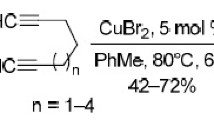
A catalytic method was developed for the synthesis of N-substituted tetrapropargylamines by reactions of α,ω-diacetylenes with 1,5,3-dioxazepanes, aldehydes (benzaldehydes, formaldehyde), and cyclic secondary amines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Multicomponent reactions represent a very common methodology for organic synthesis.1 According to literature data,2 three-component condensation of amines, aldehydes, and acetylenes (А3-соupling) provides an effective approach for the synthesis of propargylamines. A modification of this reaction is the double aminomethylation of terminal acetylenes, which leads to dipropargylamines and includes several variants: a) a reaction of 1,7-octadiyne with aromatic aldehydes and cyclic secondary amines with the formation of dipropargylamines,3 b) a reaction of terephthalaldehyde with phenylacetylene and piperidine or morpholine,4 c) a reaction of secondary diamines with benzaldehyde and phenylacetylene.5 Double aminomethylation can be also performed by using a primary amine6 or N-methylformamide.7 Di- and tetrapropargylamines were obtained by heterocyclization of piperazine, formaldehyde, and α,ω-dipropargylic alcohols.8 Multicomponent reactions of α,ω-diacetylenes with secondary amines and terephthalaldehyde or reactions of α,ω-diacetylenes with primary amines and aldehydes (polycoupling) led to the formation of polymeric products.9
Quite limited data are available on methods used for the synthesis of azatetraynes belonging to propargylamine series.8,10 We recently proposed a procedure for the preparation of cyclic and acyclic azatetraynes by reactions of N-substituted bis(ethoxymethyl)amines11 or 1,5,3-dioxazepanes12 with α,ω-diacetylenic hydrocarbons in the presence of copper salts as catalysts. Our interest toward propargylamines was motivated by their applications in the role of biologically active compounds13 and building blocks for organic synthesis.10е,14
In a continuation of our research aimed at the synthesis of azatetraynes,11,12 as well as in order to develop an effective method for the preparation of triazatetraynes belonging to the propargylamine series, we studied the reaction of N-substituted 1,5,3-dioxazepanes with α,ω-diacetylenes, aldehydes (halogenated benzaldehydes, formaldehyde), and cyclic secondary amines in the presence of СuCl as catalyst that has shown high activity in aminomethylation reactions of terminal acetylenes.15
In the case of reaction between N-(n-butyl)-1,5,3-dioxazepane, 1,7-octadiyne, piperidine (2a) as secondary amine, and p-bromobenzaldehyde (3c), it was established that this synthesis can be performed by a one-pot fourcomponent condensation of the starting reagents (Scheme 1, method I) with the formation of the target compound – 10-(4-bromophenyl)-N-[10-(4-bromophenyl)-10-(piperidin-1-yl)deca-2,8-diyn-1-yl]-N-butyl-10-(piperidin-1-yl)deca-2,8-diyne-1-amine (4с). Compound 4с can be also obtained by a two-step procedure (method II) involving isolation of azatetrayne intermediate 1,12а which subsequently reacted with piperidine (2a) and p-bromobenzaldehyde (3c).

Scheme 1
The one-pot procedure (method I) was performed by first mixing n-butyl-1,5,3-dioxazepane with 1,7-octadiyne for 6 h under argon atmosphere at 80°С in PhMe medium in the presence of СuCl catalyst (5 mol %), followed by the addition of piperidine (2a), p-bromobenzaldehyde (3c), СuCl (5 mol %), and continuing the stirring for 6 h at 80°С. Triazatetraacetylene 4с was formed under these conditions in approximately 9% yield (Table 1).
A two-step procedure (method II) involved the initial isolation of azatetrayne 1a, and in this case the yield of triazatetrayne 4с reached 51% when calculated from azatetrayne 1a. The reaction was performed in PhMe medium under argon atmosphere in the presence of СuCl (5 mol %) at 80°С over the course of 6 h. Under these conditions, the aminomethylation of azatetraynes 1a–c was performed with benzaldehydes 3a–g (m-, p-fluorobenzaldehyde, m-, p-chlorobenzaldehyde, p-bromobenzaldehyde, m-(trifluoromethyl)benzaldehyde), 1,6-hexadiyne, and morpholine (2b). As a result, method II was used to obtain triazatetraynes 4а–g in 46–65% yields (27–37% overall yield calculated from azatetrayne 1). This reaction was not successful with о-fluorobenzaldehyde (the starting azatetrayne 1а did not participate in the reaction), which could be probably explained by steric effects. The reaction with formaldehyde proceeded with the formation of target products 4h–j in 25–42% yields according to method I and in 58–69% yields according to method II (34–37% overall yields). It should be noted that the four-component reactions (method I) along with products 4а–с,h–j also gave the corresponding azatetraynes 1а,с. The reaction did not occur in the absence of catalyst.
