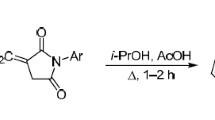
We studied the condensation of 1,2-diamino-4-phenylimidazole with N-arylmaleimides and established that this reaction occurred upon brief refluxing of reactants in isopropanol in the presence of a catalytic amount of acetic acid and produced substituted 7-amino-N-aryl-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5-b]pyridazine-4-carboxamides. Performing this reaction at room temperature led to the acyclic intermediates N-aryl-3-(1,2-diamino-4-phenylimidazol-5-yl)pyrrolidine-2,5-diones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Imidazopyridazines play an important role in modern pharmaceutical and medicinal chemistry due to their biological activity, including anticancer,1 antiepileptic,2 and antimalarial effects,3 promotion of soluble guanylyl cyclase activity,4 as well as suppression of HIV5 and influenza6 viruses.
N-Arylmaleimides are often used for molecular design of quite diverse heterocyclic matrices.7 The recyclization of N-arylmaleimides with N,N- and N,C-bisnucleophiles is known to include the stages of amino or methylеne group addition at the activated maleimide double bond and opening of imide ring with transamidation at the free amino group of the bisnucleophilic substrate.7 а–d These reactions were also performed with such cyclic bisnucleophiles as aminobenzimidazoles, aminotriazoles, and aminopyrazoles. 7 e,f, 8 These polynucleophiles reacted at the amino group or ring nitrogen8 e,f or the СН fragment of the ring (in the case of unsubstituted aminopyrazoles).8 Depending on the direction of nucleophilic attack, isomeric rings were formed with the methylene group in endo or exo positions, creating five-, six-, or seven-membered rings, respectively.7 e,f
Previously we have described methods for the synthesis of various imidazo[1,5-b]pyridazine derivatives, based on tandem reactions of 1,2-diaminoimidazole and various dielectrophiles.9 At the same time, no examples have been described for the preparation of tetrahydroimidazo[1,5-b]-pyridazines. In order to expand the synthetic potential of 1,2-diaminoimidazole in heterocyclization reactions by using other available and reactive dielectrophiles, in the current work we studied the interaction of 1,2-diamino-4-phenylimidazole (1) with N-arylmaleimides 2a–e.
As a polynucleophilic (1,3-C,N and 1,4-N,N) agent, the diaminoimidazole 1 can produce several linear products in the reaction with arylmaleimides 2a–e, as outlined in Scheme 1. The heterocyclization of diaminoimidazole 1 with N-arylmaleimides 2a–e was performed by refluxing the starting materials for 1–2 h in isopropanol in the presence of a catalytic amount of acetic acid. The reaction resulted in the formation of single products that were isolated as white solids, and identified from 1Н and 13С NMR spectra as 7-amino-N-aryl-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5-b]pyridazine-4-carboxamides 4a–e. The yields of these target products were high (70–80%) and did not depend on the nature of substituents in the aromatic ring of arylmaleimides. Performing this reaction by continuous stirring at room temperature led to the formation of acyclic product 3, the cyclization of which occurred upon refluxing for 2 h under the same conditions.

Scheme 1
The interaction of N-arylmaleimides8 and maleic anhydride10 with 5-aminopyrazoles leads to a mixture of regio-isomeric derivatives of pyrazolo[1,5-а]pyrimidine-7-carboxylic and pyrazolo[3,4-b]pyridine-4-carboxylic acids10 or carboxamides,9 depending on the reaction conditions. Thus, aminopyrazole can behave both as a 1,3-C,N and 1,3-N,Nbisnucleophile towards maleimide. Based on the polynucleophilicity of 1,2-diamino-4-phenylimidazole (1), the reaction with maleimides 2a–e may occur according to the following mechanism. In the first step, diaminoimidazole 1 adds to the double bond of arylmaleimide 2 and may produce the linearly linked products 3, 3′, and 3″, formed by attack at the СН or NH2 groups, respectively. The spectrum of compound 3b retained the proton signals of two amino groups, while the signal of imidazole ring СН group disappeared, and the signals of methylene and methyne protons from the maleimide fragment appeared at 3.24 and 4.57 ppm, respectively. These spectral features unequivocally confirmed the formation of intermediate 3 during the reaction. The further intramolecular cyclization of intermediate 3 can also proceed by two mechanisms: the route А gives tetrahydroimidazo[1,5-b]pyridazines 4, while the route В leads to dihydroimidazopyrazoles 5.
