Abstract
Understanding the factors that limit species distributions has become increasingly important in the face of rapid climate change. Many approaches have been used to predict responses of species and communities to new environmental challenges, including species distribution modelling, glasshouse and growth cabinet experiments, and small-scale field manipulations, all of which have both advantages and limitations. Here, we review the use of a powerful, direct method to predict how species and communities will respond to the changing climate: the field transplant experiment. We discuss how transplant experiments can elucidate the factors that limit species distributions; disentangle the role of genetic change vs. phenotypic plasticity in species’ responses; and improve understanding of the role of species interactions in driving community change. Several generalisations about potential species’ responses to climate change are emerging from these studies, including the critical role of specific life stages in response to warming trends, the role of natural enemies and new hosts in limiting or promoting adaptive capacity, and the role of niche saturation in conferring community stability at a functional guild level. Transplant experiments have also confirmed likely mechanisms of recent range shifts and highlighted the potential for some modelling exercises to overestimate future range changes. With the prospect that accelerating warming over the next few decades will increase extinction rates and accelerate ecosystem degradation, we urge researchers to utilise this powerful but underused method more widely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is nearly seven decades since publication of the extraordinary 30-year-long study on the ecology of the yarrow Achillea lanulosa by Jens Christen Clausen and colleagues, a paper often credited with introducing the transplant experiment as a technique for distinguishing genetic from environmental effects on species populations (Clausen et al. 1948). A decade later, Joseph Connell transplanted the barnacles Chthalamus stellatus and Balanus balanoides to different heights within a Scottish inter-tidal zone to tease out the role of physical factors vs. biological interactions in determining the species’ distributions (Connell 1961). These classic studies laid the groundwork for many hundreds, possibly thousands, of field manipulations to understand the most fundamental question in ecology—why do species live where they do?
Fast forward several decades to a time of rapid climate change when the need to address this basic question is ever more urgent. Several complementary approaches to understanding future climatic impacts on species and ecological communities are being harnessed. Species distribution models (SDMs), based on statistical relationships between present-day occurrences of species and environmental variables, are used to assess the location and size of suitable future habitats (Franklin 2010), but they have well-known limitations, including the fact that they typically do not incorporate the effects of species interactions into their predictions (Van der Putten et al. 2010). Nevertheless, there have been considerable advancements in modelling techniques, as these can now incorporate mechanistically informed, niche-based and demographic models (Wiens et al. 2009) and eco-evolutionary dynamics (Cotto et al. 2017). Experiments in which temperature, CO2, and other physical factors are manipulated in glasshouses, or in rarer cases, in the field, provide useful information about potential physical limits but tend to be short-term and in small scale (e.g., Pelini et al. 2011). Inferences about future changes in community composition and structure have also been made via observations of species turnover along latitudinal and elevational gradients, essentially using space for time substitutions to project the future (e.g., De Frenne et al. 2013).
Transplant experiments, in which individuals of a species or groups thereof are moved to new climatic habitats and their response observed, provide a very powerful direct tool to glimpse the future, complementing the approaches described above. These experiments have been used to assess likely future responses of single species or groups of species, changes in multi-trophic interactions, and shifts in community composition and structure. Here, we present an overview of the questions addressed by such techniques and synthesise how the knowledge gained complements other, more commonly used, approaches.
Box—transplant experiments |
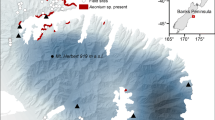
Field transplant experiments involve establishment of individuals of species, or groups thereof, in new locations where they are subject to a range of different climatic and other environmental factors compared to those at the source location (Fig. 1). In most experiments, individuals or communities have been transplanted between different locations within the known geographic range of the species or community (Fig. 1a, #1, #3). In others, transplantation has occurred at the range limit, or in a few cases, outside the current range (Fig. 1a, #2). Investigations of the relative importance of genetic vs. environmental drivers may also involve transplantation of individuals from multiple locations within the current range (Fig. 1a, #3). The majority of studies published to date have involved reciprocal transplants (Fig. 1a, #1) of plants, insects, or soil cores between colder and warmer areas within the current range of the species of interest. Most have been carried out in the Northern Hemisphere and have used transplant sites at different latitudes (Fig. 1b) or elevations (Fig. 1c) to achieve temperature changes of ~2–3 °C, thus simulating aspects of the projected climate of the near to mid-term future (~20–40 years). The most common measures of success for single species have been survival, growth, and reproduction. Relatively fewer experiments have moved individuals of species completely outside their known range or have measured changes in species interactions, community structure, or ecosystem processes. |
2 Methods
The transplant studies reviewed here (see Table 1) were obtained by conducting a literature search in December 2015 using the terms “climate change” and “transplant experiment”/“translocation” in “Web of Science” and “Scopus”. The search also yielded transplant studies that have been used to address a wide variety of ecological questions, such as to assess responses of species and communities to pollution (e.g., nitrogen, heavy metal accumulation), to study the effects of land-use changes and habitat fragmentation (e.g., urban-rural gradients), to conduct provenance trials (e.g., in forestry), or to investigate responses to increasing atmospheric CO2. In this review, we focus on a subset of 47 studies that have specifically used these techniques to address climate change-related impacts. It is possible that some relevant studies were not found using our search terms due to the use of different keywords. However, here, we do not aim for a systematic literature review but rather provide an overview of the current state of the field, synthesising what has been achieved so far, identifying knowledge gaps and limitations, and pointing to constructive ways forward.