The structures of compounds 4а–j were determined by using 1H and 13C NMR spectroscopy and mass spectrometry. 1Н and 13С NMR spectra of compounds 4а–j lacked signals that would be characteristic for terminal triple bond atoms in azatetraynes 1. The signals of magnetically nonequivalent sp-hybridized carbon atoms were assigned on the basis of 1Н–13С HMBC experiment. The formation of compounds 4а–g was deduced from the presence of tertiary carbon atom signal in 13C NMR spectrum in the range of 61.2–61.5 ppm (multiplicity determined using a DEPT-135 experiment) and 1H NMR singlet in the range of 4.50–4.54 ppm. The formed asymmetric center affected the NMR parameters of the adjacent aromatic substituent, for example, the chemical shifts of ortho-protons in the p-fluoro-substituted phenyl ring in compound 4е were observed as separate doublets. However, in the case of p-bromo-substituted analog (compound 4с), all four protons of the substituted aromatic ring were observed as a narrow singlet at 7.45 ppm, apparently due to overlapping signals of magnetically nonequivalent protons. In the case of compound 4е, 1Н–15N НМВС experiment allowed to determine the chemical shifts of two nitrogen atoms, one of which belonged to piperidine ring (303.6 ppm) and the other was part of the N-tert-butyl substituent (307.8 ppm).
Mass spectra (atmospheric pressure chemical ionization) of compounds 4a–i featured peaks of [M+H]+ ions corresponding to the molecular mass values of these compounds, which served as additional confirmation of their structures. The strongest peak in МALDI–TOF mass spectrum of compound 4g was that of protonated molecular ion [М+Н]+ with m/z 818.2719, containing two atoms of 81Br isotope, while the strongest mass spectral peak for compound 4с was that of an ion with m/z 836.1969.
Thus, we have described a СuCl-catalyzed method for the synthesis of new N-substituted tetrapropargylamines by a four-component reaction of α,ω-diacetylenes with N-substituted 1,5,3-dioxazepanes, formaldehyde, and piperidine, as well as aminomethylation reaction of dipropargylamines with aldehydes and cyclic secondary amines.
Experimental
IR spectra were recorded on a Bruker VERTEX 70v spectrometer for samples prepared as thin films. Onedimensional (1Н and 13С) and two-dimensional homonuclear (COSY, NOESY) and heteronuclear (1Н–13С HSQC, 1Н–13С HMBC) NMR spectra were acquired on Bruker Avance 400 (400 and 101 MHz) and Bruker Ascend 500 (500 and 126 MHz) spectrometers for samples in CDCl3 solutions. Residual signals of CDCl3 solvent were used as internal standards (7.28 ppm for 1Н nuclei and 77.10 ppm for 13С nuclei). 19F NMR spectra were acquired on a Bruker Avance 400 spectrometer (376 MHz), using СFCl3 as internal standard (0.0 ppm). Heteronuclear twodimensional 1Н−15N HMBC spectra were acquired on a Bruker Ascend 500 spectrometer at 51 MHz resonance frequency for 15N nuclei, using MeNO2 as standard (380 ppm, chemical shift values recalculated relative to MeNO2). GCMS analysis (EI ionization at 70 eV for compound 1с) was performed using a Shimadzu GC 2010 gas chromatograph with GCMS-QP2010 Ultra mass selective detector. Highresolution mass spectra (MALDI–TOF) of compounds 1с, 4а–j were recorded on a Bruker Autoflex III spectrometer using sinapic acid matrix, samples were prepared by the dried-droplet method from chloroform solutions (1:10). Mass spectra (APCI) of compounds 4a–i were recorded on a Shimadzu LCMS-2010 EV instrument using atmospheric pressure chemical ionization (sample injection by syringe, 0.1 ml/min, eluent MeCN–H2O, 95:5) in positive ion mode at 4.5 kV capillary voltages; interface temperature 250°С, heater temperature 200°С, evaporator temperature 230°С; the nebulizer gas (nitrogen) flow rate was 2.5 l/min. Individual compounds were purified by column chromatography on KSK silica gel (50–160 μm). TLC analysis was performed on Sorbfil PTSKh-AF-A plates, visualization in iodine vapor.
The starting materials (amines, α,ω-diacetylenes, aldehydes) with ≥98% assay were commercially available and used without additional purification. N-Alkyl-1,5,3-dioxazepanes and azatetraynes 1а–с were obtained according to a literature procedure.12a
N-(Octa-2,7-diyn-1-yl)-N-(propan-2-yl)octa-2,7-diyn-1-amine (1c). A Schlenk vessel was placed over a magnetic stirrer, flushed with argon stream, and charged with 1,5,3-dioxazepane (1 mmol), α,ω-diacetylene (2 mmol), CuCl (0.05 mmol, 5 mg), and PhMe (5 ml). The mixture was stirred at 80°С for 6 h. The reaction mixture was filtered through a layer of silica gel, the solvent was evaporated at reduced pressure on a rotary evaporator. The product was isolated by silica gel column chromatography. Yield 171 mg (64%), yellow oil, Rf 0.63 (Me2СО–PhH, 2:1). IR spectrum, ν, cm–1: 669, 755, 1118, 1216, 1326, 1385, 1432, 2118, 2957. 1H NMR spectrum (400 MHz), δ, ppm (J, Hz): 1.12 (6Н, d, 3J = 6.5, CH(CH3)2); 1.73 (4H, pent, 3J = 7.0, 2CH2CH2CH2); 1.97 (2Н, t, J = 2.5, 2C≡CH); 2.32–2.34 (8Н, m, 2CH2CH2CH2); 2.92 (1Н, pent, 3J = 6.5, CH(CH3)2); 3.50 (4H, s, 2NCH2C≡C). 13C NMR spectrum (100 MHz), δ, ppm: 17.6 and 17.9 (CH2CH2CH2); 20.2 (СН3); 27.7 (CH2CH2CH2); 39.5 (NCH2C≡C); 51.1 (CH(CH3)2); 68.8 (C≡C); 76.5 (C≡C); 83.6 (C≡C). Mass spectrum, m/z (Irel, %): 267 [M]+ (6), 252 [M–CH3]+ (100), 224 [M–CH(CH3)2]+ (63), 158 [HN(CH2C≡C)CH2C≡C(CH2)3C≡CH]+ (17), 91 [C≡C(CH2)3C≡CH]+ (42), 77 [N(CHC)CH2C≡C]+ (63). Found, m/z: 266.3389 [M–H]+. C19H24N. Calculated, m/z: 266.1909.
