Abstract
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) represents a novel approach to deliver intraperitoneal chemotherapy. We report our experience with PIPAC in patients with peritoneal metastasis (PM) from gastric cancer (GC). Data from GC patients (n = 20) included in the prospective PIPAC-OPC1 and PIPAC-OPC2 studies are reported. All patients had received prior systemic chemotherapy. The mean peritoneal cancer index (PCI) was 10.5 (range 0–39) and nine patients had diffuse GC. PIPAC with cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 were administered at 4–6-week intervals. Outcome criteria were objective tumour response, survival and adverse events. Twenty patients had 52 PIPAC procedures with a median follow-up of 10.4 months (3.3–26.5). Median survival from the time of PM diagnosis and after the first PIPAC procedure was 11.5 months and 4.7 months, respectively. Fourteen patients had repeated PIPAC (> 2), and the objective tumour response according to the histological peritoneal regression grading score (PRGS) was observed in 36%, whereas 36% had stable disease. Ten patients completed the three prescheduled sessions (per protocol group) and 40% of those displayed an objective tumour response, while 20% had stable disease. Only minor postoperative complications were noted, and none were considered causally related to the PIPAC treatment. PIPAC with low-dose cisplatin and doxorubicin can induce a quantifiable objective tumour response in selected patients with PM from GC. Survival data are encouraging and warrant further clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) represents the fifth most common malignancy worldwide, and is the third leading cause of cancer-related deaths [1]. Despite significant improvements regarding preventive strategies and curative treatment of premalignant and early neoplastic lesions, the majority of GC patients have advanced disease at the time of diagnosis [2,3,4]. Recurrences are frequently experienced despite curative therapeutic ambition, and the peritoneum is one of the most prevalent sites of metastases and recurrences. Irrespective of metastatic disease at the time of diagnosis or relapse, there is no consensus regarding direct treatment for patients with established peritoneal metastasis (PM) due to GC (GCPM). Numerous modalities have been tried to control this clinical stage, including catheter-based intraperitoneal chemotherapy or cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC), but none have provided a satisfactory clinical outcome [2, 5,6,7]. The only treatment option practiced is palliative systemic chemotherapy, but the median survival time remains short [8, 9]. Therefore, new and/or complementary therapeutic modalities are warranted. Intraperitoneal chemotherapy administration, and its further advancement and refinement into pressurised intraperitoneal aerosol chemotherapy (PIPAC), has been shown to be a safe and feasible technique to deliver cytotoxic drugs into the abdominal cavity as an aerosol under pressure [10,11,12,13,14]. PIPAC treatment may provide an objective tumour response in a significant subset of patients with PM, and PIPAC can often be administered as an outpatient procedure [14, 15]. However, specific data on the effect of PIPAC-directed treatment in GCPM, based on an objective and validated model for response evaluation, are sparse and warrant confirmation and enlargement [16, 17].
With this study, we present the additional results of PIPAC therapy in a consecutive cohort of patients with therapy-resistant GCPM. The main outcome was to evaluate the objective tumour response based on repeated peritoneal biopsies according to the peritoneal regression grading score (PRGS) [18]. Secondary outcomes included median overall survival after diagnosis and after the first PIPAC treatment, ascites formation, peritoneal lavage cytology and treatment-related adverse reactions.
Patients and methods
Patients with GCPM included in the prospective PIPAC-OPC1 and PIPAC-OPC2 trials are reported. The PIPAC-OPC1 trial has been completed and published [14], whereas the PIPAC-OPC2 trial is ongoing [19]. GCPM was documented through radiology, histology or cytology, and patients were discussed at a dedicated interdisciplinary tumour board meeting. Patients who had received first line systemic treatment with a maximum of one extraperitoneal metastasis were included; women had to be post-menopausal. Patients were older than 18 years with an Eastern Cooperative Oncology Group performance status of less than 3. Exclusion criteria were gastrointestinal tract obstruction, a history of allergic reactions to doxorubicin or platinum, renal impairment (GFR < 40 ml/min), myocardial insufficiency (NYHA class > 2), impaired liver function (bilirubin > 1.5 upper normal limit) or inadequate haematological function (absolute neutrophil count (ANC) ≤ 1.5 × 109/l and platelets ≤ 100 × 109/l).
PIPAC
Patients were scheduled for three PIPAC procedures in intervals of 4–6 weeks, but after each procedure a combination of tolerability and morphological assessment of response determined whether to continue treatment or not.