3 Questions addressed by transplant experiments
Transplant experiments have been harnessed to address questions at all levels of ecological organisation—genetic, species, community, and ecosystem, in a wide range of terrestrial and aquatic habitats. These questions can be broadly grouped into seven categories:
3.1 Can recently observed changes in species distributions be attributed to climatic change over the same period?
The number of studies published over the last two decades that attribute, or partially attribute, observed changes in species ranges, population sizes, phenology, behaviour, or physiology to climate change at the particular location over the same period now number many hundreds (Scheffers et al. 2016). Most commonly, such attribution has been based on a correlation between an observed species’ response (such as a range expansion at a cold boundary) and warming temperatures in the same location. In some such cases, transplant experiments have been used to go beyond correlation to not only confirm a hypothesis about attribution but also understand the underlying mechanism for the change.
3.2 What are the limits to future adaptive responses?
Shifts to new, more climatically suitable habitat is arguably the best-studied adaptive response to climate change, and transplant experiments have been used to understand the limits of such adaptation in plants (Etterson and Shaw 2001; Ibáñez et al. 2009; Marsico and Hellmann 2009; McCarthy-Neumann and Ibáñez 2012; Van der Veken et al. 2012), butterflies (Merrill et al. 2008; Buckley and Bridle 2014), spiders (Krehenwinkel and Tautz 2013), birds (Burger et al. 2013), and marine invertebrates (Jones et al. 2012).
3.3 What is the relative importance of biotic vs. physical factors in explaining observed shifts and/or predicting future shifts?
Successful latitudinal or elevational range shifts of species under a changing climate are also dependent on the presence of suitable biotic factors. Transplant experiments have been used to address the interplay of biotic interactions, such as herbivory (Merrill et al. 2008; Marsico and Hellmann 2009; Nooten and Hughes 2014), competition (Marsico and Hellmann 2009; McCarthy-Neumann and Ibáñez 2012; Alexander et al. 2015; Tomiolo et al. 2015), and parasitism (Prior and Hellmann 2013) in such shifts.
3.4 What is the potential for temporal or spatial mismatches in species interactions in the future?
Idiosyncratic responses of individual species to climate change mean that interactions between many species will continue to change. Two types of mismatches are commonly described: temporal mismatches refer to the decoupling of life cycle events (phenology), while spatial mismatches may arise through differential changes in distributions of interacting partners. Transplant experiments have provided useful insights into the flow-on impacts of altered interactions among plants (Alexander et al. 2015; Tomiolo et al. 2015), between plants and pollinators (Forrest and Thomson 2011), and between plants and herbivores (Garibaldi et al. 2011).
3.5 What is the capacity for climate change to fundamentally alter community composition and structure?
Three main types of transplant experiments to test community-level questions have been employed. Firstly, groups of species, such as blocks of vegetation with soil, have been moved into new areas. Secondly, individuals of single species that provide habitat for others have been transplanted into new sites and subsequent colonisation in situ monitored over time. The third type is a combination of the first two, where turf blocks with soil are transplanted into predicted climates and individuals of species added to them to assess the effects of novel and/or current competitors in plant communities (Alexander et al. 2015). The focus of experiments at the community level has generally been plants (Bruelheide 2003; Ström et al. 2011; Alexander et al. 2015), herbivorous insects (Andrew and Hughes 2007; Heimonen et al. 2014; Nooten et al. 2014), soil invertebrates (Briones et al. 1997; Todd et al. 1999; Budge et al. 2011), and more rarely, insect communities comprising both herbivores and non-herbivores (Nooten et al. 2014).
3.6 How will climate change alter ecosystem functions such as nutrient cycling?
Fundamental ecosystem processes, such as nutrient cycling and decomposition, are likely to alter as the climate continues to warm, leading to concern about the fate of carbon (C) and nitrogen (N) stocks in soil. Elevational transplantations of soil cores either in a reciprocal fashion or downslope to warmer sites have been used to investigate the response of soil C and N in warmer and drier climates (Link et al. 2003; Rey et al. 2007; Garten 2008; Bimüller et al. 2014).
3.7 What is the role of genetic vs. environmental drivers of change?
Reciprocal transplants, in which individuals are “swapped” between sites, have frequently been used to disentangle the effects of genotype, i.e., “site of origin effects”, and environmental factors, i.e., “location effects”. Individuals can be transplanted in a reciprocal fashion between contrasting areas of their range (Fig. 1a #1) or from multiple sites of origin into a warmer climate (Fig. 1a #3) to assess how they respond to new environmental conditions. Genotype or phenotype can affect species interaction strengths (Barton 2011; Garibaldi et al. 2011), phenology (Frei et al. 2014), and physiology in terms of fitness and survival (De Frenne et al. 2012; Meineri et al. 2013).