Synthesis of triazatetraacetylenes 4а–j. Method I. A two-necked flask was placed on a magnetic stirrer and charged under argon flow with 3-alkyl-1,5,3-dioxazepane (1 mmol), 1,7-octadiyne (2 mmol), CuCl (5 mg, 0.05 mmol), and PhMe (3 ml). The mixture was stirred at 80°С for 6 h, then treated by adding piperidine (2 mmol), aldehyde 3a–c,g (2 mmol), CuCl (5 mg, 0.05 mmol), and PhMe (3 ml). Stirring was continued under argon atmosphere at 80°С for 6 h. The reaction mixture was filtered through a thin silica gel layer, the solvent was removed by evaporation at reduced pressure on a rotary evaporator. The product was isolated by column chromatography.
Method II. A two-neck flask was flushed with argon and charged with azatetraacetylene 1a–c (1 mmol),12a piperidine (2а) or morpholine (2b) (2 mmol), aldehyde 3a–g (2 mmol), CuCl (5 mg, 0.05 mmol), and PhMe (3 ml). The mixture was stirred under argon atmosphere at 80°С for 6 h. The product was isolated according to method I.
N-Butyl-10-(3-fluorophenyl)-N-[10-(3-fluorophenyl)- 10-(piperidin-1-yl)-deca-2,8-diyn-1-yl]-10-(piperidin-1-yl)- deca-2,8-diyn-1-amine (4a). Yield 225 mg (65%), brown oil, Rf 0.65 (hexane–EtOAc, 1:2). IR spectrum, ν, cm–1: 764, 1089, 1111, 1155, 1283, 1466, 1590, 1614, 2933. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 0.91–0.93 (3Н, m, CH3); 1.32–1.38 (2H, m, CH2CH3); 1.40–1.48 (6Н, m, CH3CH2CH2, 2N(CH2CH2)2CH2); 1.53–1.63 (8Н, m, 2N(CH2CH2)2CH2); 1.67–1.74 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.27 (4Н, s, 2NCH2C≡CCH2(CH2)3C≡C); 2.34–2.39 (4Н, m, 2NCH2C≡C(CH2)3CH2C≡C); 2.42–2.47 (8Н, m, 2N(CH2CH2)2CH2); 2.47–2.52 (2H, m, NCH2(CH2)2CH3); 3.39 (4H, s, 2NCH2C≡C); 4.54 (2H, s, 2C≡CCHAr); 6.94– 6.97 (2Н, m, H Ar); 7.31–7.33 (6Н, m, H Ar). 13C NMR spectrum (125 MHz), δ, ppm (J, Hz): 14.0 (СH3); 18.3 (CH2C≡CCHAr, NCH2C≡CCH2); 20.6 (СН3CH2); 24.4 (N(CH2CH2)2CH2); 26.2 (N(CH2CH2)2CH2); 28.0 and 28.1 (C≡C–CH2(CH2)2CH2C≡C); 29.6 (СН3CH2CH2); 42.6 (NCH2C≡C); 50.5 (N(CH2CH2)2CH2); 52.6 (СН3(CH2)2CH2N); 61.5 (C≡CCH(Ar)N); 75.4 (NCH2C≡C); 75.9 (C≡CCHAr); 84.4 (NCH2C≡C); 87.8 (C≡CCHAr); 114.1 (d, JCF = 21.1, C Ar); 115.3 (d, JCF = 22.0, C Ar); 123.9 (C Ar); 129.2 (d, J = 8.0, C Ar); 142.2 (C Ar); 162.8 (d, JCF = 243.4, CF Ar). 19F NMR spectrum (376 MHz, CDCl3), δ, ppm: –17.96 (FC6H4). Mass spectrum (APCI), m/z (Irel, %): 692 [M+H]+ (100). Found, m/z: 692.3790 [M+H]+. C46H60F2N3. Calculated, m/z: 692.4755. Found, m/z: 714.3651 [M+Na]+. C46H59F2N3Na. Calculated, m/z: 714.4775. Found, m/z: 730.3234 [M+K]+. C46H59F2N3K. Calculated, m/z: 730.4314.
