Condensation of 1,2-diaminobenzimidazole with N-arylitaconimides was studied. It was found that 2-(10-amino-N-aryl-2-oxo-2,3,4,10-tetrahydropyrimido[1,2-a]benzimidazol-3-yl)acetamides were formed in the course of this reaction upon brief heating to reflux of the reagent mixture in 2-propanol in the presence of catalytic amounts of acetic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently, imidazopyrimidines attract the attention of researchers due to their diverse pharmacological activity. Interest in the synthesis of pyrimidobenzimidazoles arises primarily due to their structural similarity to natural biologically active compounds, particularly purine and pyrimidine bases. Pyrimidobenzimidazoles with the properties of phosphodiesterase, topoisomerase, and p38 MAP-kinase inhibitors, and also of antagonists of estrogen receptors have been discovered.1a–d Because of these properties they are used as antidepressants, vasodilators, antibiotics, antifungals, antivirals, and anticancer drugs.1e–f Of the possible options of fusing of benzimidazole and pyrimidine cycles, pyrimido[1,2-a]benzimidazoles, which can be synthesized by various methods,1e are the most common.
One method of accessing pyrimido[1,2-a]benzimidazoles is heterocyclization of pyrimidines with amino alcohols1a or 2-aminopyrimidines with bromo ketones.1d–f However, this route usually involves a large number of steps, some of them requiring the use of hard to access reagents. Therefore, constructing the pyrimidobenzimidazole system from benzimidazole derivatives, in particular of 2-aminobenzimidazole2 and 1,2-diaminobenzimidazole, appears synthetically more attractive.
The polynucleophilicity of 1,2-diaminobenzimidazole determines the various routes of its reaction with dielectrophilic reagents. To create the pyrimidine ring, 1,2-diaminobenzimidazole (1) must react with 1,3-dielectrophiles as a 1,3-NCN-dinucleophile. A two-component reaction of diaminobenzimidazole 1 with ethoxymethylene derivatives of diethyl malonate and ethyl cyanoacetate,3a as well as N-arylmaleimides3b is known. In the reaction with carboxylic acid chlorides,3c carboxylic acids and their esters,3d and 1,3-dicarbonyl compounds,3e 1,2-diaminobenzimidazole (1) reacts as a 1,4-NNCN-dinucleophile, which leads to the formation of five- and six-membered systems.3c-e
In continuation of our research on the synthesis of azaheterocyclic compounds containing an imidazole moiety, the reaction of 1,2-diaminobenzimidazole (1) with N-arylitaconimides 2a–e was studied in the present work in order to expand the synthetic potential of diaminoimidazole 1 and search for new routes of synthesizing pyrimido[1,2-a]-benzimidazole derivatives. Arylitaconimides are widely used in various heterocyclization reactions as synthetic analogs of arylmaleimides,4 but their reactions with heterocyclic N,N-dinucleophiles were not previously explored. Heterocyclization of 1,2-diaminobenzimidazole (1) with itaconimides 2a–e was performed by heating mixture of the reactants under reflux in 2-propanol in the presence of a catalytic amount of acetic acid for 1–2 h. Taking into account the polynucleophilicity of the starting diaminobenzimidazole 1, six- (compounds 6, 8), seven-(compounds 6', 7, 8', 9'), and eight-membered (compounds 7', 9) heterocyclic systems (routes a, b, c, and d, Scheme 1) can be formed in the course of the reaction via respective alternative intermediates 3–5.

Scheme 1
The presumptive mechanism of the process is based on publications devoted to the study of the reaction of aminoazoles with arylmaleimides.5 It is known that the reaction of N-arylmaleimides with aminoazoles produces regioisomeric mixtures of compounds. Pyrazolopyrimidines and pyrazolopyridines form in reactions with 5-aminopyrazoles.5 Considering the polynucleophilicity of 1,2-diaminobenzimidazole (1), its reaction with itaconic imides 2a–e can include the following sequence of reactions: the first step may be the addition of diaminoimidazole 1 to the methylidene group of the arylitaconimide with amino groups or the endocyclic nitrogen atom, which may lead to alternative linear intermediates 3, 4, or 5, which are further subjected to intramolecular cyclization.