Types of transplant experiments. (a) Individuals of species or groups thereof are either (1) transplanted reciprocally or (2) unidirectional from one source or (3) from several sources within their range or beyond their current range into a new climate, in a (b) latitudinal or (c) altitudinal fashion. Filled triangles show individuals within their range, hatched triangles show individuals moved outside of their range into a warmer climate to simulate future conditions (2; 3). Type 1 tests whether individuals of species are locally adapted and how they fare in warmer climates. Type 2 tests whether individuals of species can survive in future climatic conditions beyond their current range. Type 3 experiments can be used to investigate genotypic or environmental adaptation, by transplanting individuals of species sourced from multiple locations into a new area (3) (in or outside the range), either at a different latitude (b) or elevation (c)
4 Experimental designs
Experimental designs vary considerably among transplant studies reviewed here (Table 1) (for a more extended critique of the experimental designs used in general transplant experiments, see Hargreaves et al. 2014). Generally, individuals of a species or group thereof have been sourced from a single location by collecting seeds/seedlings (plants) or individuals (animals) and transplanted into new climates, mainly in sites at different latitudes (43% of the reviewed studies) or different elevations (40%). A few studies have transplanted along longitudinal gradients (4%), a combination of longitude and latitude (4%), or locally in close proximity (9%). Distances between source and transplant sites range from just over 100 km (Marsico and Hellmann 2009) to more than 2000 km (Ågren and Schemske 2012), corresponding to 1–20° latitude or from 50 (Bennington et al. 2012) to 1200 m (Frei et al. 2014) in elevation. Most studies have focused on exposing transplants to increases in average annual temperature (56% of studies) ranging from ~1 (Byars and Hoffmann 2009) to ~10 °C (Ågren and Schemske 2012). A minority of studies (10%) have focused on achieving significant precipitation differences ranging from 60 to 500 mm p.a. Even fewer studies (7%) have examined differences in soil moisture. About a quarter (26%) examined two climate variables in combination, generally temperature and either precipitation or soil moisture/humidity. Experimental duration has varied from ~2 months to more than 30 years, with the majority of the experiments lasting about a year. A few studies have repeated the experimental design over multiple years—for plants (Ibáñez et al. 2008; Ibáñez et al. 2009; Ågren and Schemske 2012; McCarthy-Neumann and Ibáñez 2012) and butterflies (Buckley and Bridle 2014). In these cases, the trends found were generally consistent across replicate years.
5 Emerging generalisations
The volume of published papers addressing the ecological consequences of climate change is vast and growing exponentially. A Web of Science search on the terms “climate change” and “species” or “ecosystems” over the period 1997–2006 produces a list of approximately 180,000 papers. In the following decade (2007–May 2016), over half a million papers were published. Even a cursory perusal of this literature reveals that the majority of such papers provide at best, correlative data on observed or potential impacts. Speculation about the future is rife and is rarely based on detailed understanding of the limits to, and potential of, species and community responses. While published transplant studies are a very modest proportion of this burgeoning literature, and, as noted above, have varied markedly in experimental design and duration, we argue that they are beginning to provide the necessary underpinnings to convert speculation to more confident prediction. In many cases, the results are unsurprising/intuitive but are no less valuable for that. Based on a synthesis of 47 transplant studies (Table 1), we offer the following broad generalisations:
5.1 Transplants can provide convincing evidence for attribution of recent range shifts to climate change
Expansions of species’ ranges to higher latitudes or elevations have been recorded for hundreds of species globally over the past few decades, consistent with being a response to the changing climate (Scheffers et al. 2016), and further range shifts are expected. In many cases, contractions at the warmer edges of ranges appear to be lagging behind expansions at the cooler edges. Assessment of the performance of individuals transplanted to warmer and/or cooler sections of a historical range has been used to test hypotheses about warm boundary contractions in several taxa including mussels, barnacles, and butterflies. On the US east coast, acorn barnacles (Semibalanus balanoides), for example, were moved to three transplant sites, spanning most of the species’ historical range; barnacle survival decreased dramatically at the lower latitude (warmer) site, corroborating the hypothesis that climatic unsuitability had driven the 350-km-range contraction observed over the last half century (Jones et al. 2012). Transplants were also used to investigate the 100-fold decline in abundance of the rough limpet (Collisella scabra) along its poleward 300-km-range edge (Gilman 2005). Transplantation of the limpet showed that increased mortality at the cold edge was associated with lower maturation rates, an effect particularly pronounced under limiting food conditions (Gilman 2006). In mountainous Spain, the survival of eggs of the black-veined white butterfly (Aporia crataegi) transplanted along an elevational gradient decreased at low elevation sites (Merrill et al. 2008), providing an explanation for observed contractions at the warmer range edge.