N-Butyl-10-(3-chlorophenyl)-N-[10-(3-chlorophenyl)- 10-(piperidin-1-yl)deca-2,8-diyn-1-yl]-10-(piperidin-1-yl)- deca-2,8-diyn-1-amine (4b). Yield 202 mg (56%), brown oil, Rf 0.87 (hexane–EtOAc, 1:2). IR spectrum, ν, cm–1: 685, 762, 992, 1038, 1113, 1324, 1383, 1468, 1595, 1642, 2235, 2933. 1H NMR spectrum (400 MHz), δ, ppm (J, Hz): 0.91–0.93 (3Н, m, CH3); 1.31–1.38 (2H, m, CH2CH3); 1.40–1.49 (6Н, m, CH3CH2CH2, 2N(CH2CH2)2CH2); 1.53–1.62 (8Н, m, 2N(CH2CH2)2CH2); 1.66–1.74 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.24–2.29 (4Н, m, 2NCH2C≡CCH2(CH2)3C≡C); 2.33–2.38 (4Н, m, 2NCH2C≡C(CH2)3CH2C≡C); 2.43–2.47 (8Н, m, 2N(CH2CH2)2CH2); 2.50 (2H, t, 3J = 7.5, NCH2(CH2)2CH3); 3.39 (4H, s, 2NCH2C≡C); 4.52 (2H, s, 2C≡CCHAr); 7.24–7.27 (4Н, m, H Ar); 7.46 (2Н, d, J = 7.5, H Ar); 7.58 (2H, s, H Ar). 13C NMR spectrum (100 MHz), δ, ppm: 14.0 (СH3); 18.3 (CH2C≡CCHAr, NCH2C≡CCH2); 20.6 (СН3CH2); 24.4 (N(CH2CH2)2CH2); 26.1 (N(CH2CH2)2CH2); 28.0 and 28.1 (C≡C–CH2(CH2)2CH2C≡C); 29.6 (СН3CH2CH2); 42.6 (NCH2C≡C); 50.5 (N(CH2CH2)2CH2); 52.6 (СН3(CH2)2CH2N); 61.5 (C≡CCH(Ar)N); 75.3 (NCH2C≡C); 75.7 (C≡CCHAr); 84.4 (NCH2C≡C); 88.0 (C≡CCHAr); 126.6 (C Ar); 127.4 (C Ar); 128.5 (C Ar); 129.2 (C Ar); 129.2 (C Ar); 133.9 (C Ar); 141.5 (C Ar). Mass spectrum (APCI), m/z (Irel, %): 724 [M+H]+ (100). Found, m/z: 667.4379 [M–Bu+H]+. C42H51Сl2N3. Calculated, m/z: 667.3460.
10-(4-Bromophenyl)-N-[10-(4-bromophenyl)-10-(piperidin-1-yl)deca-2,8-diyn-1-yl]-N-butyl-10-(piperidin-1-yl)- deca-2,8-diyn-1-amine (4c). Yield 207 mg (51%), brown oil, Rf 0.68 (hexane–EtOAc, 1:2). IR spectrum, ν, cm–1: 503, 767, 832, 869, 1011, 1088, 1382, 1441, 1452, 2233, 2931. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 0.91–0.93 (3Н, m, CH3); 1.29–1.37 (2H, m, CH2CH3); 1.38–1.49 (6Н, m, 2N(CH2CH2)2CH2, CH2CH2CH3); 1.63–1.72 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.20–2.29 (4Н, m, 2NCH2C≡CCH2(CH2)3C≡C); 2.30–2.37 (4Н, m, 2CH2C≡CCHAr); 2.40–2.52 (8H, m, 2N(CH2CH2)2CH2); 2.48–2.52 (2Н, t, 3J = 9.2, NCH2(CH2)2CH3); 3.39 (4H, s, 2NCH2C≡C); 4.54 (2H, s, C≡CCHAr); 7.45 (8H, s, H Ar). 13C NMR spectrum (125 MHz), δ, ppm: 14.0 (СH3); 18.3 (CH2C≡CCHAr, NCH2C≡CCH2); 20.7 (СН3CH2); 24.4 (N(CH2CH2)2CH2); 26.2 (N(CH2CH2)2CH2); 28.0 and 28.1 (C≡C–CH2(CH2)2CH2C≡CH); 29.7 (СН3CH2CH2); 42.6 (NCH2C≡C); 50.5 (N(CH2CH2)2CH2); 52.7 (СН3(CH2)2CH2N); 61.4 (C≡CCH(Ar)N); 75.4 (NCH2C≡C); 75.9 (C≡CCHCAr); 84.4 (NCH2C≡C); 87.8 (C≡CHAr); 121.1 (C Ar); 130.2 (C Ar); 131.0 (C Ar); 138.4 (C Ar). Mass spectrum (APCI), m/z (Irel, %): 812 [M+H]+ (100). Found, m/z: 834.1924/836.1969/838.1973 (35/100/58) [M+Na]+. C46H59Br2N3Na. Calculated, m/z: 834.2973/836.2953/838.2933. Found, m/z: 850.1737/852.1741/854.1514 (5/12/5) [M+K]+. C46H59Br2KN3. Calculated, m/z: 850.2713/852.2692/854.2672.