1Н NMR spectra of the isolated compounds 4 contained not only proton signals of aryl and methyl groups (for products 4b,d,e), but also the imidazole NH2 substituent signals in the region of 5.5–5.6 ppm. The methylеne proton signals appeared as double doublets and/or doublets at 2.6–2.7 and 3.01 ppm (3-СH2), while the amide proton signals were observed at stronger field. Based on the literature data,8 , 10 the six-membered structure of compounds 4а–е was confirmed by the double doublet signal of the 4-СH methine proton at 4.3–4.6 ppm (J ax = 6.2–6.9 and J eq = 1.7–1.8 Hz), which was coupled to the protons of 3-CH2 methylene group. For the five-membered ring, due to strong spin-spin coupling constants, this signal should appear as a triplet with similar constants. 13С NMR spectra of compounds 4а–е contained characteristic signals of imidazole С-7 atom at 143–144 ppm and the bridgehead С-4а atom at 111–113 ppm.11 The signals of pyridazine ring carbon atoms were observed at 33, 37 (С-3 and С-4), and 166 ppm (С-2). The presence of a two-proton singlet of NH2 group in the reaction product spectra clearly excluded the possibility of products with a seven-membered ring (route С).
The structure of compounds 4 was also confirmed by the NOESY and HMBC 2D NMR techniques. The indicators for six-membered structure were the NOESY cross peaks of methine proton at the С-4 atom with the amide proton (Fig. 1). Such a correlation would be impossible in the case of pyrazole ring. The absence of a correlation in HMBC spectra (Fig. 2) between the protons of amide fragment in maleimide and the С-3 carbon atom also allowed to confirm the formation of pyridazine ring. This cross peak should be present in the case of a fivemembered system.
Mass spectral analysis of the reaction products did not detect the molecular ion of compounds 4с,d. These molecular structures did produce ions with m/z 295 and also characteristic ions for all compounds 4 with m/z 227.
The likely fragmentation mechanism for compounds 4с,d is presented in Scheme 2. The first step probably involves the cleavage of aromatic nucleus, leading to the relatively stable tetrahydroimidazopyridazine ion (m/z 227). This ion is subject to further fragmentation.

Scheme 2
Thus, we have discovered a new, completely regioselective heterocyclization reaction of 1,2-diamino-4-phenylimidazole with N-arylmaleimides that provided 7-amino-N-aryl-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5-b]pyridazine-4-carboxamides.
Experimental
1Н and 13С NMR spectra were acquired on a Bruker DRX spectrometer (500 and 125 MHz, respectively) in DMSO-d 6 , with TMS as internal standard. The mixing time for NOESY spectrum was 1.0 s. Mass spectra were recorded on a Finnigan MAT Incos 50 spectrometer (EI ionization, 70 eV). Elemental analysis was performed on a Carlo Erba NA 1500 instrument. Melting points were determined on a Stuart SMP30 apparatus. The individuality of starting materials and products, as well as qualitative composition of reaction mixtures were determined by TLC on Merck TLC plates Silica gel 60 F254; eluents: methanol, chloroform, and various mixtures of methanol with chloroform. Visualization under UV light and with iodine vapor.
The starting diaminoimidazole 1 was obtained according to a published procedure.12 Compounds 2а–е were obtained from Acros Organics.
3-(1,2-Diamino-4-phenylimidazol-5-yl)-1-(4-isopropylphenyl) pyrrolidine-2,5-dione (3b). A mixture of diaminoimidazole 1 (0.87 g, 5 mmol), N-arylmaleimide 2b (1.08 g, 5 mmol), isopropanol (5 ml), and 1–2 drops of acetic acid was stirred for 3 h at room temperature. The precipitate that formed was filtered off and recrystallized from a 2:1 mixture of i-PrOH–DMF. Yield 1.75 g (90 %), white powder, mp 215 °C. 1H NMR spectrum, δ, ppm (J, Hz): 1.23 (6Н, d, J = 6.9, (СН 3)2CH); 2.91–3.00 (2Н, m, (ССН3)2CH, СН2); 3.24 (1Н, dd, J = 17.8, J = 9.8, СН2); 4.57 (1Н, dd, J = 9.8, J = 5.6, СН); 5.39 (2Н, s, NH2); 5.46 (2Н, s, NH2); 7.19–7.23 (3Н, m, Н Ar); 7.33–7.38 (4Н, m, Н Ar); 7.52 (2Н,dd, J = 8.3, J = 1.2, о-Н Ph). 13C NMR spectrum, δ, ppm: 23.6 (CH3); 33.0 (CH2); 35.2 (CН); 36.8 (CН); 119.0 (C-5); 125.5, 126.0, 126.4, 126.8, 127.1, 127.4, 127.8, 128.3, 130.5, 135.5 (С Ar); 148.5 (C-4); 149.0 (С-2); 175.8 (СО); 176.9 (CO). Mass spectrum, m/z (I rel, %): 389 [M] + (100). Found, %: C 67.44; H 5.93; N 17.89. C22H23N5O2. Calculated, %: C 67.85; H 5.95; N 17.98.