5.2 Importance of climatic impacts on vulnerable overwintering stages in determining range expansion and contractions
In addition to providing empirical evidence of climate attribution for range changes, some experiments have been able to identify the likely mechanism by which the change has occurred. The wasp spider Argiope bruennichi, for example, has recently undergone a range expansion at the cooler edge of its distribution in Europe (Krehenwinkel and Tautz 2013). A reciprocal transplant experiment in which egg-sacs of the spider were collected from populations at the warm- and cool-range edges revealed an increased overwintering survival of cool-edge versus warm-edge spiders. A similar release from overwintering vulnerability is the proposed mechanism by which the sachem, a small skipper butterfly (Atalopedes campestris), has expanded its range northwards, colonising areas of the Pacific Northwest USA over the past half century. Transplantation of caterpillars at the cool-range edge and beyond revealed that recent warming of winter temperatures (~1–2 °C over the last century) accounted for the expansion by increasing survivorship (Crozier 2004).
5.3 Suitable habitats exist for many species beyond their current ranges even under present-day climate
The notion that the fundamental niche of a species (the range it could occupy based purely on physical factors) is generally more extensive than the niche it actually realises (due to limiting biotic factors) has been a core tenet of ecology since elucidated by G. Evelyn Hutchinson in 1957 (Hutchinson 1959). But despite the long history of the concept, our understanding of exactly how physical and biological factors limit the ranges of most species remains scant. This poor understanding now significantly limits our ability to confidently predict the capacity of individual species to shift range, given either direct changes in climate or changes in climate-mediated biotic interactions. Experiments that found high survivorship of herbaceous species transplanted to suitable habitat polewards of their current range limit in Belgium (Van der Veken et al. 2012) and in western North America (Marsico and Hellmann 2009) provide examples of potential adaptive capacity.
5.4 Presence or absence of new enemies will significantly influence the capacity for some species to establish in new, climatically suitable habitats
Transplant experiments have also shown that exclusion of natural enemies such as parasites, predators, or herbivores (enemy release) or competitors can facilitate the establishment of plant species in novel areas and enhance growth (McCarthy-Neumann and Ibáñez 2012; Lakeman-Fraser and Ewers 2013). For example, the New Zealand native Kawakawa tree (Macropiper excelsum) showed increased survival and growth when transplanted polewards beyond its current range limit, attributed to lack of herbivory by the caterpillar of the Kawakawa looper (Cleora scriptaria) (Lakeman-Fraser and Ewers 2013). The jumping oak gall wasp (Neuroterus saltatorius), introduced on Vancouver Island in Canada, had higher survival rates in the introduced than in the native region, supporting the enemy release hypothesis (Prior and Hellmann 2013); however, a translocation experiment revealed increased background mortality of the wasp in its native range under exclusion of its parasitoid enemies, suggesting that other factors limit the species in its native range and contribute to its success in its introduced range. Interspecific competition within the same trophic level had either a negative effect by reducing plant survival for four herbaceous common forest understorey species when individuals were transplanted beyond their range limit in Europe (Van der Veken et al. 2012) or a positive effect by increasing germination success for poleward-transplanted individuals of Lomatium species in Canada (Marsico and Hellmann 2009).
5.5 Presence or absence of new hosts will determine the success of range shifts for herbivores
Release from climatically limiting temperatures at a cooler range edge has been found to result in host plant shifts for several herbivore species such as butterflies. The brown argus butterfly (Aricia agestis), for example, was observed to shift host plant preference from rockrose (Cistaceae) to Geraniaceae species throughout its expanding range (Buckley and Bridle 2014). Butterflies were reciprocally transplanted onto locally occurring host plant patches within the historical range on rockrose and in the expanding range on Geraniaceae. Butterflies originating from the expanding range showed reduced egg laying and survival when put onto rockrose plants, whereas butterflies originating from the historical range were able to lay eggs on both plants (Buckley and Bridle 2014). This experiment also indicated that the recent shift of host plant preference has led to a reduced potential for future adaptation because the expanding butterflies performed more poorly on the non-local host plant species.
5.6 Phenological plasticity reduces the likelihood of uncoupled species interactions
A frequently voiced concern is that decoupling of the life cycles of plants and pollinators may have significant negative consequences for provision of pollination services in both native and agricultural systems (Menzel et al. 2006). While much work needs to be done, an experiment in which the overwintering stages of individuals of eight bee species native to the Rocky Mountains were reciprocally transplanted to different elevations within their range revealed that emergence in spring was a phenotypically plastic response, with earlier emergence at warmer sites matching the onset of flowering of the local alpine plants (Forrest and Thomson 2011).
5.7 Species distribution models can overestimate capacity for adaptive range changes
Species distribution modelling is generally considered a useful tool to assess the potential availability of future, climatically suitable habitat for individual species. SDMs applied to 18 North American forest tree species consistently indicated the potential for range expansions under future climate scenarios (Ibáñez et al. 2008). Seedlings of species from the dominant genera Quercus, Acer, Liquidambar, and Pinus, representing both locally resident species and species predicted to migrate into the study area, were transplanted into multiple sites in the Southern Appalachian Mountains and in the Duke Forest Piedmont, North Carolina. In contrast to predictions from the SDMs, seedlings showed lower survival in plots with lower soil moisture at all sites, indicating that they may not survive or recruit under predicted future climate conditions (Ibáñez et al. 2008). Furthermore, the growth rate of seedlings, from both the resident species and those species that may potentially expand into the sites, was similarly low, indicating that under drier future climates, neither migrant nor resident species may prevail, potentially leading to a decline in local species richness (Ibáñez et al. 2008).
