3Н, s, NCH3); 3.55–3.64 (1Н, m, CH pyrrolidine); 4.22–4.31 (2Н, m, ОСН2CH3); 4.40–4.46 (1Н, m, CH pyrrolidine); 5.32 (1Н, t, J = 6.8, CH pyrrolidine). 13C NMR spectrum, δ, ppm: 12.5; 14.5; 31.0; 41.4; 42.7; 59.9; 60.6; 90.0; 102.9; 110.1; 116.3; 132.1; 136.1; 164.8. Mass spectrum, m/z (Irel, %): 330 [М+Н]+ (100). Found, %: C 51.18; H 6.19; N 12.83. С14H20СlN3O4. Calculated, %: C 50.99; H 6.11; N 12.74.
Ethyl 5-chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1-propyl-1 H -pyrrole-3-carboxylate (4b). Yield 2.94 g (82%), light-yellow oil. IR spectrum, ν, cm–1: 1693 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.96 (3Н, t, J = 7.6, СН2СН2CH3); 1.37 (3Н, t, J = 7.2 ОСН2CH3); 1.64–1.75 (2H, m, СН2СН2CH3); 2.44 (3Н, s, 2-CH3); 2.51 (3Н, s, NCH3); 2.64 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.07 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.14 (1Н, t, J = 8.4, CH pyrrolidine); 3.59–3.66 (1Н, m, CH pyrrolidine); 3.84–3.89 (2H, m, СН2СН2CH3); 4.25–4.33 (2Н, m, ОСН2CH3); 4.42–4.51 (1Н, m, CH pyrrolidine); 5.34 (1Н, t, J = 6.8, CH pyrrolidine). 13C NMR spectrum, δ, ppm: 10.6; 12.0; 14.1; 22.9; 41.0; 42.0; 45.4; 59.4; 59.6; 60.2; 89.2; 100.2; 109.8; 115.6; 135.2; 164.5. Mass spectrum, m/z (Irel, %): 358 [М+Н]+ (100). Found, %: C 53.88; H 6.87; N 11.83. С16H24СlN3O4. Calculated, %: C 53.71; H 6.76; N 11.74.
Ethyl 1-butyl-5-chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylate (4с). Yield 2.98 g (80%), light-yellow oil. IR spectrum, ν, cm–1: 1691 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.96 (3Н, t, J = 7.6, CH3); 1.35–1.41 (5Н, m, ОСН2CH3, СН2); 1.59–1.65 (2H, m, СН2); 2.45 (3Н, s, 2-CH3); 2.50 (3Н, s, NCH3); 2.62 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.06 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.13 (1Н, t, J = 8.4, CH pyrrolidine); 3.58–3.64 (1Н, m, CH pyrrolidine); 3.82–3.90 (2H, m, СН2); 4.26–4.31 (2Н, m, ОСН2CH3); 4.41–4.48 (1Н, m, CH pyrrolidine); 5.34 (1Н, t, J = 6.8, CH pyrrolidine). 13C NMR spectrum, δ, ppm: 12.0; 13.2; 14.1; 19.4; 31.7; 41.0; 42.1; 43.8; 59.4; 59.6; 60.2; 89.6; 109.8; 115.5; 115.9; 135.1; 164.4. Mass spectrum, m/z (Irel, %): 372 [М+Н]+ (100). Found, %: C 55.08; H 6.97; N 11.40. С17H26СlN3O4. Calculated, %: C 54.91; H 7.05; N 11.30.
Ethyl 1-benzyl-5-chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylate (4d). Yield 3.37 g (83%), light-yellow oil. IR spectrum, ν, cm–1: 1693 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 1.37 (3Н, t, J = 7.2, ОСН2CH3); 2.44 (3H, s, 2-СН3); 2.49 (3H, s, NСН3); 2.71 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.11 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.20 (1Н, t, J = 8.4, CH pyrrolidine); 3.63–3.69 (1Н, m, CH pyrrolidine); 4.26–4.33 (2Н, m, ОСН2CH3); 4.47–4.55 (1Н, m, CH pyrrolidine); 5.17 (2Н, s, СН2Ph); 5.38 (1Н, t, J = 6.8, CH pyrrolidine); 7.00 (2Н, d, J = 7.6, H Ph); 7.27–7.38 (3H, m, H Ph). 13C NMR spectrum, δ, ppm: 12.6; 14.5; 41.4; 42.6; 47.6; 59.9; 60.0; 60.6; 89.9; 110.7; 116.6; 117.1; 126.0 (2C); 127.8; 128.9 (2C); 135.8; 136.4; 164.9. Mass spectrum, m/z (Irel, %): 406 [М+Н]+ (100). Found, %: C 58.88; H 6.07; N 10.46. С20H24СlN3O4. Calculated, %: C 59.19; H 5.96; N 10.35.
Ethyl 5-chloro-1-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-2-phenyl-1 H -pyrrole-3-carboxylate (4е). Yield 3.33 g (85%), light-yellow oil. IR spectrum, ν, cm–1: 1697 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.84 (3Н, t, J = 7.2, CH3); 2.46 (3H, s, СН3); 2.67 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.12 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.17 (1Н, t, J = 8.4, CH pyrrolidine); 3.34 (3H, s, NСН3); 3.60–3.68 (1Н, m, CH pyrrolidine); 3.62–3.98 (2Н, m, ОСН2CH3); 4.43–4.50 (1Н, m, CH pyrrolidine); 5.44 (1Н, t, J = 6.8, CH pyrrolidine); 7.22–7.29 (2Н, m, H Ph); 7.40–7.49 (3H, m, H Ph). 13C NMR spectrum, δ, ppm: 13.6; 32.4; 41.5; 42.7; 59.7; 59.9; 60.8; 90.1; 111.2; 117.0; 118.1; 128.1 (2С); 128.7; 130.5 (2С); 132.3; 139.0; 164.4. Mass spectrum, m/z (Irel, %): 392 [М+Н]+ (100). Found, %: C 58.08; H 5.77; N 10.86. С19H22СlN3O4. Calculated, %: C 58.24; H 5.66; N 10.72.