Synthesis of 7-amino- N -aryl-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5- b ]pyridazine-4-carboxamides 4а–е (General method). A mixture of diaminoimidazole 1 (0.87 g, 5 mmol), N-arylmaleimide 2а–е (5 mmol), isopropanol (5 ml), and 1–2 drops of acetic acid was refluxed for 1–2 h. The precipitate that formed was filtered off and recrystallized from a 2:1 mixture of i-PrOH–DMF, giving compounds 4а–е as a white powder.
7-Amino-2-oxo- N ,5-diphenyl-1,2,3,4-tetrahydroimidazo-[1,5- b ]pyridazine-4-carboxamide (4а). Yield 70%, mp 259°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.65 (1Н, dd, J = 15.8, J = 1.8) and 3.01 (1Н, dd, J = 16.0, J = 6.7, 3-СН2); 4.35 (1Н, dd, J = 6.8, J = 1.7, 4-СН); 5.55 (2Н, s, NH2); 7.09 (1Н, t, J = 7.3, p-Н N-Ph); 7.17 (1Н, t, J = 7.4, p-Н 5-Ph); 7.33 (4Н, dt, J = 7.7, J = 2.8, m-Н Ph); 7.56 (2Н, d, J = 7.3, o-Н N-Ph); 7.60 (2Н, d, J = 7.6, о-Н 5-Ph); 10.43 (1Н, s, NHCO); 11.40 (1Н, br. s, 1-NН). 13C NMR spectrum, δ, ppm: 33.7 (С-3); 37.9 (С-4); 112.3 (С-4а); 119.6, 123.8, 125.3, 125.8, 128.5, 128.9, 134.6 (С Ar); 138.8 (С-5); 143.1 (С-7); 166.7 (С-2); 169.7 (NHCO). Mass spectrum, m/z (I rel, %): 347 [M]+ (100). Found, %: C 66.32; H 4.91; N 20.06. C19H17N5O2. Calculated, %: C 65.70; H 4.93; N 20.16.
7-Amino- N -(4-isopropylphenyl)-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5- b ]pyridazine-4-carboxamide (4b). Yield 73%, mp 261°C. 1H NMR spectrum, δ, ppm (J, Hz): 1.17 (6Н, d, J = 6.9, (СН 3)2CH)); 2.84 (1H, quin, J = 6.9, (СН3)2CH); 2.62 (1Н, dd, J = 15.8, J = 1.8) and 2.99 (1H, dd, J = 15.8, J = 6.9, 3-СН2); 4.32 (1H, dd, J = 6.9, J = 1.8, 4-CH); 5.52 (2H, s, NH2); 7.14–7.22 (3H, m, Н Ar); 7.31–7.35 (2Н, m, Н Ph); 7.50 (2H, d, J = 8.5, о-Н Ar); 7.55 (2H, d, J = 7.6, о-Н Ph); 10.33 (1Н, s, NHCO); 11.30 (1Н, br. s, 1-NН). 13C NMR spectrum, δ, ppm: 24.0 (2CH3); 33.0 (CH); 33.6 (C-3); 37.8 (C-4); 112.3 (C-4a); 119.7, 125.3, 125.8, 126.6, 128.4, 134.6 (С Ar); 136.5 (C-5); 143.1 (С Ar); 144.0 (C-7); 166.7 (С-2); 169.5 (NHCO). Mass spectrum, m/z (I rel, %): 389 [M]+ (100). Found, %: C 67.44; H 5.94; N 17.92. C22H23N5O2. Calculated, %: C 67.85; H 5.95; N 17.98.
Compound 4b was also obtained from the intermediate 3b according to the following procedure: compound 3b (5 mmol) was dissolved in isopropanol (5 ml), 1–2 drops of acetic acid were added, and the mixture was refluxed for 2 h. The precipitate that formed was recrystallized from a 2:1 mixture of i-PrOH–DMF, giving compound 4b (1.56 g, 80%).