5.8 Beta diversity of many communities along environmental gradients is high, and community composition is capable of extremely rapid change in relation to climate
Transplants of host plants have provided evidence that many herbivorous invertebrates are readily able to colonise novel hosts and that invertebrate community structure and composition can be driven by both climate and host plant identity (Andrew and Hughes 2007; Heimonen et al. 2014; Nooten et al. 2014). Monitoring of insect colonisation on plant hosts transplanted to warmer sites along the east coast of Australia, for example, revealed a marked turnover (~90%) of species composition compared to the communities supported by the host plants within their native ranges. Insect colonisation of plants of three rare Banksia species, transplanted to wetter and cooler sites ~40 km from their native populations in southwest Australia, came to closely resemble those on closely related, locally occurring individuals of plant species after just 3 years (Moir et al. 2012). In Sweden, the community composition on turves of riparian vegetation communities transplanted to deeper levels in the riparian zone to simulate projected changes in water levels came to closely resemble that at the local (deeper) site after only 6 years (Ström et al. 2011).
5.9 Niche saturation of insect herbivore communities promotes functional stability
While considerable species-level turnover of invertebrates has been found on transplanted host plants, communities can also maintain surprising consistency in structure at a functional level (Andrew and Hughes 2007; Nooten et al. 2014). Experiments in which colonisation of invertebrate communities on transplanted hosts has been monitored over time indicate that while profound changes in the composition of herbivorous insect communities may be expected in the future (Andrew and Hughes 2007; Heimonen et al. 2014; Nooten et al. 2014), niche saturation in these communities means that as individual species drop out of a community, they are rapidly replaced by others of similar ecology, thus promoting functional stability at the community level.
5.10 Changes in soil communities may be more affected by moisture than by changes in temperature
Reciprocal transplants of soil cores between sites differing in temperature and/or precipitation have indicated that communities of soil invertebrates respond more strongly to changes in precipitation than to temperature (Briones et al. 1997; O’Lear and Blair 1999; Sohlenius and Boström 1999). Transplants resulting in changed soil moisture conditions have generally led to shifts in the vertical distribution of invertebrate taxa, such as nematodes and mites, changing both community composition (O’Lear and Blair 1999; Sohlenius and Boström 1999) and structure (Todd et al. 1999). These experiments overall show similar species-specific responses to those of aboveground taxa (Briones et al. 1997; O’Lear and Blair 1999). At the level of functional guilds, responses depend on the feeding type, with herbivore guilds showing the strongest and most consistent positive responses to increased soil moisture (Todd et al. 1999).
5.11 Climate change may have serious consequences for loss of soil carbon and nitrogen
Transplants of plant-soil-microbe mesocosms to warmer sites in beech forests in southern Germany showed a decrease in stable soil N (Bimüller et al. 2014). A loss of organic C content in forest soils was found when soil cores were transplanted from high to low elevation sites in the southern Appalachian Mountains in the US (Garten 2008). Transplanting soil cores from mountainous and intermediate sites into warmer coastal sites led to a decline in both soil C and N in a rye grass- and clover-dominated semi-natural grassland system (Rey et al. 2007); in addition to C and N decline, the greater the warming, the higher were the CO2 emissions of the transplanted soils, indicating positive feedbacks to the climate system. Intact soil-herbaceous-plant cores, transplanted between low and high elevation sites in a shrub-steppe ecosystem in southern Washington State, lead to a decline in N and C content in semi-arid soils under warmer and drier conditions, whereas no effect was found for production characteristics of the associated plants in terms of density and shoot biomass (Link et al. 2003). In a Californian grassland system, decomposition rates in plant litterbags transplanted to simulate a drier climate decreased, indicating a shift in the composition of the microbial community (Allison et al. 2013). These kinds of experiments suggest that under future climate conditions, soils in many ecosystems, ranging from forests to grasslands, might release key nutrients, leading to a decreased availability of N and C for standing vegetation and potentially driving changes of plant community composition and structure.