PIPAC treatment with cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 was performed in the setting of a diagnostic laparoscopy and has been described in detail previously [13]. For safe open access to the abdominal cavity, all procedures were preceded by percutaneous ultrasound and the patients received prophylactic antibiotics. The extent of PM was evaluated according to Sugarbaker’s peritoneal cancer index (PCI) [5], but only at the index procedure. If present, ascites fluid was evacuated; otherwise, the peritoneal cavity was irrigated with 200 ml of saline, which was subsequently evacuated and analysed for malignant cells (peritoneal lavage fluid). Peritoneal biopsies from each affected quadrant of the abdominal cavity were captured, and biopsy sites were marked by metal clips allowing for repeated biopsies from the same site at the following PIPAC procedure. A CE certified nebuliser (CapnoPen®, Capnomed, Villingendorf, Germany) was used to aerosolise the chemotherapeutics at a flow rate of 0.5–0.7 ml/s and a maximum pressure of 300 PSI. Due to a protocol amendment in the PIPAC-OPC2 trial, 13 of the patients were treated by standard PIPAC treatment (30 min of simple diffusion), while seven patients were treated by ePIPAC. With ePIPAC, the same steps regarding safety and chemotherapy administration were followed, but after intraperitoneal delivery of chemotherapy, the Ultravision generator (Ultravision, Alesi Surgical Ltd., UK) was turned on, and electrostatic precipitation was performed for at least one minute (or until the aerosol was cleared completely by visual inspection). Following evacuation of CO2 through a closed air waste system, the patients were sutured according to departmental guidelines.
Surgical complications within 30 days were graded according to the Clavien-Dindo [19] classification and adverse events were graded according to CTCAE version 4.0 [20].
A contrast-enhanced multi-slice CT of the thorax and abdomen was performed after three PIPAC treatments. Treatment was continued for another three courses if the CT did not show extra-peritoneal disease progression and if the patient had responded to treatment (see below).
Evaluation of treatment response
The response to PIPAC treatment was based on a histological assessment of repeated peritoneal biopsies and the cytological assessment of ascites/peritoneal lavage fluid retrieved before each PIPAC procedure. As secondary criteria, the patient should maintain the activities of everyday living without being hampered by treatment-related toxicities.
Each peritoneal biopsy was fixed in formalin and embedded in paraffin and analysed by a dedicated gastrointestinal pathologist allocated to the study. The biopsies were processed as follows: After fixation in formalin (6–24 h) and embedding in paraffin, three step sections (thickness 4 microns) were cut and stained with haematoxylin and eosin (H&E), followed by a section immunostained for epithelial cell adhesion molecule (Ep-CAM) and a final series of three step sections stained with H&E. Before staining, the sections were mounted on FLEX IHC microscope slides, dried at room temperature and baked at 60 °C for 60 min before immunostaining. Staining was automated using the Dako Omnis immunostainer with EnVision FLEX + DAB detection with a mouse or rabbit linker (Dako/Agilent, Glostrup, Denmark). The primary antibody used was clone BS14 epithelial-specific antigen/CD326 (code: BSH-7402-1) from Nordic Biosite Aps (Copenhagen, Denmark) at a dilution of 1:600. Antigen retrieval was performed using target retrieval solution (TRS) with high pH (9.0) for 30 min at 97 °C. Incubation was done at 32 °C for 20 min. Nuclear counterstaining was performed using haematoxylin FLEX on the Dako Omnis platform. Finally, slides were washed, dehydrated and coverslipped using an automated Dako cover slipper (Dako/Agilent, Glostrup, Denmark).
The PRGS was used [21] for the evaluation of histological regression. A decrease in the mean PRGS during the course of therapy was considered a response to treatment, while the mean PRGS remained unchanged in stable disease. Complete response was defined as PRGS = 1 in all biopsies from respective abdominal quadrants, and the absence of malignant cells at peritoneal cytology.
A five-tier score was used for cytological evaluation: malignant cells, suspicious cells, atypical cells, no malignant cells, other. Malignant and suspicious cells were defined as positive cytology.
Statistics
Values are given as means or medians where appropriate. The survival analyses used traditional Kaplan Meier plots. Otherwise, only descriptive statistics have been applied. The statistical software Stata version 13 (Stata Corp, Texas, USA) was used for the statistical analyses.
Ethics
The studies were conducted according to predefined protocols and the Helsinki Declaration. Oral and written informed consent was obtained from each patient. The study protocols were approved by the Regional Committees on Health Research Ethics for Southern Denmark (Project-ID: S-20140211 and S-20160100), the Danish Medicines Agency (Code number: 2016083464) and the Danish Data Protection Agency (14/52603 and 16/23653) and registered at www.clinicaltrials.gov (ClinicalTrials.gov identifier: NCT02320448 and NCT03287375) and the European Clinical Trials Database (EudraCT) number 2016-003394-18.