Ethyl 5-chloro-4-(1-methyl-4-nitropyrrolidin-3-yl)-2-phenyl-1-propyl-1 H -pyrrole-3-carboxylate (4f). Yield 3.32 g (79%), light-yellow oil. IR spectrum, ν, cm–1: 1695 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.73 (3Н, t, J = 7.2, CH3); 0.79 (3Н, t, J = 7.2, CH3); 1.51–1.59 (2H, m, СН2СН2СН3); 2.43 (3H, s, NСН3); 2.70 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.14 (1Н, dd, 1J = 10.0, 2J = 8.4, CH pyrrolidine); 3.21 (1Н, t, J = 8.4, CH pyrrolidine); 3.59–3.66 (3Н, m, СН2СН2СН3, CH pyrrolidine); 3.88–3.94 (2Н, m, ОСН2CH3); 4.44–4.51 (1Н, m, CH pyrrolidine); 5.43 (1Н, t, J = 6.8, CH pyrrolidine); 7.18–7.29 (2Н, m, H Ph); 7.38–7.45 (3H, m, H Ph). 13C NMR spectrum, δ, ppm: 10.9; 13.4; 23.7; 41.4; 42.8; 46.7; 59.5; 59.9; 60.6; 89.9; 111.3; 116.9; 117.4; 127.9 (2С); 128.5; 130.5 (2С); 132.3; 138.8; 164.4. Mass spectrum, m/z (Irel, %): 420 [М+Н]+ (100). Found, %: C 59.88; H 6.02; N 9.86. С21H26СlN3O4. Calculated, %: C 60.07; H 6.24; N 10.01.
Synthesis of compounds 5a–f (General method). KOH (0.50 g, 9 mmol) was added to a solution of carboxylate 4a–f (3 mmol) in 1,4-dioxane–H2O 1:1 mixture (10 ml), and the resulting solution was heated under reflux for 3 h. The solvent was evaporated under reduced pressure, the resulting residue was dissolved in 1% aqueous KOH (20 ml), and the solids were filtered off. The filtrate was acidified with 10% aqueous HCl to pH 5. The formed precipitate was filtered off, dried, and recrystallized from 50% aqueous AcOH.
5-Chloro-1,2-dimethyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylic acid (5а). Yield 0.72 g (80%), light-yellow powder, mp 120–121°С. IR spectrum, ν, cm–1: 1682 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.30 (3Н, s, NCH3); 2.46–2.50 (1Н, m, CH pyrrolidine); 2.48 (3H, s, 2-СН3); 2.87–2.91 (1Н, m, CH pyrrolidine); 3.07 (1Н, t, J = 6.8, СH pyrrolidine); 3.53–3.67 (4Н, m, NCH3, CH pyrrolidine); 4.27–4.33 (1Н, m, СH pyrrolidine); 5.39 (1Н, t, J = 6.8, СH pyrrolidine). 13C NMR spectrum, δ, ppm: 11.8; 32.7; 40.1; 40.4; 41.4; 60.8; 66.8; 90.5; 117.1; 125.8; 138.5; 165.7. Mass spectrum, m/z (Irel, %): 302 [М+Н]+ (100). Found, %: C 47.96; H 5.45; N 14.06. С12H16СlN3O4. Calculated, %: C 47.77; H 5.35; N 13.93.
5-Chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1-propyl-1 H -pyrrole-3-carboxylic acid (5b). Yield 0.77 g (78%), light-yellow powder, mp 158–159°С. IR spectrum, ν, cm–1: 1680 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.87 (3Н, t, J = 7.6, СН2СН2СН3); 1.58–1.63 (2Н, m, СН2СН2СН3); 2.28 (3Н, s, 2-CH3); 2.43–2.48 (4Н, m, NCH3, CH pyrrolidine); 2.84 (1Н, dd, J = 11.0, J = 3.6, CH pyrrolidine); 2.98 (1Н, t, J = 6.6, CH pyrrolidine); 3.53 (1Н, d, J = 11.0, CH pyrrolidine); 3.88 (2Н, t, J = 7.6, NCH2); 4.20 (1Н, dd, J = 11.0, J = 3.6, CH pyrrolidine); 5.30 (1Н, t, J = 6.6, CH pyrrolidine). 13C NMR spectrum, δ, ppm: 10.8; 11.9; 22.9; 41.0; 42.2; 45.2; 59.2; 60.2; 90.0; 110.0; 114.6; 116.1; 135.7; 165.8. Mass spectrum, m/z (Irel, %): 330 [М+Н]+ (100). Found, %: C 51.16; H 6.05; N 12.89. С14H20СlN3O4. Calculated, %: C 50.99; H 6.11; N 12.74.