N-Butyl-(10-piperidin-1-yl)-N-{10-(piperidin-1-yl)- 10-[3-(trifluoromethyl)phenyl]deca-2,8-diyn-1-yl}-10-[3-(trifluoromethyl) phenyl]deca-2,8-diyn-1-amine (4d). Yield 233 mg (59%), brown oil, Rf 0.6 (hexane–EtOAc, 1:2). IR spectrum, ν, cm–1: 702, 769, 1073, 1163, 1269, 1330, 1444, 1616, 2250, 2858, 2931. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 0.90–0.94 (3Н, m, CH3); 1.30–1.36 (2H, m, CH2CH3); 1.40–1.48 (6Н, m, CH2CH2CH3, N(CH2CH2)4CH2); 1.52–1.61 (8Н, m, N(CH2CH2)2CH2); 1.68–1.74 (8Н, m, C≡CCH2(CH2)2CH2C≡C); 2.27 (4Н, br. s, NCH2C≡CCH2); 2.36–2.41 (4H, m, NCH2C≡C(CH2)3CH2); 2.42–2.51 (10Н, m, 2N(CH2CH2)2CH2, NCH2(CH2)2CH3); 3.37 (4H, s, NCH2C≡C); 4.50 (2H, s, C≡CCH(Ar)N); 7.46 (2Н, t, J = 7.5, CH(Ar)); 7.53 (2Н, d, J =7.5, CH(Ar)); 7.78 (2H, d, J = 7.5, CH(Ar)); 7.86 (2H, s, CH(Ar)). 13C NMR spectrum (125 MHz), δ, ppm: 14.0 (СH3); 18.2 (CH2C≡CCH(Ar)N); 18.3 (NCH2C≡CCH2); 20.6 (СН3CH2); 24.4 (N(CH2CH2)2CH2); 26.1 (N(CH2CH2)2CH2); 28.0 and 28.1 (C≡CCH2(CH2)2CH2C≡C); 29.6 (NСН2CH2CH2CH3); 42.6 (NCH2C≡C); 50.5 (N(CH2CH2)2CH2); 52.6 (CH2CH2CH2N); 61.5 (CH2C≡CCH(Ar)N); 75.4 (NCH2C≡C); 75.6 (C≡CCHCAr); 84.4 (NCH2C≡C); 88.3 (C≡CCHAr); 124.1 (CH Ar); 125.2 (CH Ar); 125.4 (CF3); 128.4 (CH Ar); 130.4 (CCF3 Ar); 131.8 (CH(Ar)); 140.5 (C Ar). Mass spectrum (APCI), m/z (Irel, %): 792 [M+H]+ (100). Found, m/z: 792.5355 [M+H]+. C48H60F6N3. Calculated, m/z: 792.4691.
N-(tert-Butyl)-10-(4-fluorophenyl)-N-[10-(4-fluorophenyl)- 10-(piperidin-1-yl)deca-2,8-diyn-1-yl]-10-(piperidin-1-yl)deca-2,8-diyn-1-amine (4е). Yield 183 mg (53%),brown oil, Rf 0.65 (hexane–EtOAc, 1:2). IR spectrum, ν, cm–1: 773, 856, 1024, 1037, 1143, 1267, 1390, 1601, 2257, 2856, 2934. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 1.19 (9Н, s, С(CH3)3); 1.40–1.46 (4H, m, 2N(CH2CH2)2CH2); 1.51–1.63 (8Н, m, 2N(CH2CH2)2CH2); 1.67–1.72 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.22–2.27 (4Н, m, 2NCH2C≡CCH2); 2.34–2.37 (4Н, m, 2CH2C≡CСHAr); 2.42–2.46 (8H, m, 2N(CH2CH2)2CH2); 3.60 (4H, s, 2NCH2C≡C); 4.52 (2H, s, 2C≡C–CHAr); 7.32–7.33 (4Н, dd, J = 8.5, H Ar); 7.53–7.55 (4Н, t, J = 8.5, H Ar). 13C NMR spectrum (125 MHz), δ, ppm (J, Hz): 18.4 (CH2C≡CCHAr); 18.5 (NCH2C≡CCH2); 24.5 (N(CH2CH2)2CH2); 26.1 (N(СН2CH2)2CH2); 27.5 (CH3); 27.9 and 28.2 (C≡CCH2(CH2)2CH2C≡CH); 36.7 (NCH2C≡C); 50.4 (N(CH2CH2)2CH2); 54.9 (СН3)3CN); 61.2 (–CH2C≡CCH); 76.3 (NCH2C≡C); 77.9 (C≡CCHAr); 83.6 (C≡CCHAr); 87.6 (NCH2C≡C); 114.6 (d, J = 21.3, CH Ar); 130.0 (d, J = 7.9, CH Ar); 134.9 (C Ar); 162.1 (d, J = 243.8, CF Ar). 15N NMR (51 MHz), δ, ppm: 303.6 (N(CH2CH2)2CH2);
307.8 (NCH2C≡C). Mass spectrum (APCI), m/z (Irel, %):692 [M+H]+ (100). Found, m/z: 692.2827 [M+H]+. C46H60F2N3. Calculated, m/z: 692.4755. Found, m/z: 714.2365 [M+Na]+. C46H59NaF2N3. Calculated, m/z: 714.4575. Found, m/z: 730.2128 [M+K]+. C46H59KF2N3. Calculated, m/z: 730.4314.