Results
Patients were included from March 2015 to October 2018 and the last PIPAC was completed on 26 October 2018. Twenty patients with GCPM were scheduled for PIPAC, but in six of these patients, only one PIPAC procedure could be completed due to severe deterioration of the patient’s general condition. Accordingly, treatment response was evaluated in the 14 patients in whom ≥ 2 therapy sessions could be finalised. The preoperative and procedure-related patient characteristics are summarised in Table 1. Five patients had undergone gastric resection previously, while the remaining 15 patients had their tumour in situ when PM was diagnosed, and no patient had extra-peritoneal metastases. Nine of the patients had a diffuse type GC histology. Nineteen patients had received palliative systemic chemotherapy prior to PIPAC, whereas in 14 (74%) patients it was given after the first line treatment. Nine patients received a combination of systemic treatment and PIPAC (bidirectional treatment).
The total number of PIPAC procedures amounted to 52 (11 ePIPAC, 41 PIPAC procedures) with a median PIPAC operating time of 72 min (range 52–90) for ePIPAC and 94 min (range 70–142) for PIPAC during which no intraoperative complications were recorded.
Fourteen patients completed two PIPAC procedures and 10 patients completed three PIPAC procedures. Three patients had more than three procedures and one had eight treatments.
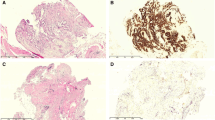
Histological regression was seen in five patients (36%, intension to treat (ITT) 25%) while five (36%, ITT 25%) had stable disease after the first PIPAC procedure. Histological regression was noted in four patients (40%, ITT 20%) and two (20%, ITT 10%) had stable disease after the second PIPAC (representative tissue sections are shown in Fig. 1).
Microscopic findings in peritoneal quadrant biopsies prior to PIPAC treatment 1 (a–c) and prior to PIPAC treatment 3 (d–f) from a patient with diffuse gastric cancer (patient no. 5; Table 2). a Diffusely infiltrating tumour cells, accompanied by slight amounts of fibrosis, according to PRGS score of 3 at baseline. The rectangle indicates the area shown in (c) (H&E, scale bar: 1 mm). b Deeper step section of the biopsy shown in (a), immunostained for EpCAM, highlighting tumour cells (scale bar: 1 mm). c Higher magnification of the tumour cells shown in (a) (H&E, scale bar: 100 µm). d The biopsy from one quadrant shows regression of tumour cells (PRGS 1), which have been replaced by fibrosis. The rectangle indicates the area shown in (f) (H&E, scale bar: 1 mm). e Deeper step section of the biopsy shown in (d, distance between (d) and (e) is around 100 µm), immunostained for EpCAM, supporting the absence of tumour cells. On the lower left, unspecific and weak light brown staining of degenerated striated muscle cells is shown (scale bar: 1 mm). f Higher magnification of the regressive fibrosis shown in (d) (H&E, scale bar: 100 µm)
Three of the four patients who had histological regression after the second PIPAC procedure were diagnosed with diffuse type gastric cancer (Table 2).
No patients had a complete response (mean PRGS = 1 and negative cytology).
Eight patients (57%) had ascites at the time of the first PIPAC procedure which prevailed in five patients (36%) of those who completed more than two sessions. The amount of ascites was reduced from the first PIPAC (median 25, range 10–2400 ml) to the last PIPAC (median 20, range 10–250 ml) session. If no ascites was present, peritoneal lavage was performed and all fluids were analysed for malignancy. One patient (11%) converted from positive to negative cytology, while four patients (44%) converted from negative to positive cytology. (Table 3).
Only minor postoperative adverse events/reactions were noted. Among the CTCAE 1-2, we observed minor reversible neuropathy, urinary retention, nausea and pain, which were probably related to the PIPAC treatment. One case of CTCAE grade 3 occurred in the form of abdominal wound dehiscence and one case of grade 4 was caused by mechanical bowel obstruction that required in hospital care, but this was not considered to be causally related to the PIPAC treatment.
The time interval from diagnosis to first PIPAC procedure was 6.1 months (1.1–22.0). The median follow-up was 10.4 months (3.3–26.5), during which time we recorded a median survival of 11.5 months from the time of PM diagnosis (Fig. 2a), whereas this was 4.7 months (0.7–15.6) following the first PIPAC session (Fig. 2b). Six patients were alive at the end of the follow-up period.