1-Butyl-5-chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylic acid (5с). Yield 0.81 g (79%), light-yellow powder, mp 137–138°С. IR spectrum, ν, cm–1: 1679 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.90 (3Н, t, J = 7.6, СН2СН2СН2СН3); 1.28–1.33 (2Н, m, СН2СН2СН2СН3); 1.54–1.57 (2Н, m, СН2СН2СН2СН3); 2.30 (3Н, s, 2-CH3); 2.45–2.49 (4Н, m, NCH3, CH pyrrolidine); 2.85 (1Н, d, J = 10.2, CH pyrrolidine); 3.00 (1Н, t, J = 6.4, CH pyrrolidine); 3.55 (1Н, d, J = 10.2, CH pyrrolidine); 3.90 (2Н, t, J = 7.6, NCH2); 4.21 (1Н, dd, J = 10.2, J = 3.2, CH pyrrolidine); 5.30 (1Н, t, J = 6.4, CH pyrrolidine). 13C NMR spectrum, δ, ppm: 11.8; 13.5; 19.3; 31.7; 40.9; 42.2; 43.7; 60.2; 66.4; 89.9; 110.1; 114.6; 116.1; 135.7; 165.7. Mass spectrum, m/z (Irel, %): 344 [М+Н]+ (100). Found, %: C 52.16; H 6.55; N 12.09. С15H22СlN3O4. Calculated, %: C 52.40; H 6.45; N 12.22.
1-Benzyl-5-chloro-2-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-1 H -pyrrole-3-carboxylic acid (5d). Yield 0.88 g (78%), light-yellow powder, mp 134–135°С. IR spectrum, ν, cm–1: 1683 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.30 (3Н, s, 2-CH3); 2.41 (3Н, s, NCH3); 2.46–2.50 (1H, m, CH pyrrolidine); 2.88 (1Н, dd, 1J = 9.8, 2J = 2.8, CH pyrrolidine); 3.03 (1Н, t, J = 6.4, CH pyrrolidine); 3.53 (1Н, d, J = 10.0, CH pyrrolidine); 4.26 (1Н, dd, 1J = 9.8, 2J = 2.8, CH pyrrolidine); 5.24 (2Н, s, СН2Ph); 5.35 (1Н, t, J = 6.4, CH pyrrolidine); 6.97–7.02 (2Н, m, H Ph); 7.28–7.36 (3Н, m, H Ph). 13C NMR spectrum, δ, ppm: 12.0; 41.0; 42.2; 46.9; 59.2; 60.3; 90.0; 110.8; 115.2; 116.5; 126.0 (2С); 127.5; 128.8 (2С); 136.1; 136.5; 165.8. Mass spectrum, m/z (Irel, %): 378 [М+Н]+ (100). Found, %: C 57.46; H 5.26; N 10.99. С18H20СlN3O4. Calculated, %: C 57.22; H 5.34; N 11.12.
5-Chloro-1-methyl-4-(1-methyl-4-nitropyrrolidin-3-yl)-2-phenyl-1 H -pyrrole-3-carboxylic acid (5е). Yield 0.84 g (77%), light-yellow powder, mp 185–187°С. IR spectrum, ν, cm–1: 1686 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.30 (3Н, s, NCH3); 2.48–2.51 (1H, m, CH pyrrolidine); 2.89 (1Н, dd, 1J = 9.8, 2J = 2.8, CH pyrrolidine); 3.07 (1Н, t, J = 6.4, CH pyrrolidine); 3.52–3.57 (4Н, m, NCH3, CH pyrrolidine); 4.29 (1Н, dd, 1J = 9.8, 2J = 2.8, CH pyrrolidine); 5.39 (1Н, t, J = 6.4, CH pyrrolidine); 7.33–7.44 (5Н, m, H Ph). 13C NMR spectrum, δ, ppm: 21.1; 32.3; 41.0; 59.4; 66.4; 90.0; 111.4; 116.8; 125.4; 128.0 (2С); 128.3; 129.0; 130.7 (2С); 138.2; 165.3. Mass spectrum, m/z (Irel, %): 364 [М+Н]+ (100). Found, %: C 56.30; H 5.06; N 11.30. С17H18СlN3O4. Calculated, %: C 56.13; H 4.99; N 11.55.
5-Chloro-4-(1-methyl-4-nitropyrrolidin-3-yl)-2-phenyl-1-propyl-1 H -pyrrole-3-carboxylic acid (5f). Yield 0.93 g (79%), light-yellow powder, mp 140–141°С. IR spectrum, ν, cm–1: 1685 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 0.63 (3Н, t, J = 7.6, СН2СН2СН3); 1.43–1.46 (2Н, m, СН2СН2СН3); 2.30 (3Н, s, NCH3); 2.47–2.51 (1H, m, CH pyrrolidine); 2.88 (1Н, dd, 1J = 9.8, 2J = 3.0, CH pyrrolidine); 3.08 (1Н, t, J = 6.6, CH pyrrolidine); 3.53 (1Н, d, J = 10.0, CH pyrrolidine); 3.67 (2Н, t, J = 7.6, NCH2); 4.28 (1Н, dd, 1J = 9.8, 2J = 3.0, CH pyrrolidine); 5.39 (1Н, t, J = 6.6, CH pyrrolidine); 7.32–7.44 (5Н, m, H Ph). 13C NMR spectrum, δ, ppm: 10.5; 22.9; 40.8; 41.8; 45.9; 59.2; 60.2; 89.9; 111.6; 115.8; 116.7; 127.8; 128.3 (2С); 130.4; 131.5 (2С); 137.8; 165.1. Mass spectrum, m/z (Irel, %): 392 [М+Н]+ (100). Found, %: C 58.46; H 5.76; N 10.55. С19H22СlN3O4. Calculated, %: C 58.24; H 5.66; N 10.72.