7-Amino- N -(2,3-dichlorophenyl)-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5- b ]pyridazine-4-carboxamide (4c). Yield 76%, mp 246°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.70 (1Н, dd, J = 16.0, J = 1.9) and 3.02 (1H, dd, J = 16.0, J = 6.9, 3-СН2); 4.56 (1H, dd, J = 6.8, J = 1.7, 4-CH); 5.58 (2H, s, NH2); 7.20 (1H, t, J = 7.4, p-Н Ar); 7.37 (3Н, dt, J = 8.2, J = 2.8, Н Ar); 7.49 (1H, dd, J = 8.1, J = 1.5, о-Н Ph); 7.61–7.66 (3H, m, Н Ar); 10.10 (1Н, s, NHCO); 11.32 (1Н, br. s, 1-NН). 13C NMR spectrum, δ, ppm: 33.2 (С-3); 37.3 (C-4); 111.6 (C-4a); 124.9, 125.4, 125.7, 125.9, 127.3, 128.2, 128.5, 132.0, 134.5 (С Ar); 136.3 (C-5); 143.2 (C-7); 166.3 (С-2); 170.1 (NHCO). Mass spectrum, m/z (I rel, %): 295 [M–C4H2Cl2]+ (100). Found, %: C 54.49; H 3.62; N 16.74. C19H15Cl2N5O2. Calculated, %: C 54.82; H 3.63; N 16.82.
7-Amino- N -(2-methylphenyl)-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5- b ]pyridazine-4-carboxamide (4d). Yield 72%, mp 252°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.17 (3Н, s, СН3); 2.69 (1Н, d, J = 15.4) and 3.01 (1H, dd, J = 15.9, J = 6.8, 3-СН2); 4.44 (1H, d, J = 6.2, 4-CH); 5.60 (2H, s, NH2); 7.09–7.13 (1H, m, p-Н Ar); 7.15–7.24 (3Н, m, Н Ar); 7.37 (3H, t, J = 7.6, Н Ar); 7.64 (2H, d, J = 7.8, o-Н Ph); 9.76 (1Н, s, NHCO); 11.50 (1Н, br. s, 1-NH). 13C NMR spectrum, δ, ppm: 17.7 (CH3); 33.6 (С-3); 37.4 (С-4); 112.2 (С-4а); 125.3, 125.8, 126.1, 126.4, 126.5, 128.4, 130.5, 132.2, 134.7 (С Ar); 135.8 (С-5); 143.1 (С-7); 166.3 (С-2); 169.7 (NHCO). Mass spectrum, m/z (I rel, %): 295 [M–C5H6] + (100). Found, %: C 66.07; H 5.28; N 19.31. C20H19N5O2. Calculated, %: C 66.47; H 5.30; N 19.38.
7-Amino- N -(5-chloro-2-methylphenyl)-2-oxo-5-phenyl-1,2,3,4-tetrahydroimidazo[1,5- b ]pyridazine-4-carboxamide (4e).Yield 70%, mp 228°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.17 (3Н, s, СН3); 2.72 (1Н, d, J = 15.7) and 3.01 (1H, dd, J = 15.9, J = 6.8, 3-СН2); 4.49 (1H, d, J = 6.4, 4-СН); 5.62 (2H, s, NH2); 7.15–7.27 (4H, m, Н Ar); 7.36 (1Н, t, J = 7.5, Н Ph); 7.51 (1Н, s, Н Ar); 7.62 (2H, d, J = 7.6,
Н Ar); 9.83 (1Н, s, NHCO); 11.35 (1Н, br. s, 1-NH). 13C NMR spectrum, δ, ppm: 17.2 (CH3); 33.4 (С-3); 37.3 (С-4); 111.9 (С-4а); 124.3, 125.2, 125.3, 125.9, 128.5, 130.0, 130.5, 131.9, 134.6 (С Ar); 137.1 (С-5); 143.1 (С-7); 166.5 (С-2); 169.9 (NHCO). Mass spectrum, m/z (I rel, %): 395 [M]+ (100). Found, %: C 60.33; H 4.56; N 17.63. C20H18ClN5O2. Calculated, %: C 60.68; H 4.58; N 17.69.
The results were obtained within the framework of Decree No. 218 by the Government of Russian Federation, contract 02.G25.31.0007, with support from the Ministry of Education and Science of the Russian Federation.