N-(tert-Butyl)-9-(4-chlorophenyl)-N-[9-(4-chlorophenyl)- 9-(piperidin-1-yl)nona-2,7-diyn-1-yl]-9-(piperidin-1-yl)nona-2,7-diyn-1-amine (4f). Yield 167 mg (48%),brown oil, Rf 0.87 (Me2СО–PhH, 1:1). IR spectrum, ν, cm–1: 598, 770, 1017, 1089, 1201, 1265, 1390, 1430, 1453, 1608, 2254, 2868, 2933. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 1.21 (9Н, m, C(CH3)3); 1.42–1.50 (4H, m, 2N(CH2CH2)2CH2); 1.55–1.80 (8Н, m, 2C≡CCH2CH2CH2C≡C, N(CH2CH2)2CH2); 2.30–2.52 (16Н, m, 2C≡CCH2CH2CH2C≡C, 2N(CH2CH2)2CH2); 3.72 (4H, s, 2NCH2C≡C); 4.61 (2H, s, C≡CCHAr); 7.32 (4H, d, 3J = 8.0, H Ar); 7.56 (4H, br. s, H Ar). 13C NMR spectrum (125 MHz), δ, ppm: 18.2; 18.4; 24.1; 25.7; 27.5; 28.0; 37.2; 50.5; 55.3; 61.3; 77.2; 77.3 (overlapping with СDCl3 signals); 83.2; 83.5; 128.2 (C Ar); 130.1; 130.8; 133.0. Mass spectrum (APCI), m/z (Irel, %):696 [M+H]+ (100). Found, m/z: 694.4112 [M–H]+. C44H54Cl2N3. Calculated 694.3695.
10-(4-Bromophenyl)-N-[10-(4-bromophenyl)-10-(morpholin-4-yl)deca-2,8-diyn-1-yl]-N-(tert-butyl)-10-(morpholin-4-yl)deca-2,8-diyn-1-amine (4g). Yield 188 mg (46%), brown oil, Rf 0.75 (PhH–CHCl3–hexane–Et2O, 1:1:1:2). IR spectrum, ν, cm–1: 731, 817, 854, 1167, 1364, 1453, 1589, 2259, 2825, 2857, 2942. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 1.20 (9Н, s, C(CH3)3); 1.63–1.71 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.20–2.35 (8Н, m, 2C≡CCH2(CH2)2CH2C≡C); 2.51 (8H, s, 2N(CH2CH2)2O); 3.64 (4H, s, 2NCH2C≡C); 3.69–3.72 (8H, m, 2N(CH2CH2)2O); 4.49 (2H, s, 2C≡CCHAr); 7.45 (8H, dd, 3J = 8.8, H Ar). 13C NMR spectrum (125 MHz), δ, ppm: 18.3 (CH2C≡CCH–Ar); 18.5 (NCH2C≡CCH2); 27.4 (CH3); 27.9 and 28.1 (C≡CCH2(CH2)2CH2C≡C); 36.9 (NCH2C≡C); 49.7 (CHAr); 55.3 (C(CH3)3); 61.0 (N(CH2CH2)2O); 67.1 (N(CH2CH2)2O); 75.2 (NCH2C≡C); 76.8 (C≡CCHC–Ar); 84.2 (NCH2C≡C); 88.7 (C≡CHAr); 121.5 (C Ar); 130.2 (CH Ar); 131.0 (CH Ar); 137.5 (C Ar). Mass spectrum (APCI), m/z (Irel, %): 816 [M+H]+(100). Found, m/z: 816.4077/818.4026/820.4202 (16/43/16) [M+H]+. C44H56Br2N3O2. Calculated, m/z: 816.2739/818.2719/820.2698.