Synthesis of compounds 6a–f (General method). SnCl2·2H2O (5.65 g, 25 mmol) was added to a solution of carboxylate 4а–f (5 mmol) in EtOAc (10 ml), and the resulting mixture was heated at 50°С for 3 h. The reaction mixture was cooled, and 20% aqueous KOH (5 ml) was added to pH 10–11. The organic layer was extracted with EtOAc (2×15 ml), dried over anhydrous Na2SO4, and evaporated.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-5-chloro-1,2-dimethyl-1 Н -pyrrole-3-carboxylate (6а). Yield 1.01 g (73%), light-yellow oil. IR spectrum, ν, cm–1: 1682 (С=О), 3309 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 1.34 (3H, t, J = 7.2, ОСН2СН3); 1.52–1.67 (2H, br. s, NH2); 2.38 (3H, s, 2-CH3); 2.46 (3H, s, NCH3); 2.64 (1H, dd, J = 9.4, J = 3.8, СH pyrrolidine); 2.61–2.68 (1H, m, СH pyrrolidine); 2.87–2.94 (2H, m, СH pyrrolidine); 3.44 (3Н, s, NCH3); 3.60–3.65 (1Н, m, СH pyrrolidine); 3.72–3.79 (1H, m, СH pyrrolidine); 4.27 (2H, q, J = 7.2, ОСН2СН3). 13C NMR spectrum, δ, ppm: 12.3; 14.5; 30.8; 39.9; 42.4; 47.0; 59.5; 60.6; 67.5; 110.9; 114.8; 118.5; 135.2; 165.2. Mass spectrum, m/z (Irel, %): 300 [М+Н]+ (100). Found, %: C 56.22; H 7.52; N 13.89. С14H22СlN3O2. Calculated, %: C 56.09; H 7.40; N 14.02.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-5-chloro-2-methyl-1-propyl-1 Н -pyrrole-3-carboxylate (6b). Yield 1.24 g (76%), light-yellow oil. IR spectrum, ν, cm–1: 1682 (С=О), 3302 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 0.94 (3H, t, J = 7.2, СН2СН2СН3); 1.34 (3H, t, J = 7.2, ОСН2СН3); 1.57–1.64 (2H, m, СН2СН2СН3); 2.18–2.65 (2H, br. s, NH2); 2.41 (3H, s, 2-CH3); 2.46 (3H, s, NCH3); 2.77 (1H, dd, J = 9.4, J = 3.8, СH pyrrolidine); 2.95 (1H, t, J = 7.6 СH pyrrolidine); 2.92–2.99 (2H, m, СH pyrrolidine); 3.57–3.65 (1Н, m, СH pyrrolidine); 3.75–3.79 (1Н, m, СH pyrrolidine); 3.81–3.87 (2H, m, NСН2СН2СН3); 4.27 (2Н, q, J = 7.2, ОСН2СН3). 13C NMR spectrum, δ, ppm: 12.6; 13.5; 14.4; 19.2; 32.2; 42.0; 43.8; 57.4; 59.4; 60.3; 65.1; 110.7; 114.2; 118.1; 134.5; 165.1. Mass spectrum, m/z (Irel, %): 328 [М+Н]+ (100). Found, %: C 58.94; H 8.14; N 12.61. С16H26СlN3O2. Calculated, %: C 58.62; H 7.99; N 12.82.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-1-butyl-5-chloro-2-methyl-1 Н -pyrrole-3-carboxylate (6с). Yield 1.56 g (91%), light-yellow oil. IR spectrum, ν, cm–1: 1684 (С=О), 3310 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 0.94 (3H, t, J = 7.2, CH2СН2СН2СН3); 1.21–1.37 (5H, m, CH2СН2СН2СН3, ОСН2СН3); 1.57–1.63 (2H, m, CH2СН2СН2СН3); 2.10–2.28 (2H, br. s, NH2); 2.39 (3H, s, 2-CH3); 2.45 (3H, s, NCH3); 2.69 (1H, dd, J = 9.4, J = 3.8, СH pyrrolidine); 2.78 (1H, t, J = 7.6, СH pyrrolidine); 2.92–2.99 (2H, m, СH pyrrolidine); 3.57–3.65 (1Н, m, СH pyrrolidine); 3.75–3.79 (1Н, m, СH pyrrolidine); 3.81–3.87 (2H, m, NСН2СН2СН2СН3); 4.27 (2Н, q, J = 7.2, ОСН2СН3). 13C NMR spectrum, δ, ppm: 12.2; 13.7; 14.5; 19.9; 32.2; 42.3; 44.0; 47.0; 57.4; 59.6; 60.5; 65.3; 111.0; 114.4; 118.3; 134.7; 165.2. Mass spectrum, m/z (Irel, %): 342 [М+Н]+ (97). Found, %: C 59.90; H 8.34; N 12.09. С17H28СlN3O2. Calculated, %: C 59.72; H 8.26; N 12.29.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-1-benzyl-5-chloro-2-methyl-1 Н -pyrrole-3-carboxylate (6d). Yield 1.61 g (86%), light-yellow oil. IR spectrum, ν, cm–1: 1686 (С=О), 3305 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 1.37 (3H, t, J = 7.2, ОСН2СН3); 1.72–1.86 (2H, br. s, NH2); 2.41 (3H, s, 2-CH3); 2.42 (3H, s, NCH3); 2.69 (1H, dd, J = 9.4, J = 3.8, СH pyrrolidine); 2.81 (1H, t, J = 9.6, СH pyrrolidine); 2.92–3.03 (2H, m, СH pyrrolidine); 3.64–3.72 (1Н, m, CH pyrrolidine); 3.80–3.85 (1Н, m, СH pyrrolidine); 4.30 (2H, q, J = 7.2, ОСН2СН3); 5.16 (2Н, s, CH2Ph); 7.02 (2H, d, J = 7.6, Н Ph); 7.27–7.38 (3H, m, Н Ph). 13C NMR spectrum, δ, ppm: 12.4; 14.5; 42.4; 47.1; 47.3; 57.7; 59.7; 60.7; 65.5; 111.6; 114.8; 119.1; 126.0 (2С); 127.6; 128.9 (2С); 135.4; 136.2; 165.2. Mass spectrum, m/z (Irel, %): 376 [М+Н]+ (100). Found, %: C 64.04; H 7.10; N 11.02. С20H26СlN3O2. Calculated, %: C 63.91; H 6.97; N 11.18.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-5-chloro-1-methyl-2-phenyl-1 Н -pyrrole-3-carboxylate (6е). Yield 1.37 g (76%), light-yellow oil. IR spectrum, ν, cm–1: 1690 (С=О), 3297 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 0.89 (3H, t, J = 7.2, ОСН2СН3); 1.88–2.03 (2H, br. s, NH2); 2.44 (3H, s, NCH3); 2.72 (1H, dd, J = 9.4, J = 3.8, CH pyrrolidine); 2.87 (1H, t, J = 9.6, CH pyrrolidine); 2.97–3.08 (2H, m, CH pyrrolidine); 3.34 (3Н, s, NCH3); 3.62–3.69 (1Н, m, CH pyrrolidine); 3.81–3.89 (1Н, m, CH pyrrolidine); 3.98 (2H, q, J = 7.2, ОСН2СН3); 7.19–7.32 (2H, m, Н Ph); 7.37–7.51 (3H, m, Н Ph). 13C NMR spectrum, δ, ppm: 13.5; 32.4; 41.3; 42.4; 59.6; 59.7; 60.4; 67.5; 111.0; 116.6; 118.2; 128.0 (2С); 128.6; 130.4 (2С); 132.1; 139.1; 164.4. Mass spectrum, m/z (Irel, %): 362 [М+Н]+ (100). Found, %: C 63.21; H 6.81; N 11.48. С19H24СlN3O2. Calculated, %: C 63.06; H 6.69; N 11.61.