References
(a) Huang, W.-S.; Metcalf, C. A.; Sundaramoorthi, R.; Wang, Y.; Zou, D.; Thomas, R. M.; Zhu, X.; Cai, L.; Wen, D.; Liu, S.; Romero, J.; Qi, J.; Chen, I.; Banda, G.; Lentini, S. P.; Das, S.; Xu, Q.; Keats, J.; Wang, F.; Wardwell, S.; Ning, Y.; Snodgrass, J. T.; Broudy, M. I.; Russian, K.; Zhou, T.; Commodore, L.; Narasimhan, N. I.; Mohemmad, Q. K.; Iuliucci, J.; Rivera, V. M.; Dalgarno, D. C.; Sawyer, T. K.; Clackson, T.; Shakespeare, W. C. J. Med. Chem. 2010, 53, 4701. (b) Peterson, E. A.; Boezio, A. A.; Andrewa, P. S.; Boezio, C. M.; Bush, T. L.; Cheng, A. C.; Choquette, D.; Coats, J. R.; Colletti, A. E.; Copeland, K. W.; DuPont, M.; Graceffa, R.; Grubinska, B.; Kim, J. L.; Lewis, R. T.; Liu, J.; Mullady, E. L.; Potashman, M. H.; Romero, K.; Shaffer, P. L.; Stanton, M. K.; Stellwagen, J. C.; Teffera, Y.; Yi, S.; Cai, T.; La, D. S. Bioorg. Med. Chem. Lett. 2012, 22, 4967. (c) Miller, G. D.; Woessner, D. W.; Sirch, M. J.; Lim C. S., Mol. Pharmaceutics 2013, 10, 3475. (d) Foulks, J. M.; Carpenter, K. J.; Luo, B.; Xu, Y.; Senina, A.; Nix, R.; Chan, A.; Clifford, A.; Wilkes, M.; Vollmer, D.; Brenning, B.; Merx, S.; Lai, S.; McCullar, M. V.; Ho, K.-K.; Albertson, D. J.; Call, L. T.; Bearss, J. J.; Tripp, S.; Liu, T.; Stephens, B. J.; Mollard, A.; Warner, S. L.; Bearss, D. J.; Kanner, S. B. Neoplasia 2014, 16, 403.
(a) Moreau, S.; Coudert, P.; Rubat, C.; Vallee-Goyet, D.; Gardette, D.; Gramain, J.-C.; Couquelet, J., Bioorg. Med. Chem. 1998, 6, 983. (b) Rimoli, M. G.; Russo, E.; Cataldi, M.; Citraro, R.; Ambrosino, P.; Melisi, D.; Curcio, A.; De Lucia, S.; Patrignani, P.; De Sarro, G.; Abignente, E. Neuropharmacology 2009, 56, 637.
Chapman, T. M.; Osborne, S. A.; Bouloc, N.; Large, J. M.; Wallace, C.; Birchall, K.; Ansell, K. H.; Jones, H. M.; Taylor, D.; Clough, B.; Green, J. L.; Holder, A. A. Bioorg. Med. Chem. Lett. 2013, 23, 306
Roberts, L. R.; Bradley, P. A.; Bunnage, M. E.; England, K. S.; Fairman, D.; Fobian, Y. M.; Fox, D. N. A.; Gymer, G. E.; Heasley, S. E.; Molette, J.; Smith, G. L.; Schmidt, M. A.; Tones, M. A.; Dack, K. N. Bioorg. Med. Chem. Lett. 2011, 21, 6515.
Livermore, D. G. H.; Bethell, R. C.; Cammack, N.; Hancock, A. P.; Harm, M. M.; Green, D. V. S.; Lamont, R. B.; Noble, S. A.; Orr, D. C.; Payne, J. J.; Ramsay, M. V. J.; Shingler, A. H.; Smith, C.; Storer, R.; Williamson, C.; Willson, T. J. Med. Chem. 1993, 36, 3784.
Knight, D. J.; Scopes, D. I. C.; Storer, R.; Holman, S. DE 3541358. (Chem. Abstr. 1986, 105, 173001r).
(a) Shih, M.-H. Tetrahedron, 2002, 58, 10437. (b) Costanzo, A.; Bruni, F.; Auzzi, G.; Selleri, S.; Pecori Vettori, L. J. Heterocycl. Chem. 1990, 27, 695. (c) Grandberg, I. I.; Py, D. V.; Kost, A. N. Zh. Obshch. Khim., 1961, 31, 2311. (d) Rudenko, R. V.; Komykhov, S. A.; Musatov, V. I.; Konovalova, I. A.; Shishkin, O. V.; Desenko, S. M. J. Heterocycl. Chem. 2011, 48, 888. (e) Kovygi, Yu. A.; Krylski, D. V.; Zorina, A. V.; Shikhaliev, Kh. S. Chem. Heterocycl. Compd. 2004, 40, 1222. [Khim. Geterotsikl. Soedin., 2004, 1404.] (f) Zorina, A. V.; Shikhaliev, Kh. S.; Kovygin, Yu. A. Vestnik VGU. Seriya: Khim. Biol. Farm., 2005, 1, 39.