6 Situations where generalisations are proving elusive
6.1 Role of local adaptation
The capacity for species to adapt to the rapidly changing climate, whether in situ or by shifting to habitat elsewhere, is likely to be constrained if the genetic correlation between multiple traits is antagonistic to the direction of selection (Etterson and Shaw 2001), or if local populations exhibit a high degree of adaptation to current, local conditions (O’Neil et al. 2014; Boshier et al. 2015). Transplant experiments in which the performance of plants from multiple local populations has been compared among control and sites with different climates have produced a wide variety of outcomes. In some cases, the local genotype performed best, indicating strong local adaptation (Ågren and Schemske 2012; Bennington et al. 2012; De Frenne et al. 2012), whereas in others, significant phenotypic plasticity resulted in positive performance under changed environmental conditions at the transplant sites (Byars and Hoffmann 2009; Pluess et al. 2011; Meineri et al. 2013; Frei et al. 2014). Differences in home site advantage for different species within a single experiment have been found, e.g., translocation experiments with two species of butterflies, the Propertius duskywing (Erynnis propertius) and the Anise swallowtail (Papilio zelicaon) in British Columbia (Canada), indicated local adaptation for fitness-related parameters for one species but not for the other (Pelini et al. 2009). The importance of examining the genetic level when evaluating local adaptation revealed a follow-up study that compared whole transcriptome expression of the same butterfly species, identifying population-specific patterns in both species (O’Neil et al. 2014). Species-specific and population-specific differences may continue to inhibit generalisations about questions of local adaptation.
6.2 Interaction strength of species interactions
Transplant experiments have also been used to assess how species interactions, such as herbivory or predation, might be affected by future climates. No generalisations have emerged as yet in regards to herbivory, with both positive and negative changes reported. Reciprocally transplanted seedlings of the southern beech, Nothofagus pumillo, to different elevations in the Andes experienced increased herbivore pressure at warmer sites (Garibaldi et al. 2011). In contrast, little change in herbivore type and pressure was experienced by shrubby understorey plants transplanted to warmer sites in southeast Australia (Andrew and Hughes 2007; Nooten and Hughes 2014).
7 Limitations and caveats
Transplant experiments, like any other approach to predicting the future, have limitations. For example, when individuals of species are transplanted towards the equator or downslope in elevation into a new climate, there will always be a level of uncertainty as to the match of future conditions. Transplanted individuals may also be affected by a range of uncontrolled variables, including differences in soil properties, photoperiod, biotic interactions, and community dynamics, that confound interpretation of results. Transplants of individuals outside the species’ current range may also face new enemies and competitors, which could affect their survival. Many of these factors can be minimised by careful selection of appropriate field sites, e.g., in a similar habitat, soil, and vegetation structure, but caveats on interpretation will always be necessary.
8 The power of the transplant: conclusions and next steps
Published transplant studies still comprise a small portion of the published literature on climate change impacts on species and ecological communities (see above). Among the likely reasons for this include the fact that these experiments tend to be more costly, especially in terms of labour, than other methods of understanding the ecological future. Challenges in finding suitable transplant sites and then in gaining landholder permission to perform the work can also present barriers. Notwithstanding these practical difficulties, these experiments provide a range of advantages over the more traditional glasshouse or growth chamber experiments for addressing the challenges of understanding future impacts on multiple, interacting species in communities, because multiple factors—abiotic and biotic—can be assessed simultaneously, and over longer time frames.
When such experiments have been employed, they have provided empirical corroboration of what would otherwise be speculation and new insights into the relative importance of biotic vs. abiotic factors in determining species ranges; the role of genotype vs. phenotypic plasticity in shaping population and species responses; potential changes in both composition and structure of communities; and how fundamental ecosystem processes may alter.
Transplant experiments in the future could increasingly incorporate new genetic tools, such as those employed by O’Neil et al. (2014), using expression assays to identify putative genes of local adaptation to climate. Further, transplant experiments could be combined with in situ experiments using localised temperature manipulation or open/closed top chambers. They could also prove useful to test cutting-edge model predictions such as those incorporating niche-based and eco-evolutionary dynamics (Cotto et al. 2017).
Of the papers reviewed here, the majority (>70%) describe experiments that moved individuals within the species’ existing geographic range. Given that the world is very likely to warm least ~2–4 °C in the next century (IPCC 2014), we need to push these limits further, undertaking bolder, more co-ordinated, multi-taxa and multi-biome experiments, taking species into climates likely to prevail in the second half of the 21st century. The prospect that a 4 °C warming could put a sixth of the earth’s species at increased risk of extinction (Urban 2015) adds urgency to this task.