Discussion
Large population-based studies have shown that patients with GCPM have a median overall survival of 4–5 months, and that only one in four patients receive palliative systemic chemotherapy [23, 24]. New treatment modalities are desperately needed, and PIPAC directed therapy has emerged as an option in these patients. This study adds results to the hitherto limited amount of data assessing the outcome of PIPAC with low-dose cisplatin and doxorubicin in patients with chemotherapy-resistant GCPM. An objective tumour response and stable disease was documented in 40% and 20%, respectively, after two PIPAC directed treatments. The median overall survival after the diagnosis of GCPM was 11.5 months while this was 4.7 months after the first PIPAC procedure. These results agree well with the quite recently presented experiences from German centres [17] recording an overall survival of 6.7 months in the ITT analysis and a complete or major regression on histology in 36% of patients.
These results, observed in a selected group of very advanced primary and recurrent gastric cancers, deliver further evidence suggesting that PIPAC can induce the regression of resistant PM, which meets the clinical need for new and better therapies for patients with such a fatal cancer disease. Our results also provide some evidence that PIPAC therapy might even be effective in treating patients with recurrent, chemo-resistant gastric peritoneal metastasis, including the aggressive diffuse type of gastric cancer histology.
Given the fact that patients with GCPM have a poor prognosis, the present outcomes must be put into perspective. A population-based study found a median overall survival of 4.6 months from the time of diagnosis of PM only, but in patients with metastases additional to the PM, the corresponding survival was only 3.3 months [23]. Similar poor survival data can be extracted from other studies [25], while an overall survival as long as 13 months was reported in a Russian trial where GCPM patients were treated with bidirectional chemotherapy (combining XELOX with PIPAC with cisplatin and doxorubicin) as a first line option [26]. Our data are in agreement with a recently published study of 24 GCPM patients treated with PIPAC, that showed a median overall survival at 121 days after the first PIPAC procedure, while the patients who received > 3 PIPAC procedures had significantly higher median survival (450 days) [27]. These data suggest that PIPAC treatment can prolong survival in patients with GCPM and suggest that treatment must be initiated early in the course of disease. Nevertheless, more information is warranted on the factors determining the local response to PIPAC as reflected by the PRGS, which until proven otherwise seems to be a critical parameter to objectively assess the response to the therapeutic intervention. As it has been alleged that prior and/or concomitant systemic therapy induces peritoneal fibrotic reactions, it has to be studied whether the clinical effects of PIPAC are dependent on corresponding peritoneal reactions [28]. According to the currently applied scientific protocol for the evaluation of PIPAC, we did not capture information on more diffuse peritoneal reactions to systemic and local chemotherapies.
Despite the documented survival data and an observed 40% tumour response rate (ITT 20%), further studies are required to document that these tumour tissue parameters possess true relevance for the overall clinical response to therapy. Furthermore, this study is limited by the predefined use of low-dose of cisplatin and doxorubicin. The publication of data from a recent dose-escalation study [29] pave the way for complementary studies on the efficacy of PIPAC in GCPM. As the inclusion criteria for the PIPAC-OPC1 and PIPAC-OPC2 trial were almost identical, we found it ethically justified to include data from both studies in this subgroup analysis. The fact that the patients were treated with either PIPAC or ePIPAC introduces some uncertainties in the interpretation of data and the external validity of the study outcomes. Nevertheless, the accumulated amount of data emphasise the urgent need for large scale clinical trials to assess the value of adding PIPAC to systemic chemotherapy in corresponding disease states [16].
In conclusion, PIPAC with low-dose cisplatin and doxorubicin can induce objective tumour response in selected patients with GCPM and survival data are encouraging but need to be further substantiated and expanded upon in large scale clinical trials.
References
International WCRF (2019) Worldwide cancer data https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data2018 [updated Jan. 2019; cited 2019 Jan]. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data.