Rudenko, R. V.; Komykhov, S. A.; Desenko, S. M.; Musatov, V. I.; Shishkin, O. V.; Konovalova, I. A.; Vashchenko, E. V.; Chebanov, V. A. Synthesis, 2011, 783.
(a) Vandyshev, D. Yu.; Shikalyev, Kh. S.; Potapov, A. Yu.; Krysin, M. Yu. Chem. Heterocycl. Compd. 2015, 51, 573 [Khim. Geterotsikl. Soedin. 2015, 51, 573.] (b) Kolos, N. N.; Orlov, V. D.; Paponov, B. V.; Shishkin, O. V. Chem. Heterocycl. Compd. 1999, 35, 1207. [Khim. Geterotsikl. Soedin. 1999, 1388.] (c) Kolos, N. N.; Orlov, V. D.; Paponov, B. V.; Baumer, V. N. Chem. Heterocycl. Compd. 1998, 34, 1189. [Khim. Geterotsikl. Soedin. 1998, 1397.] (d) Orlov, V. D.; Papiashvili, I. Z.; Povstyanoi, M. V.; Kruglenko, V. P. Chem. Heterocycl. Compd. 1984, 20, 1152. [Khim. Geterotsikl. Soedin. 1984, 1396.] (e) Brückner, R.; Lavergne, J.-P.; Viallefont, P. Liebigs Ann. Chem. 1979, 639. (f) Plaskon, A. S.; Ryabukhin, S. V.; Volochnyuk, D. M.; Shivanyuk, A. N.; Tolmachev, A. A. Heterocycles 2008, 75, 1765. (g) Kolos, N. N.; Beryozkina, T. V.; Orlov, V. D. Mendeleev Commun. 2002, 12, 91. (h) Kolos, N. N.; Kovalenko, L. Yu.; Shishkina, S. V.; Shishkin, O. V.; Konovalova, I. S. Chem. Heterocycl. Compd. 2007, 43, 1397. [Khim. Geterotsikl. Soedin. 2007, 1646.] (i) Lipson, V. V.; Svetlichnaya, N. V.; Shishkina, S. V.; Shishkin, O. V. Mendeleev Commun. 2008, 18, 141. (j) Lipson, V. V.; Svetlichnaya, N. V.; Shirobokov, M. G.; Musatov, V. I.; Shishkin, O. V.; Shishkina, S. V. Russ. J. Org. Chem. 2012, 48, 273. [Zh. Org. Khim. 2012, 48, 281.] (k) Vandyshev, D. Yu.; Shikhaliev, Kh. S.; Potapov, A. Yu.; Krysin, M. Yu. Chem. Heterocycl. Compd. 2014, 50, 1316. [Khim. Geterotsikl. Soedin. 2014, 1428.]
Filimonov, S. I.; Korsakov, M. K.; Chirkova, Zh. V.; Abramov, I. G.; Stashina, G. A.; Firgang, S. I.; Kovygin, Yu. A.; Shikhaliev, Kh. S. Chem. Heterocycl. Compd. 2013, 49, 993. [Khim. Geterotsikl. Soedin. 2013, 1065.]
Lipson, V. V.; Svetlichnaya, N. V.; Shishkina, S. V.; Shishkin, O. V. Mendeleev Commun. 2008, 18, 141.
Ivashchenko, A. V.; Lazareva, V. T.; Prudnikova, E. K.; Ivashchenko, S. P.; Rumyantsev, V. G. Chem. Heterocycl. Compd. 1982, 18, 185. [Khim. Geterotsikl. Soedin. 1982, 236.]
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(9), 829–833
Rights and permissions
About this article
Cite this article
Vandyshev, D.Y., Shikhaliev, K.S., Potapov, A.Y. et al. Condensation of 1,2-diamino-4-phenylimidazole and N-arylmaleimides with the formation of new tetrahydroimidazo[1,5-b]pyridazines. Chem Heterocycl Comp 51, 829–833 (2015). https://doi.org/10.1007/s10593-015-1782-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1782-6