However, in our studies the reaction led to the formation of single products, which were assigned the structure of 2-(10-amino-N-aryl-2-oxo-2,3,4,10-tetrahydropyrimido[1,2-a]benzimidazol-3-yl)acetamides 6a–e on the basis of 1H and 13C, NOESY, and HMBC NMR spectroscopy data. The product yields were high (80-95%) and independent of the substituents on the aromatic ring of arylitaconimides 2a–e.
The 1H NMR spectra of synthesized compounds 6a–e feature the signals of the imidazole exocyclic amino group at 5.63 ppm together with the signals of the CH groups of the benzimidazole fragment. In addition to the signals of protons of the aryl substituents, the signals of the methylene group protons appear as a doublet of doublets at 2.44–2.50 ppm (partially overlapping with the signal of DMSO protons) and at 2.93–2.95 ppm, as well as a triplet and a doublet of doublets at 3.90 and 4.47–4.50 ppm, respectively. The singlets of the amide proton signals appear in 9.87–10.36 ppm range. Based on the previous experimental work,3b , 5 the multiplet character of the signal of the methine proton of 3-CH group at 3.05–3.16 ppm split by the protons of the methylene group of the pyrimidine ring confirms the six-membered cyclic structure of compounds 6a–e. 13C NMR spectra of compounds 6a–e show the characteristic signals of nodal atoms C-5a, C-9a, C-10a at 132, 133, and 154 ppm. Signals of the tetrahydropyrimidine ring atoms are observed at 35, 42 (CH2 and C-3), and 176 (C-2) ppm. Preserving of the signal of an amino group in the 1H NMR spectra of the reaction products eliminates the possibility of the formation of compounds 7, 7'and 9, 9' (route b and d) and suggests the formation of intermediate compounds 3 or 4. Further intramolecular cyclization of these intermediates can take place according to two routes a or c, with the formation of a six- (compounds 6a-e, 8) or seven-membered (compounds 6', 8') rings.
The cross peak between the signals of the methylene proton of the atom C-4 and the proton at atom C-6 of the benzene ring in the NOESY spectrum (Fig. 1 a) proves that the reaction takes route a. If products 8 or 8' were formed (route c), this correlation would not be possible, which completely eliminates the formation of intermediate 4. The absence of cross peaks for signal at 175.9 ppm with signals of the proton of the NH group and ortho protons of the aromatic ring in 1H–13C HMBC spectra allows to assign this signal to the carbonyl group (C-2) of the pyrimidine ring. The presence of four cross peaks between the signals of the two protons at the C-4 atom and exocyclic methylene protons and the signal of the carbon nucleus C-2 (175.9 ppm) in 1H–13C HMBC spectrum (Fig. 1 b) indicates the formation of a tricyclic compound 6 (route a). Two correlations should be observed for the seven-membered structure 6'.
A major problem in the study of the cascade processes is determining the sequence of reactions that lead to the desired products. This requires information about the structure of the intermediates, however, their separation from the reaction mixture is done very rarely. In recent years, monitoring by mass spectrometry (electrospray ionization), including in combination with liquid chromatography for analyzing the composition of the intermediates and reaction products occurring in the liquid phase, is used to solve this problem.
We carried out HPLC-MS analysis in combination with UV detection of the composition of the reaction mixture for pyrimido[1,2-a]benzimidazole 6c by determining the molecular weight of intermediates and products. Samples were taken from the reaction mixture at regular intervals of 1, 60, and 120 min.
It can be seen from the chromatogram (Fig. 2) that a peak of a possible intermediate 3c is present having a mass of the protonated molecular ion of 370. Based on the reactivity of the nucleophilic centers of 1,2-diaminobenzimidazole (1),3 as well as on the analysis of 1H, 13C, NOESY, and 1H–13C HMBC NMR data, we made the following observations during the interpretation of the results of the chromatographic analysis.