Yonemura Y, Endou Y, Sasaki T et al (2010) Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol 36(12):1131–1138. https://doi.org/10.1016/j.ejso.2010.09.006
Glehen O, Gilly FN, Arvieux C et al (2010) Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17(9):2370–2377. https://doi.org/10.1245/s10434-010-1039-7
El-Sedfy A, Brar SS, Coburn NG (2014) Current role of minimally invasive approaches in the treatment of early gastric cancer. World J Gastroenterol 20(14):3880–3888. https://doi.org/10.3748/wjg.v20.i14.3880
Sugarbaker PH (2016) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 48:42–49. https://doi.org/10.1016/j.ctrv.2016.06.007
Kitayama J, Ishigami H, Yamaguchi H et al (2018) Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg 2(2):116–123. https://doi.org/10.1002/ags3.12060
Ishigami H, Fujiwara Y, Fukushima R et al (2018) Phase III Trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol 36(19):1922–1929. https://doi.org/10.1200/JCO.2018.77.8613
Miyashiro I, Furukawa H, Sasako M et al (2011) Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer 14(3):212–218. https://doi.org/10.1007/s10120-011-0027-3
Sarela AI, Miner TJ, Karpeh MS et al (2006) Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg 243(2):189–195. https://doi.org/10.1097/01.sla.0000197382.43208.a5
Esquis P, Consolo D, Magnin G et al (2006) High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg 244(1):106–112. https://doi.org/10.1097/01.sla.0000218089.61635.5f
Shinkai M, Imano M, Chiba Y et al (2018) Intraperitoneal and systemic chemotherapy for patients with gastric cancer with peritoneal metastasis: a Phase II Trial. Anticancer Res 38(10):5975–5981. https://doi.org/10.21873/anticanres.12945
Solass W, Kerb R, Murdter T et al (2014) Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 21(2):553–559. https://doi.org/10.1245/s10434-013-3213-
Nowacki M, Alyami M, Villeneuve L et al (2018) Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol 44(7):991–996. https://doi.org/10.1016/j.ejso.2018.02.014
Graversen M, Detlefsen S, Bjerregaard JK et al (2018) Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol. https://doi.org/10.1177/1758835918777036
Graversen M, Lundell L, Fristrup C, Pfeiffer P, Mortensen MB (2018) Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as an outpatient procedure. Pleura Peritoneum. https://doi.org/10.1515/pp-2018-0128
Garg PK, Jara M, Alberto M, Rau B (2019) The role of Pressurized IntraPeritoneal Aerosol Chemotherapy in the management of gastric cancer: a systematic review. Pleura Peritoneum 4(1):20180127
Struller F, Horvath P, Solass W et al (2019) Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol 11:1758835919846402. https://doi.org/10.1177/1758835919846402
Solass W, Sempoux C, Detlefsen S et al (2016) Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 1(2):99–107. https://doi.org/10.1515/pp-2016-0011
Clavien PA, Strasberg SM (2009) Severity grading of surgical complications. Ann Surg 250(2):197–198. https://doi.org/10.1097/SLA.0b013e3181b6dcab
National Institutes of Health NCI (2009) Common Terminology Criteria for Adverse Events (CTCAE). 4.0 [published Online First: 28.05.2009]
Solass W, Sempoux C, Carr N et al (2019) Reproducibility of the Peritoneal Regression Grading Score (PRGS) for assessment of response to therapy in peritoneal metastasis. Histopathology 74(7):1014–1024. https://doi.org/10.1111/his.13829
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Thomassen I, van Gestel YR, van Ramshorst B et al (2014) Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 134(3):622–628. https://doi.org/10.1002/ijc.28373
Yang D, Hendifar A, Lenz C et al (2011) Survival of metastatic gastric cancer: significance of age, sex and race/ethnicity. J Gastrointest Oncol 2(2):77–84. https://doi.org/10.3978/j.issn.2078-6891.2010.025
Coccolini F, Celotti A, Ceresoli M et al (2016) Hyperthermic intraperitoneal chemotherapy (HIPEC) and neoadjuvant chemotherapy as prophylaxis of peritoneal carcinosis from advanced gastric cancer-effects on overall and disease free survival. J Gastrointest Oncol 7(4):523–529. https://doi.org/10.21037/jgo.2016.06.05
Khomyakov VRA, Ivanov A, Bolotina L, Utkina A et al (2016) Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 1(3):159–166
Gockel I, Jansen-Winkeln B, Haase L et al (2018) Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in gastric cancer patients with peritoneal metastasis (PM): results of a single-center experience and register study. J Gastric Cancer 18(4):379–391. https://doi.org/10.5230/jgc.2018.18.e37
Graversen M, Detlefsen S, Pfeiffer P et al (2018) Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): report of two cases and literature survey. Clin Exp Metastasis 35(3):103–108. https://doi.org/10.1007/s10585-018-9895-9
Tempfer CB, Hilal Z, Dogan A et al (2018) Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal metastasis. Eur J Surg Oncol 44(7):1112–1117. https://doi.org/10.1016/j.ejso.2018.04.020
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ellebæk, S.B., Graversen, M., Detlefsen, S. et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: a descriptive cohort study. Clin Exp Metastasis 37, 325–332 (2020). https://doi.org/10.1007/s10585-020-10023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-020-10023-5