References
Ågren J, Schemske DW (2012) Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytol 194:1112–1122
Alexander JM, Diez JM, Levine JM (2015) Novel competitors shape species’ responses to climate change. Nature 525:515–518
Allison SD, Lu Y, Weihe C, Goulden ML, Martiny AC, Treseder KK, Martiny JBH (2013) Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 94:714–725
Andrew NR, Hughes L (2007) Potential host colonization by insect herbivores in a warmer climate: a transplant experiment. Glob Chang Biol 13:1539–1549
Barton BT (2011) Local adaptation to temperature conserves top-down control in a grassland food web. Proceedings of the Royal Society B-Biological Sciences 278:3102–3107
Bennington CC, Fetcher N, Vavrek MC, Shaver GR, Cummings KJ, McGraw JB (2012) Home site advantage in two long-lived arctic plant species: results from two 30-year reciprocal transplant studies. J Ecol 100:841–851
Bimüller C, Dannenmann M, Tejedor J, von Lützow M, Buegger F, Meier R, Haug S, Schroll R, Kögel-Knabner I (2014) Prolonged summer droughts retard soil N processing and stabilization in organo-mineral fractions. Soil Biol Biochem 68:241–251
Boshier D, Broadhurst L, Cornelius J, Gallo L, Koskela J, Loo J, Petrokofsky G, St Clair B (2015) Is local best? Examining the evidence for local adaptation in trees and its scale. Environmental Evidence 4:20
Briones MJI, Ineson P, Piearce TG (1997) Effects of climate change on soil fauna; responses of enchytraeids, diptera larvae and tardigrades in a transplant experiment. Appl Soil Ecol 6:117–134
Bruelheide H (2003) Translocation of a montane meadow to simulate the potential impact of climate change. Appl Veg Sci 6:23–34
Buckley J, Bridle JR (2014) Loss of adaptive variation during evolutionary responses to climate change. Ecol Lett 17:1316–1325
Budge K, Leifeld J, Egli M, Fuhrer J (2011) Soil microbial communities in (sub)alpine grasslands indicate a moderate shift towards new environmental conditions 11 years after soil translocation. Soil Biol Biochem 43:1148–1154
Burger C, Nord A, Nilsson JA, Gilot-Fromont E, Both C (2013) Fitness consequences of northward dispersal as possible adaptation to climate change, using experimental translocation of a migratory passerine. PLoS One 8:e83176
Byars SG, Hoffmann AA (2009) Lack of strong local adaptation in the alpine forb Craspedia lamicola in Southeastern Australia. Int J Plant Sci 170:906–917
Byars SG, Papst W, Hoffmann AA (2007) Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 61:2925–2941
Clausen J, Keck DD, Heisey WM (1948) Experimental studies on the nature of species. III. Environmental responses of climatic races of Achillea. Carnegie Institution, Washington D.C
Connell JH (1961) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42:710–723
Cotto O et al (2017) A dynamic eco-evolutionary model predicts slow response of alpine plants to climate warming. Nat Commun 8:15399
Crozier LG (2004) Warmer winters drive butterfly range expansion by increasing survivorship. Ecology 85:231–241
De Frenne P, Graae BJ, Brunet J, Shevtsova A, Schrijver AD, Chabrerie O, Cousins SAO, Decocq G, Diekmann M, Hermy M, Heinken T, Kolb A, Nilsson C, Stanton S, Verheyen K (2012) The response of forest plant regeneration to temperature variation along a latitudinal gradient. Ann Bot 109:1037–1046
De Frenne P, Graae BJ, Rodríguez-Sánchez F, Kolb A, Chabrerie O, Decocq G, De Kort H, De Schrijver A, Diekmann M, Eriksson O, Gruwez R, Hermy M, Lenoir J, Plue J, Coomes DA, Verheyen K (2013) Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J Ecol 101:784–795
Etterson JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 294:151–154
Forrest JRK, Thomson JD (2011) An examination of synchrony between insect emergence and flowering in the Rocky Mountains. Ecol Monogr 81:469–491
Franklin J (2010) Mapping species distribution: spatial inference and prediction. Cambridge University Press, Cambridge
Frei ER, Ghazoul J, Matter P, Heggli M, Pluess AR (2014) Plant population differentiation and climate change: responses of grassland species along an elevational gradient. Glob Chang Biol 20:441–455
Garibaldi LA, Kitzberger T, Chaneton EJ (2011) Environmental and genetic control of insect abundance and herbivory along a forest elevational gradient. Oecologia 167:117–129
Garten CT Jr (2008) Changes in carbon following forest soil transplants along an altitudinal gradient. Commun Soil Sci Plant Anal 39:2883–2893
Gilman SE (2005) A test of Brown’s principle in the intertidal limpet Collisella scabra (Gould, 1846). J Biogeogr 32:1583–1589
Gilman SE (2006) Life at the edge: an experimental study of a poleward range boundary. Oecologia 148:270–279
Hargreaves AL, Samis KE, Eckert CG (2014) Are species’ range limits simply niche limits writ large? A review of transplant experiments beyond the range. Am Nat 183:157–173
Heimonen K, Valtonen A, Kontunen-Soppela S, Keski-Saari S, Rousi M, Oksanen E, Roininen H (2014) Colonization of a host tree by herbivorous insects under a changing climate. Oikos 124:1013–1022
Hutchinson GE (1959) Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat 93:145–159
Ibáñez I, Clark JS, Dietze MC (2008) Evaluating the sources of potential migrant species: implications under climate change. Ecol Appl 18:1664–1678
Ibáñez I, Clark JS, Dietze MC (2009) Estimating colonization potential of migrant tree species. Glob Chang Biol 15:1173–1188