When mixing the reagent and heating under reflux for 1 min, intermediate 3c (peak at 2.514 min) and the final product 6c (peak at 2.929 min) (Fig. 2) begin to form immediately. After 30 min, the concentration of the starting materials has decreased, and final product 6c begins to precipitate.
After 2 h of heating under reflux the resulting precipitate was filtered and analyzed. The chromatogram contains the signal of the final reaction product 6c at 2.907 min (m/z 370.1295 [M+H]+). To conclude, a novel heterocyclization reaction of 1,2-diaminobenzimidazole with N-arylitaconimides is described which proceeds regioselectively to form 2-(10-amino-N-aryl-2-oxo-2,3,4,10-tetrahydropyrimido[1,2-a]benzimidazol-3-yl)acetamides.
Experimental
1H and 13C NMR spectra were acquired on a Bruker DRX spectrometer (500 and 125 MHz, respectively) in DMSO-d 6, with TMS as internal standard. Mass spectra were recorded on an Agilent Technologies LCMS 6230B (ESI) system. Melting points were determined on a Stuart SMP30 apparatus. Assessment of the purity of reagents and synthesized compounds as well as the analysis of the reaction mixtures were done by TLC on Merck TLC Silica gel 60 F254 plates; eluents: methanol, chloroform, and their mixtures in different ratios. Visualization was done under UV light or in an iodine chamber. Kinetic studies of the reactions were performed by LC-MS (an Agilent 1269 Infinity LC system with a time-of-flight high resolution mass detector Agilent 6230 TOF LC/MS). Tandem electrospray ionization, registration in positive polarity mode, m/z range 50–2000 Da. Voltages: 4.0 kV capillary, 191 V fragmentor, 66 V skimmer, 750 V OctRF. Poroshell 120 EC-C18 (4.6× 50 mm, 2.7 mm) column. Gradient elution: acetonitrile–water (0.1% formic acid), flow 0.4 ml/min. MassHunter Workstation/Data Acquisition V.06.00 program was used for data collection and processing.
Starting 1,2-diaminobenzimidazole (1) and arylitaconimides 2a–e were synthesized according to published methods.6
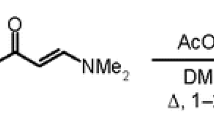
Synthesis of 2-(10-amino- N -aryl-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamides 6a–e (General method). A mixture of 1,2-diaminobenzimidazole (1) (0.74 g, 5 mmol), the respective N-arylitaconimide 2a–e (5 mmol), 2-propanol (5 ml), and AcOH (1–2 drops) was heated under reflux for 1–2 h. The precipitate that formed was filtered off and recrystallized from 2-PrOH–DMF, 2:1 mixture. Compounds 6a–e were obtained in the form of white powders.
2-(10-Amino- N -(3-chlorophenyl)-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamide (6a). Yield 1.67 g (90%). Mp 221–223°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.49 (1H, dd, J = 7.6, J = 15.7) and 2.95 (1H, dd, J = 5.4, J = 15.8, CH2CO); 3.08–3.15 (1H, m, 3-CH); 3.90 (1H, t, J = 12.1) and 4.50 (1H, dd, J = 7.5, J=12.1, 4-CH2); 5.63 (2H, s, NH2); 7.09 (1H, dd, J =1.5, J = 7.3, H Ar); 7.18–7.24 (2H, m, H-7,8); 7.33 (1H, dd, J = 8.1, J = 8.1, H-9); 7.34–7.39 (2H, m, H-6, H Ar); 7.44 (1H, dd, J = 1.1, J = 8.2, H Ar); 7.86 (1H, t, J = 2.0, o-H Ar); 10.24 (1H, s, CONH). 13C NMR spectrum, δ, ppm: 35.2 (C-4); 35.3 (CH2); 42.7 (C-3); 108.7, 109.1 (C-7,8); 117.3, 118.4, 120.0 (C Ar); 122.2, 122.6 (C-6,9); 127.3, 130.3 (C Ar); 131.4, 132.9 (C-5a,9a); 140.6 (C Ar); 154.1 (C-10a); 169.9 (CO); 175.9 (C-2). Found, m/z: 370.0389 [M+H]+. C18H16ClN5O2. Calculated, m/z: 370.1066.