Ethyl 4-(4-amino-1-methylpyrrolidin-3-yl)-5-chloro-2-phenyl-1-propyl-1 Н -pyrrole-3-carboxylate (6f). Yield 1.64 g (84%), light-yellow oil. IR spectrum, ν, cm–1: 1687 (С=О), 3301 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 0.64 (3H, t, J = 7.2, СН2СН2СН3); 0.76 (3H, t, J = 7.2, ОСН2СН3); 1.65–2.00 (2H, br. s, NH2); 1.41–1.54 (2H, m, СН2СН2СН3); 2.31 (3H, s, NCH3); 2.61 (1H, dd, J = 9.4, J = 3.8, CH pyrrolidine); 2.74 (1H, t, J = 9.6, CH pyrrolidine); 2.84–2.98 (2H, m, CH pyrrolidine); 3.42–3.59 (3Н, m, CH pyrrolidine, ОСН2СН3); 3.42–3.59 (3Н, m, CH pyrrolidine, ОСН2СН3); 3.67–3.88 (3Н, m, CH pyrrolidine, NCH2СН2СН3); 7.08–7.22 (2H, m, Н Ph); 7.26–7.34 (3H, m, Н Ph). 13C NMR spectrum, δ, ppm: 10.9; 13.5; 23.7; 42.3; 46.4; 46.9; 57.8; 59.2; 60.6; 65.5; 112.4; 115.5; 119.3; 127.7 (2С); 128.2; 130.6 (2С); 132.6; 137.5; 164.5. Mass spectrum, m/z (Irel, %): 390 [М+Н]+ (100). Found, %: C 64.54; H 7.36; N 10.69. С21H28СlN3O2. Calculated, %: C 64.69; H 7.24; N 10.78.
Synthesis of compounds 7а–f (General method). EtONa (0.14 g, 2 mmol) was added to a solution of amino carboxylate 6а–f (2 mmol) in anhydrous EtOH (10 ml), and the resulting mixture was heated under reflux for 10 h. The reaction mixture was evaporated, and H2O (10 ml) was added to the residue. The resulting precipitate was filtered off, dried, and recrystallized from MeCN.
8-Chloro-2,6,7-trimethyl-2,3,3а,4,7,8b-hexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7а). Yield 0.35 g (89%), white powder, mp 208–210°С. IR spectrum, ν, cm–1: 1661 (С=О), 3217 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 2.42 (3H, s, 6-CH3); 2.44 (3H, s, NCH3); 2.63–2.71 (2H, m, CH pyrrolidine); 2.79–2.85 (2H, m, CH pyrrolidine); 3.19 (1H, t, J = 9.6, CH pyrrolidine); 3.35–3.42 (4H, m, CH pyrrolidine, NCH3); 7.58 (1H, s, NH). 13C NMR spectrum, δ, ppm: 10.6; 30.0; 40.4; 43.7; 54.1; 56.0; 58.2; 107.9; 111.4; 117.4; 133.0; 165.9. Mass spectrum, m/z (Irel, %): 254 [М+Н]+ (100). Found, %: C 56.94; H 6.22; N 16.41. С12H16СlN3O. Calculated, %: C 56.81; H 6.36; N 16.56.
8-Chloro-2,6,-dimethyl-7-propyl-2,3,3а,4,7,8b-hexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7b). Yield 0.39 g (69%), white powder, mp 175–177°С. IR spectrum, ν, cm-1: 1663 (С=О), 3219 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 0.83 (3H, t, J = 7.2, СН2СН2СН3); 1.35–1.47 (2H, m, СН2СН2СН3); 2.41 (3H, s, 6-CH3); 2.43 (3H, s, NCH3); 2.64–2.83 (4H, m, CH pyrrolidine); 3.18 (1H, t, J = 9.6, CH pyrrolidine); 3.30–3.38 (3H, m, CH pyrrolidine, NCH2); 7.66 (1H, s, NH). 13C NMR spectrum δ, ppm: 10.8; 10.9; 26.1; 40.8; 44.0; 54.5; 56.5; 58.6; 62.9; 108.3; 111.8; 117.6; 133.4; 166.3. Mass spectrum, m/z (Irel, %): 282 [М+Н]+ (100). Found, %: C 59.55; H 7.23; N 14.77. С14H20СlN3O. Calculated, %: C 59.67; H 7.15; N 14.91.