IPCC (2014) Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 1–32
Jones SJ, Southward AJ, Wethey DS (2012) Climate change and historical biogeography of the barnacle Semibalanus balanoides. Glob Ecol Biogeogr 21:716–724
Krehenwinkel H, Tautz D (2013) Northern range expansion of European populations of the wasp spider Argiope bruennichi is associated with global warming-correlated genetic admixture and population-specific temperature adaptations. Mol Ecol 22:2232–2248
Lakeman-Fraser P, Ewers RM (2013) Enemy release promotes range expansion in a host plant. Oecologia 172:1203–1212
Link SO, Smith JL, Halvorson JJ, Bolton H Jr (2003) A reciprocal transplant experiment within a climatic gradient in a semiarid shrub-steppe ecosystem: effects on bunchgrass growth and reproduction, soil carbon, and soil nitrogen. Glob Chang Biol 9:1097–1105
Marsico TD, Hellmann JJ (2009) Dispersal limitation inferred from an experimental translocation of Lomatium (Apiaceae) species outside their geographic ranges. Oikos 118:1783–1792
McCarthy-Neumann S, Ibáñez I (2012) Tree range expansion may be enhanced by escape from negative plant-soil feedbacks. Ecology 93:2637–2649
Meineri E, Spindelböck J, Vandvik V (2013) Seedling emergence responds to both seed source and recruitment site climates: a climate change experiment combining transplant and gradient approaches. Plant Ecol 214:607–619
Menzel A et al (2006) European phenological response to climate change matches the warming pattern. Glob Chang Biol 12:1969–1976
Merrill RM, Gutiérrez D, Lewis OT, Gutiérrez J, Díez SB, Wilson RJ (2008) Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J Anim Ecol 77:145–155
Moir ML, Vesk PA, Brennan KEC, Hughes L, Keith DA, McCarthy MA, Coates DJ, Barrett S (2012) Considering extinction of dependent species during translocation, ex situ conservation, and assisted migration of threatened hosts. Conserv Biol 26:199–207
Nooten SS, Hughes L (2014) Potential impacts of climate change on patterns of insect herbivory on understorey plant species: a transplant experiment. Austral Ecology 39:668–676
Nooten SS, Andrew NR, Hughes L (2014) Potential impacts of climate change on insect communities: a transplant experiment. PLoS One 9:e85987
O’Lear HA, Blair JM (1999) Responses of soil microarthropods to changes in soil water availability in tallgrass prairie. Biol Fertil Soils 29:207–217
O’Neil ST et al (2014) Gene expression in closely related species mirrors local adaptation: consequences for responses to a warming world. Mol Ecol 23:2686–2698
Pelini SL, Dzurisin JDK, Prior KM, Williams CM, Marsico TD, Sinclair BJ, Hellmann JJ (2009) Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc Natl Acad Sci U S A 106:11160–11165
Pelini SL, Bowles FP, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR (2011) Heating up the forest: open-top chamber warming manipulation of arthropod communities at Harvard and Duke forests. Methods Ecol Evol 2:534–540
Pluess AR, Frei E, Kettle CJ, Hahn T, Ghazoul J (2011) Plant growth and fitness of Scabiosa columbaria under climate warming conditions. Plant Ecol Diversity 4:379–389
Prior KM, Hellmann JJ (2013) Does enemy loss cause release? A biogeographical comparison of parasitoid effects on an introduced insect. Ecology 94:1015–1024
Rey M, Guntinas E, Gil-Sotres F, Leiros MC, Trasar-Cepeda C (2007) Translocation of soils to stimulate climate change: CO2 emissions and modifications to soil organic matter. Eur J Soil Sci 58:1233–1243
Scheffers BR, De Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, Butchart SHM, Pearce-Kelly P, Kovacs KM, Dudgeon D, Pacifici M, Rondinini C, Foden WB, Martin TG, Mora C, Bickford D, Watson JEM (2016) The broad footprint of climate change from genes to biomes to people. Science 354:6313
Sohlenius B, Boström S (1999) Effects of global warming on nematode diversity in a Swedish tundra soil—a soil transplantation experiment. Nematology 1:695–709
Ström L, Jansson R, Nilsson C, Johansson ME, Xiong SJ (2011) Hydrologic effects on riparian vegetation in a boreal river: an experiment testing climate change predictions. Glob Chang Biol 17:254–267
Todd TC, Blair JM, Milliken GA (1999) Effects of altered soil-water availability on a tallgrass prairie nematode community. Appl Soil Ecol 13:45–55
Tomiolo S, Van Der Putten WH, Tielborger K, Allison SD (2015) Separating the role of biotic interactions and climate in determining adaptive response of plants to climate change. Ecology 96:1298–1308
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–573
Van der Putten WH, Macel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc Lond Ser B Biol Sci 365:2025–2034
Van der Veken S, De Frenne P, Baeten L, Van Beek E, Verheyen K, Hermy M (2012) Experimental assessment of the survival and performance of forest herbs transplanted beyond their range limit. Basic and Applied Ecology 13:10–19
Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA (2009) Niches, models, and climate change: assessing the assumptions and uncertainties. Proc Natl Acad Sci U S A 106:19729–19736
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nooten, S.S., Hughes, L. The power of the transplant: direct assessment of climate change impacts. Climatic Change 144, 237–255 (2017). https://doi.org/10.1007/s10584-017-2037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-017-2037-6