2-(10-Amino- N -(3,4-dichlorophenyl)-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamide (6b). Yield 1.92 g (95%). Mp 223–225°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.50 (1H, dd, J = 7.6, J = 15.7) and 2.94 (1H, dd, J = 5.4, J = 15.8, CH2CO); 3.08–3.16 (1H, m, 3-CH); 3.90 (1H, t, J = 12.1) and 4.50 (1H, dd, J = 7.5, J =12.1, 4-CH2); 5.63 (2H, s, NH2); 7.18–7.24 (2H, m, H-7,8); 7.37 (2H, dd, J = 6.6, J = 6.6, H-6,9); 7.48 (1H, dd, J = 2.3, J = 8.8, H Ar); 7.56 (1H, d, J = 8.8, H Ar); 8.04 (1H, d, J = 2.3, o-H Ar); 10.36 (1H, s, CONH). 13C NMR spectrum, δ, ppm: 35.3 (C-4); 35.5 (CH2); 42.9 (C-3); 108.9, 109.2 (C-7,8); 119.1, 120.2 (C Ar); 122.3, 122.4 (C-6,9); 124.4, 127.5, 130.7 (C Ar); 131.0, 131.5 (C-5a,9a); 139.4 (C Ar); 154.2 (C-10a); 170.3 (CO); 176.1 (C-2). Found, m/z: 404.0067 [M+H]+. C18H15Cl2N5O2. Calculated, m/z: 404.0676.
2-(10-Amino- N -(4-chlorophenyl)-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamide (6c). Yield 1.61 g (87%). Mp 208–210°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.48 (1H, dd, J = 7.6, J = 15.7) and 2.95 (1H, dd, J = 5.3, J = 15.8, CH2CO); 3.08–3.15 (1H, m, 3-CH); 3.90 (1H, t, J = 12.1) and 4.49 (1H, dd, J = 7.5, J = 12.1, 4-CH2); 5.63 (2H, s, NH2); 7.18–7.24 (2H, m, H-7,8); 7.34–7.38 (4H, m, H-6,9, H Ar); 7.64 (2H, dt, J = 8.9, J = 2.1, H Ar); 10.20 (1H, s, CONH). 13C NMR spectrum, δ, ppm: 35.2 (C-4); 35.3 (CH2); 42.8 (C-3); 108.7, 109.1 (C-7,8); 120.5 (C Ar); 122.1, 122.2 (C-6,9); 126.4, 127.3, 128.5 (C); 131.4 (C-5a,9a); 138.2 (C Ar); 154.1 (C-10a); 169.7 (CO); 176.0 (C-2). Found, m/z: 370.0632 [M+H]+. C18H16ClN5O2. Calculated, m/z: 370.1066.
2-(10-Amino- N -(3,4-dimethylphenyl)-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamide (6d). Yield 1.55 g (85%). Mp 226–228°C. 1H NMR spectrum, δ, ppm (J, Hz): 2.16 (3H, s, CH3); 2.18 (3H, s, CH3); 2.44 (1H, dd, J = 7.6, J = 15.7) and 2.93 (1H, dd, J = 5.0, J = 15.7, CH2CO); 3.05–3.12 (1H, m, 3-CH); 3.90 (1H, t, J = 12.0) and 4.47 (1H, dd, J = 7.4, J = 12.1, 4-CH2); 5.62 (2H, s, NH2); 7.03 (1H, d, J = 8.1, H Ar); 7.18–7.24 (2H, m, H-7,8); 7.30 (1H, dd, J = 2.1, J = 8.2, H Ar); 7.35–7.38 (2H, m, H-6,9); 7.40 (1H, d, J = 2.1, o-H Ar); 9.87 (1H, s, CONH). 13C NMR spectrum, δ, ppm: 19.2 (CH3); 20.0 (CH3); 35.7 (C-4); 35.8 (CH2); 43.3 (C-3); 109.2, 109.5 (C-7,8); 117.0, 120.7 (C Ar); 122.6, 122.7 (C-6,9); 127.8, 129.8 (C Ar); 131.0, 131.9 (C-5a,9a); 136.5, 137.4 (C Ar); 154.6 (C-10a); 169.6 (CO); 176.6 (C-2). Found, m/z: 364.1176 [M+H]+. C20H21N5O2. Calculated, m/z: 364.1769.