N-Butyl-10-(piperidin-1-yl)-N-[10-(piperidin-1-yl)deca-2,8-diyn-1-yl]deca-2,8-diyn-1-amine (4h). Yield 163 mg (65%), brown oil, Rf 0.4 (Et2O–CHCl3–Me2СО, 1:1:1). IR spectrum, ν, cm–1: 1115, 1203, 1324, 1443, 1454, 2234, 2858, 2932. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 0.83 (3Н, t, 3J = 7.2, CH3); 1.25 (2H, q, 3J = 7.2, CH2CH3); 1.30–1.36 (6Н, m, 2N(CH2CH2)2CH2, CH2CH2CH3); 1.52 (16Н, br. s, 2N(CH2CH2)2CH2, 2C≡CCH2(CH2)2CH2C≡C); 2.13 (8H, s, 2NCH2C≡CCH2(CH2)2CH2C≡C); 2.38 (10Н, br. s, NCH2(CH2)2CH3, N(CH2CH2)2CH2); 3.12 (4H, s, 2NCH2C≡C); 3.26 (4Н, s, N(CH2C≡C)2). 13C NMR spectrum (125 MHz), δ, ppm: 13.9 (СH3); 18.2 (CH2C≡CCH–Ar, NCH2C≡CCH2); 20.5 (СН3CH2); 24.9 (N(CH2CH2)2CH2); 25.8 (N(CH2CH2)2CH2); 27.8 and 27.9 (C≡C–CH2(CH2)2CH2C≡CH); 29.5 (СН3CH2CH2); 42.4 (N(CH2C≡C)2); 47.9 (NCH2C≡C); 52.5 (СН3(CH2)2CH2N); 53.2 (N(CH2CH2)2CH2); 75.2 (C≡C); 75.4 (C≡C); 84.4 (C≡C); 87.6 (C≡C). Mass spectrum (APCI), m/z (Irel, %):504 [M+H]+ (100). Found, m/z: 504.3535 [M+H]+. C34H54N3. Calculated, m/z: 504.4318.
N-Butyl-11-(piperidin-1-yl)-N-[11-(piperidin-1-yl)- undeca-2,9-diyn-1-yl]undeca-2,9-diyn-1-amine (4i). Yield 183 mg (69%), brown oil, Rf 0.68 (PhH–Me2СО–i-PrOH, 2:1:0.5). IR spectrum, ν, cm–1: 993, 1104, 1116, 1179, 1325, 1340, 1366, 1453, 2257, 2857, 2932. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 0.88–0.91 (3Н, m, CH3); 1.28–1.37 (2H, m, CH2CH3); 1.38–1.55 (18Н, m, 2N(CH2CH2)2CH2, 2N(CH2CH2)2CH2, CH2CH2CH3, 2C≡C(CH2)2CH2(CH2)2C≡C); 1.56–1.65 (8Н, m, 2C≡CCH2CH2CH2CH2CH2C≡C); 2.15–2.20 (8Н, br. s, 2C≡CCH2(CH2)3CH2C≡C); 2.20–2.25 (2Н, m, NCH2(CH2)2CH3); 2.40–2.50 (8H, m, 2N(CH2CH2)2CH2); 3.17–3.20 (4Н, m, 2NCH2C≡C); 3.34 (4H, s, 2NCH2C≡C). 13C NMR spectrum (125 MHz), δ, ppm: 14.0 (СH3); 18.6 and 19.1 (C≡CCH2(CH2)3CH2C≡C); 20.6 (СН3CH2); 24.0 (N(CH2CH2)2CH2); 25.8 (N(CH2CH2)2CH2); 27.8, 28.0, and 28.4 (C≡C–CH2(CH2)3CH2C≡CH); 29.6 (СН3CH2CH2); 42.5 (N(CH2C≡C)2); 48.0 (2NCH2C≡C); 52.6 (СН3(CH2)2CH2N); 53.3 (N(CH2CH2)2CH2); 75.1 (NCH2C≡C), 75.3 (C≡CCH2); 84.7 (NCH2C≡C); 84.9 (C≡CH2). Mass spectrum (APCI), m/z (Irel, %): 532 [M+H]+ (100). Found, m/z: 554.4797 [M+Na]+. C36H57N3Na. Calculated, m/z: 554.4450. Found, m/z: 570.4558 [M+K]+. C36H57N3K. Calculated, m/z: 570.4190.
N-Isopropyl-(9-piperidin-1-yl)-N-[9-(piperidin-1-yl)nona-2,7-diyn-1-yl]nona-2,7-diyn-1-amine (4j). Yield 134 mg (58%), yellow oil, Rf 0.35 (Et2O–Me2СО–CHCl3, 1:1:1). IR spectrum, ν, cm–1: 993, 1038, 1104, 1068, 1104, 1168, 1251, 1310, 1325, 1365, 1439, 1451, 1466, 2257, 2854, 2931. 1H NMR spectrum (500 MHz), δ, ppm (J, Hz): 1.11 (6H, d, 3J = 6.5, CH(CH3)2); 1.44 (4H, br. s, 2N(CH2CH2)2CH2); 1.64 (8Н, t, 3J = 5.8, 2N(CH2CH2)2CH2); 1.69–1.73 (4Н, m, 2C≡CCH2CH2CH2C≡C); 2.30–2.34 (8Н, m, 2C≡CCH2CH2CH2C≡C); 2.50 (8H, br. s, 2N(CH2CH2)2CH2); 2.89 (1Н, pent, 3J = 6.5, CH(CH3)2); 3.24 (4H, s, 2NCH2C≡C), 3.47 (4H, s, N(CH2C≡C)2). 13C NMR spectrum (125 MHz), δ, ppm: 18.0, 18.1 (CH2CH2CH2); 20.3 (СН3); 23.9 (N(CH2CH2)2CH2); 25.8 (N(CH2CH2)2CH2); 28.1 (CH2CH2CH2); 39.5 (N(CH2C≡C)2); 48.0 (2NCH2C≡C); 50.9 (CHN); 53.3 N(CH2CH2)2CH2); 75.6 (C≡CCH2N(CH2CH2)2CH2); 76.5 (NCH2C≡C); 83.6 (NCH2C≡C); 84.3 (C≡CCH2N(CH2CH2)2CH2). Found, m/z: 460.4028 [M–H]+. C31H46N3. Calculated, m/z: 460.3692.