7-Butyl-8-chloro-2,6-dimethyl-2,3,3а,4,7,8b-hexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7с). Yield 0.40 g (67%), white powder, mp 151–153°С. IR spectrum, ν, cm–1: 1664 (С=О), 3220 (N–H). 1H NMR spectrum, δ, ppm: 0.83 (3H, t, J = 7.2, CH2СН2СН2СН3); 1.17–1.39 (4H, m, CH2СН2СН2СН3); 2.39 (3H, s, 6-CH3); 2.40 (3H, s, NCH3); 2.58–2.69 (2H, m, CH pyrrolidine); 2.72–2.85 (2H, m, CH pyrrolidine); 3.07–3.16 (2H, m, NCH2); 3.26–3.47 (2H, m, CH pyrrolidine); 7.64 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.3; 13.6; 19.2; 29.7; 34.8; 40.8; 43.4; 53.9; 55.8; 57.9; 107.6; 111.1; 117.0; 132.8; 165.6. Mass spectrum, m/z (Irel, %): 296 [М+Н]+ (100). Found, %: C 61.05; H 7.53; N 14.01. С15H22СlN3O. Calculated, %: C 60.91; H 7.50; N 14.21.
7-Benzyl-8-chloro-2,6-dimethyl-2,3,3а,4,7,8b-hexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7d). Yield 0.47 g (79%), white powder, mp 182–184°С. IR spectrum, ν, cm–1: 1662 (С=О), 3218 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 2.39 (3H, s, 6-CH3); 2.43 (3H, s, NCH3); 2.61–2.93 (4H, m, CH pyrrolidine); 3.20 (1H, t, J = 9.6, CH pyrrolidine); 3.36–3.46 (1H, m, CH pyrrolidine); 5.15 (2Н, s, СН2Ph); 6.99–7.05 (2Н, m, Н Ph); 7.26–7.41 (3Н, m, Н Ph); 7.70 (1H, s, NH). 13C NMR spectrum, δ, ppm: 12.9; 42.8; 46.0; 48.4; 56.4; 58.4; 60.5; 110.4; 114.4; 120.2; 128.4 (2C); 129.7; 131.1 (2С); 135.4; 139.2; 168.1. Mass spectrum, m/z (Irel, %): 330 [М+Н]+ (100). Found, %: C 65.41; H 6.22; N 12.60. С18H20СlN3O. Calculated, %: C 65.55; H 6.11; N 12.74.
8-Chloro-2,7-dimethyl-6-phenyl-2,3,3а,4,7,8b-hexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7e). Yield 0.58 g (91%), white powder, mp 196–198°С. IR spectrum, ν, cm–1: 1660 (С=О), 3215 (N–H). 1H NMR spectrum, δ, ppm (J, Hz): 2.43 (3H, s, NCH3); 2.67 (1H, t, J = 9.6, CH pyrrolidine); 2.74–2.83 (2H, m, CH pyrrolidine); 2.87–2.94 (1H, m, CH pyrrolidine); 3.27 (1H, t, J = 9.6, CH pyrrolidine); 3.35 (3H, s, NCH3); 3.44–3.51 (1H, m, CH pyrrolidine); 7.38–7.46 (5Н, m, H Ph); 7.73 (1H, s, NH). 13C NMR spectrum, δ, ppm: 31.7; 40.6; 43.9; 54.2; 56.1; 57.9; 110.4; 112.6; 118.5; 127.7 (2C); 128.2; 130.4; 130.8 (2C); 135.6; 164.3. Mass spectrum, m/z (Irel, %): 316 [М+Н]+ (100). Found, %: C 64.49; H 5.88; N 13.19. С17H18СlN3O. Calculated, %: C 64.66; H 5.75; N 13.31.
8-Chloro-2-methyl-6-phenyl-7-propyl-2,3,3а,4,7,8bhexahydrodipyrrolo[3,4- b :3',4'- d ]pyridin-5(1 Н )-one (7f). Yield 0.57 g (82%), white powder, mp 157–159°С. IR spectrum, ν, cm–1: 1657 (С=О), 3213 (N–H). 1H NMR spectrum, δ, ppm: 0.82 (3H, t, J = 7.2, СН2СН2СН3); 1.38–1.43 (2H, m, СН2СН2СН3); 2.40 (3H, s, NCH3); 2.63–2.89 (4H, m, CH pyrrolidine); 3.19 (1H, t, J = 9.6, CH pyrrolidine); 3.35 (2H, q, J = 7.2, NCH2); 3.38–3.50 (1H, m, CH pyrrolidine); 6.97–7.03 (2Н, m, Н Ph); 7.22–7.37 (3Н, m, Н Ph); 7.65 (1H, s, NH). 13C NMR spectrum, δ, ppm: 10.8; 26.1; 40.9; 44.0; 54.5; 56.4; 58.5; 62.9; 108.5; 112.5; 118.3; 126.4 (2C); 127.8; 129.2 (2С); 133.5; 137.3; 166.2. Mass spectrum, m/z (Irel, %): 344 [М+Н]+ (100). Found, %: C 66.52; H 6.52; N 12.04. С19H22СlN3O. Calculated, %: C 66.37; H 6.45; N 12.22.