2-(10-Amino- N -(4-ethylphenyl)-2-oxo-2,3,4,10-tetrahydropyrimido[1,2- a ]benzimidazol-3-yl)acetamide (6e). Yield 1.55 g (85%). Mp 222–224°C. 1H NMR spectrum, δ, ppm (J, Hz): 1.15 (3H, t, J = 7.6, CH2CH 3); 2.45 (1H, dd, J = 7.6, J = 15.7, CH2CO); 2.55 (2H, q, J = 7.5, CH 2CH3); 2.94 (1H, dd, J = 5.1, J = 15.7, CH2CO); 3.06–3.13 (1H, m, 3-CH); 3.90 (1H, t, J = 11.9) and 4.48 (1H, dd, J = 7.5, J = 12.1, 4-CH2); 5.63 (2H, s, NH2); 7.13 (2H, d, J = 8.4, H Ar); 7.17–7.24 (2H, m, H-7,8); 7.37 (2H, dd, J = 7.1, J = 7.1, H-6,9); 7.51 (2H, d, J = 8.4, H Ar); 9.96 (1H, s, CONH). 13C NMR spectrum, δ, ppm: 15.8 (CH2 CH3); 27.6 (CH2CH3); 35.4 (C-4); 35.5 (CH2); 42.9 (C-3); 108.8, 109.2 (C-7,8); 119.2 (C Ar); 122.4 (2C, C-6,9); 127.5, 127.8, 127.9 (C Ar); 131.4, 131.5 (C-5a,9a); 137.1, 138.4 (C Ar); 154.2 (C-10a); 169.4 (CO); 176.3 (C-2). Found, m/z: 364.2258 [M+H]+. C20H21N5O2. Calculated, m/z: 364.1769.
Supplementary information file to this article containing the 1H and 13C NMR spectra of the synthesized compounds is available at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
References
(a) Gala, D.; DiBenedetto, D. J.; Kugelman, M.; Mitchell, M. B. Tetrahedron Lett. 2003, 44, 2721. (b) Abdel-Mohsen, H. T.; Regab, F. A. F.; Ramla, M. M.; El Diwani, H. I. Eur. J. Med. Chem. 2010, 45, 2336. (c) Chandra; Puttaraju, K. B.; Mahesh, S. S.; Shivashankar, K.; Lokanath, N. K.; Madegowda, M. J. Biomed. Inform. 2014, 10, 288. (d) Rupert, K. C.; Henry, J. R.; Dodd, J. H.; Wadsworth, S. A.; Cavender, D. E.; Olini, G. C.; Fahmy, B.; Siekierka, J. J. Bioorg. Med. Chem. Lett. 2003, 13, 347. (e) Clements-Jewery, S.; Dansawan, G.; Gardener, C. R.; Matharu, S. S.; Murdoch, R.; Tully, W. R.; Westwood, R. J. Med. Chem. 1988, 31, 1220. (f) Meshram, H. M.; Kumar, A. S; Kumar, G. S.; Swetha, A.; Reddy, B. Ch.; Ramesh, P. Pharma Chem. 2012, 4, 956.