References
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168.
a Yoo, W.-J.; Zhao, L.; Li, C.-J. Aldrichimica Acta 2011, 44, 43. (b) Liu, Yu. ARKIVOC 2014, (i), 1. (c) Sun, L.; Wu, M.; Huang, X.; Wang, J.; Song, G. Chem. Heterocycl. Compd. 2018, 54, 355. [Khim. Geterotsikl. Soedin. 2018, 54, 355.]
Cammarata, J. R.; Rivera, R.; Fuentes, F.; Otero, Yo.; Ocando-Mavarez, E.; Arce, A.; Garcia, J. M. Tetrahedron 2017, 58, 4078.
Tajbaksh, M.; Farhang, M.; Mardani, H. R.; Hosseinzadeh, R.; Sarrafi, Ya. Chin. J. Catal. 2013, 34, 2217.
Periasamy, M.; Reddy, P. O.; Sanjeevakumar, N. Eur. J. Org. Chem. 2013, 3866.
(a) Bonfield, E. R.; Li, Ch.-J. Adv. Synth. Catal. 2008, 350, 370. (b) Grirrane, A.; Álvarez, E.; García, H.; Corma, A. Chem.–Eur. J. 2018, 24, 16356.
Park, K.; Heo, Yu.; Lee, S. Org. Lett. 2013, 15, 3322.
(a) Pang, T.; Yang, Q.; Gao, M.; Wang, M.; Wu, A. Synlett 2011, 3046. (b) Thirunarayanan, A.; Rajakumar, P. Synlett 2014, 2127.
Liu, Ya.; Gao, M.; Lam, Ja. W. Y.; Hu, R.; Tang, B. Zh. Macromolecules 2014, 47, 4908.
(a) Gorman, I. E.; Willer, R. L.; Kemp, L. K.; Storey, R. F. Polymer 2012, 53, 2548. (b) Jin, P.-Yu.; Jin, P.; Ruan, Yi.-A.; Ju, Yo.; Zhao, Yu.-F. Synlett 2007, 3003. (c) Groaz, E.; Banti, D.; North, M. Tetrahedron 2008, 64, 204. (d) Korbad, B. L.; Lee, S.-H. Eur. J. Org. Chem. 2014, 46, 5089. (e) Shi, W.-J.; Liu, J.-Yo.; Ng, D. K. P. Chem.–Asian J. 2012, 7, 196.
Khabibullina, G. R.; Zaynullina, F. T.; Valiakhmetova, А. R.; Ibragimov, A. G.; Dzhemilev, U. M. Synthesis 2016, 2294.
(a) Khabibullina, G. R.; Zaynullina, F. T.; Karamzina, D. S.; Ibragimov, A. G.; Dzhemilev, U. M. Tetrahedron 2017, 73, 2367. (b) Khabibullina, G. R.; Zaynullina, F. T.; Kutepov, B. I.; Ibragimov, A. G.; Dzhemilev, U. M. Chem. Heterocycl. Compd. 2018, 54, 86. [Khim. Geterotsikl. Soedin. 2018, 54, 86.]
Zoccarato, F.; Cappellotto, M.; Alexandre, A. J. Bioenerg. Biomembr. 2008, 40, 289.
Samadi, A.; Estrada, M.; Pérez, C.; Rodríguez-Franco, M. I.; Iriepa, I.; Moraleda, I.; Chioua, M.; Marco-Contelles, J. Eur. J. Med. Chem. 2012, 57, 296.
(а) Shaibakova, M. G.; Titova, I. G.; Ibragimov, A. G.; Dzhemilev, U. M. Russ. J. Org. Chem. 2011, 47, 161. [Zh. Org. Khim. 2011, 47, 173.] (b) Khabibullina, G. R.; Yanybin, V. M.; Ibragimov, A. G.; Dzhemilev, U. M. Chem. Heterocycl. Compd. 2014, 50, 726. [Khim. Geterotsikl. Soedin. 2014, 788.]
This work was performed with financial support from the Grants Council of the President of the Russian Federation (grant NSh-5240.2018.3), Russian Foundation for Basic Research (Project No. 18-33-00837-mol-a), and within the framework of the Project part of the State Assignment АААА-А17-117012610060-7 and АААА-А17-117011910027-0.
Structural characterization of the compounds was performed at the Collective Use Center “Agidel” at the Institute of Petrochemistry and Catalysis, Russian Academy of Sciences. APCI mass spectra of compounds 4a–i were recorded at the Collective Use Center “Chemistry” of the Ufa Institute of Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(1), 97–102
Rights and permissions
About this article
Cite this article
Khabibullina, G.R., Zaynullina, F.T., Tyumkina, T.V. et al. Synthesis of N-substituted tetrapropargylamines by catalytic aminomethylation of α,ω-diacetylenes. Chem Heterocycl Comp 55, 97–102 (2019). https://doi.org/10.1007/s10593-019-02425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02425-5