X-ray structural analysis of a single crystal of compound 7а was performed at room temperature on a Bruker Smart Apex II diffractometer (MoKα radiation, graphite monochromator, θmax 25.7°). The linear dimensions of the single crystal were 0.19 × 0.26 × 0.35 mm; empirical formula C12H16ClN3O·H2O; M 271.74; the crystals are rhombic; space group Рbcn; а 7.8977(6), b 17.6141(15), c 19.5007(15) Å; V 2712.8(4) Å3; Z 8; dcalc 1.331 g·cm–3; μ 0.280 mm–1; F(000) 1152). A total of 12364 reflections were collected, of which 2588 were independent (R factor 0.0413). The absorption was corrected using the SADABS program using the multiscan method (ratio Тmin/Tmax = = 0.6776/0.7453). The structure was solved by the direct method and refined by the least-squares technique in the full-matrix anisotropic approximation using the Bruker SHELXTL software package.17 The positions of all hydrogen atoms of the CH groups were calculated geometrically and refined using the riding model. A total of 2588 independent reflections were used in the refinement, of which 1833 reflections were with I > 2σ(I) (178 refinable parameters, used weighting scheme ω = 1/(σ2(Fo2) + (0.1P)2 + 0.1575P), where Р = (Fo2 + + 2Fc2)/3, the ratio of maximum least-squares shift to error in the final refinement cycle 0.015(0.000). The final values of probability factors: R1(F) 0.0517, wR2(F2) 0.1444 over reflections with I > 2σ(I), R1(F) 0.0790, wR2(F2) 0.1651, GOF 1.037 for all independent reflections. Residual electron density from the difference Fourier series after the last refinement cycle was 0.42 and –0.22 е/Å3. The full set of X-ray structural data was deposited at the Cambridge Crystallographic Data Center (deposit CCDC 2113396).
Supplementary information file containing 1H and 13C NMR spectra of all the synthesized compounds is available at the journal website http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
(a) Michael, J. P. Nat. Prod. Rep. 2005, 22, 603. (b) Fukuda, T.; Sudoh, Y.; Tsuchiya, Y.; Okuda, T.; Igarashi, Y. J. Nat. Prod. 2014, 77, 813.
Hovinga, C. A. Pharmacotherapy 2001, 21, 1375. a Lauster, C. D.; McKaveney, T. D.; Muenсh, S. V. Am. J. Health Syst. Pharm. 2007, 64, 1265.
(а) Smolobochkin, A.; Gazizov, A.; Sazykina, M.; Akylbekov, N.; Chugunova, E.; Sazykin, I.; Gildebrant, A.; Voronina, J.; Burilov, A.; Karchava, S.; Klimova, M.; Voloshina, A.; Sapunova, A.; Klimanova, E.; Sashenkova, T.; Allayarova, U.; Balakina, A.; Mishchenko, D. Molecules 2019, 24, 3086. a Wallach, J.; Colestock, T.; Agramunt, J.; Claydon, M. D. B.; Dybek, M.; Filemban, N.; Chatha, M.; Halberstadt, A. L.; Brandt, S. D.; Lodge, D.; Bortolotto, Z. A.; Adejare, A. Eur. J. Pharmacol. 2019, 857, 172427. b Kasturi, S.; Surarapu, S.; Uppalanchi, S.; Anireddy, J. S.; Dwivedi, S.; Anantaraju, H. S.; Perumal, Y.; Sigalapalli, D. K.; Babu, B. N.; Ethiraja, K. S. Bioorg. Med. Chem. Lett. 2017, 27, 2818. c Meyers, M. J.; Liu, J.; Xu, J.; Leng, F.; Guan, J.; Liu, Z.; McNitt, S. A.; Qin, L.; Dai, L.; Ma, H.; Adah, D.; Zhao, S.; Li, X.; Polino, A. J.; Nasamu, A. S.; Goldberg, D. E.; Liu, X.; Lu, Y.; Tu, Z.; Chen, X.; Tortorella, M. D. J. Med. Chem. 2019, 62, 3503. d Curtin, M. L.; Pliushchev, M. A.; Li, H.-Q.; Torrent, M.; Dietrich, J. D.; Jakob, C. G.; Zhu, H.; Zhao, H.; Wang, Y.; Ji, Z.; Clark, R. F.; Sarris, K. A.; Selvaraju, S.; Shaw, B.; Algire, M. A.; He, Y.; Richardson P. L.; Sweis, R. F.; Sun, C.; Chiang, G. C.; Michaelides, M. R. Bioorg. Med. Chem. Lett. 2017, 27, 1576. e Kumar, R. S.; Almansour, A. I.; Arumugam, N.; Althomili, D. M. Q.; Altaf, M.; Basiri, A.; Kotresha, D.; Manohar, T. S.; Venketesh, S. Bioorg. Chem. 2018, 77, 263.
(a) Chalyk, B. A.; Butko, M. V.; Yanshyna, O. O.; Gavrilenko, K. S.; Druzhenko, T. V.; Mykhailiuk, P. K. Chemistry 2017, 23, 16782. (b) Odusami, J. A.; Ikhile, M. I.; Izunobi, J. U.; Olasupo, I. A.; Osunsanmi, F. O.; Opoku, A. R.; Fotsing, M. C. D.; Asekun, O. T.; Familoni, O. B.; Ndinteh, D. T. Bioorg. Chem. 2020, 105, 104340. c Kaminski, K. Curr. Top. Med. Chem. 2017, 17, 858. d Campello, H. R.; Parker, J.; Perry, M.; Ryberg, P.; Gallagher, T. Org. Lett. 2016, 18, 4124. e Zhao, Q.; Vuong, T. M. H.; Bai, X.-F.; Pannecoucke, X.; Xu, L.-W.; Bouillon, J.-P.; Jubault, T. M. H. Chemistry 2018, 24, 5644. f Martinez-Bailen, M.; Carmona, A. T.; Patterson-Orazem, A. C.; Lieberman, R. L.; Ide, D.; Kubo, M.; Kato, A.; Robina, I.; Moreno-Vargas, A. J. Bioorg. Chem. 2019, 86, 652.