(a) Rudenko, R. V.; Komykhov, S. A.; Musatov, V. I.; Konovalova, I. A.; Shishkin, O. V.; Desenko, S. M. J.Heterocycl. Chem. 2011, 48, 888. (b) Shikhaliev, Kh. S.; Potapov, A. Yu.; Kryl'skii, D. V. Russ. Chem. Bull., Int. Ed. 2007, 56, 367. [Izv. Akad. Nauk, Ser. Khim. 2007, 355.] (c) Shikhaliev, Kh. S.; Kryl'skii, D. V.; Potapov, A. Yu.; Krysin, M. Yu.; Trefilova, I. N. Izv. Vuzov Khim. i Khim. Tekhnol. 2004, 47(3), 149. (d) Kovygin, Yu. A.; Shikhaliev, Kh. S.; Potapov, A. Yu.; Kryl'skii, D. V. Izv. Vuzov Khim. i Khim. Tekhnol. 2005, 48(1), 59.
(a) Romano, C.; Cuesta, E.; Avendano, C. Heterocycles 1990, 31 , 267. (b) Vandyshev, D. Yu.; Shikhaliyev, H. S.; Potapov, A. Yu. Eur. Chem. Bull. 2015, 4, 424. (c) Morkovnik, A. S.; Kuz'menko, T. A.; Divaeva, L. N.; Borodkin, G. S. Rus. J. Org. Chem. 2013, 49, 895. [Zh. Org. Khim. 2013, 909.] (d) Klyuev, N. A.; Povstyanoi, M. V.; Orlov, V. M.; Gnidets,. P.; Kruglenko, V. P. Chem. Heterocycl. Compd. 1992, 28, 779. [Khim. Geterotsikl. Soedin. 1992, 937.] (e) Kuz'menko, T. A.; Kuz'menko, V. V.; Pozharskii, A. F.; Simonov, A. M. Chem. Heterocycl. Compd. 1988, 24, 880. [Khim. Geterotsikl. Soedin. 1988, 1070.]
Medway, A. M.; Sperry, J. Green Chem. 2014, 16, 2084.
(a) Filimonov, S. I.; Korsakov, M. K.; Chirkova, Zh. V.; Abramov, I. G.; Stashina, G. A.; Firgang, S. I.; Kovygin, Yu. A.; Shikhaliev, Kh. S. Chem. Heterocycl. Compd. 2013, 49, 993. [Khim. Geterotsikl. Soedin. 2013, 1065.] (b) Rudenko, R. V.; Komykhov, S. A.; Desenko, S. M.; Musatov, V. I.; Shishkin, O. V.; Konovalova, I. A.; Vashchenko, E. V.; Chebanov, V. A. Synthesis 2011, 783.
(a) Pozharskii, A. F.; Anisimova, V. A.; Tsupak, E. B. Laboratory Experiments in Heterocyclic Chemistry [in Russian]; Rostov University, 1985, p. 106. (b) Oishi, T. Polym. J. 1980, 12, 719. (b) Abdel-Naby, A. S. J. Appl. Polym. Sci. 2011, 121, 169. (c) Hegazy, M.-E. F.; Shishido, K.; Hirata, T. Tetrahedron: Asymmetry 2006, 17, 1859. (d) Leow, D.; Lin, S.; Chittimalla, S. K.; Fu, X.; Tan, C.-H. Angew. Chem., Int. Ed. 2008, 47, 5641. (e) Zhang, X.; Li, Z.-C.; Li, K.-B.; Du, F.-S.; Li, F.-M. J. Am. Chem. Soc. 2004, 126, 12200.
This work was supported by the Federal targeted program ''Research and Development in the Priority Areas of Development of the Russian Scientific and Technological Complex for years 2014–2020.'' (Agreement № 14.577.21.0182, the unique identifier for applied scientific research RFMEFI57715X0182).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(7), 493–497
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 738 kb)
Rights and permissions
About this article
Cite this article
Vandyshev, D.Y., Shikhaliev, K.S., Kokonova, A.V. et al. A novel method for the synthesis of pyrimido[1,2-a]benzimidazoles. Chem Heterocycl Comp 52, 493–497 (2016). https://doi.org/10.1007/s10593-016-1914-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1914-7