(a) Bucsh, R. A.; Domagala, J. M.; Laborde, E.; Sesnie, J. C. J. Med. Chem. 1993, 36, 4139. (b) Thewlis, K. M.; Aldegheri, L.; Harries, M. N.; Mookherjee, C.; Oliosi, B.; Ward, S. E. Bioorg. Med. Chem. Lett. 2010, 20, 7116. (c) Fox, B. M.; Natero, R.; Richard, K.; Connors, R.; Roveto, P. M.; Beckmann, H.; Haller, K.; Golde, J.; Xiao, S.-H.; Kayser, F. Bioorg. Med. Chem. Lett. 2011, 21, 2460. (d) Rajesh, S. M.; Perumal, S.; Menendez, J. C.; Yogeeswari, P.; Sriram, D. MedChemComm 2011, 2, 626.
Nyerges, M.; Balaw, L.; Kadas, I.; Toth, G.; Toke, L. Tetrahedron 1995, 51, 11489.
Nyerges, M.; Balazs, L; Kadas, I.; Bitter, I.; Kovesdi, I.; Toke, L. Tetrahedron 1995, 51, 6783. a Alimohammadi, K.; Sarrafi, Y.; Tajbakhsh, M.; Yeganegi, S.; Hamzehloueian, M. Tetrahedron 2011, 67, 1589. b Chen, G.; Yang, J.; Gao, S.; He, H.; Li, Sh.; Di, Y.; Chang, Y.; Lu, Y.; Hao, X. Mol. Diversity 2012, 16, 151. c Gayen, G.; Banerji, A. Monatsh. Chem. 2014, 145, 1953.
(a) Baumann, M.; Baxendale, I. R.; Ley, S. V. Synlett 2010, 749. (b) Baumann, M.; Baxendale, I. R.; Kirschning, A.; Ley, S. V.; Wegner, J. Heterocycles 2011, 82, 1297.
Baumann, M.; Baxendale, I. R.; Kuratli, C.; Ley, S. V.; Martin, R. E.; Schneider, J. ACS Comb. Sci. 2011, 13, 405.
Deprez, P.; Royer, J.; Hussоn, H.-P. Synthesis 1991, 759.
Chornous, V. A.; Mel'nik, O. Ya.; Mel'nik, D. A.; Rusanov, E. B.; Vovk, M. V. Russ. J. Org. Chem. 2015, 51, 1423.
Cascioferro, S.; Raimondi, M. V.; Cusimano, M. G.; Raffa, D.; Maggio, B.; Daidone, G.; Scillaci, D. Molecules 2015, 20, 21658.
(a) Massa, S.; Artico, M.; Corelli, F.; Mai, A.; Di Santo, R.; Cortes, S.; Marongiu, M. E.; Pani, A.; La Colla, P. J. Med. Chem. 1990, 33, 2845. (b) Raimondi, M. V.; Schillaci, D.; Petruso, S. J. Heterocycl. Chem. 2004, 44, 1407. (c) Raimondi, M. G.; Listro, R.; Cusimano, M. G.; La Franca, M.; Faddetta, T.; Gallo, G.; Schillaci, D.; Collina, S.; Leonchiks, A.; Barone, G. Bioorg. Med. Chem. 2019, 27, 721. (d) Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M. V.; Barraja, P.; Montalbano, A. Eur. J. Med. Chem. 2020, 208, 112783. (e) Valderama, K.; Pradel. E.; Firsov, A. M.; Drobecq, H.; Bauderlique-le Roy, H.; Villemagne, B.; Antonenko, Yu. N.; Hartkoorn, R. C. Antimicrob. Agents Chemother. 2019, 63, e01450-19.
Grozav, A. N.; Fedoriv, M. Z.; Chornous, V. A.; Palamar, A. A.; Bratenko, M. K.; Vovk, M. V. Chem. Heterocycl. Compd. 2019, 55, 435.
Hasvold, L. A.; Sheppard, G. S.; Wang, L.; Fidanze, S. D.; Liu, D.; Pratt, J. K.; Mantei, R. A.; Wada, C. K.; Hubbard, R.; Shen, Yu.; Lin, X.; Huang, X.; Warder, S. E.; Wilcox, D.; Li, L.; Buchanan, F. G.; Smithee, L.; Albert, D. H.; Magoc, T. J.; Park, C. H.; Petros, A. M.; Panchal, S. C.; Sun, C.; Kovar, P.; Soni, N. B.; Elmore, S. W.; Kati, W. M.; McDaniel, K. F. Bioorg. Med. Chem. Lett. 2017, 27, 2225.
(а) Fevig, J. M.; Feng, J.; Ahmad, S. US Patent 20060014777A1. a Fevig, J. M.; Feng, J.; Rossi, K. A.; Miller, K. J.; Wu, G.; Hung, C.-P.; Ung, T.; Malmstrom, S. E.; Zhang, G.; Keim, W. J.; Cullen, M. J.; Rohrbach, K. W.; Qu, Q.; Gan, J.; Pelleymounter, M. A.; Rodi, J. A. Bioorg. Med. Chem. Lett. 2013, 23, 330.
Sheldrick, G. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, А64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(1), 24–31
Supplementary Information
ESM 1
(PDF 2965 kb)
Rights and permissions
About this article
Cite this article
Grozav, A.N., Kemskiy, S.V., Fedoriv, M.Z. et al. Synthesis, hydrolysis, and reductive cyclization of ethyl 5-chloro-4-(4-nitropyrrolidin-3-yl)pyrrole-3-carboxylates. Chem Heterocycl Comp 58, 24–31 (2022). https://doi.org/10.1007/s10593-022-03052-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03052-3