Abstract
Climate is considered to be the predominant driver shaping species distributions at macroecological scales, yet the importance of incorporating biotic interactions in predicting future range margins under climate change scenarios is increasingly being recognised. We used translocation studies to investigate how survival and growth patterns of an understory shrub planted at latitudes within its range, at its range limit and beyond its polewards boundary (in areas it may colonise as a result of shifting climate envelopes) are affected by the presence of a primary herbivore. Specifically, we tested the null hypotheses that: (1) biotic interactions do not exert a significant role in limiting survival and growth rates across the limits of a host plant’s latitudinal range, and (2) at smaller spatial scales biotic interactions do not exert a significant role in determining survival and growth rates at edge versus interior position within a forest fragment. We found that the understory shrub Macropiper excelsum is able to survive polewards of its current latitudinal limit within the first year after transplant; in fact, growth is higher outside the plant’s current natural range than within its present-day distribution. This trend is particularly pronounced in forest core environments and corresponds closely to patterns of reduced herbivory outside the plant’s range. The absence of the primary herbivore, Cleora scriptaria, and concomitant reduction in the suppressive effects of herbivory outside of the plant’s range appear to be supporting enhanced growth and survival. If host plants are able to successfully track their climatic niche and disperse into novel areas prior to the arrival of their natural predators, it is possible that ‘enemy release’ may facilitate the establishment of plant species. These findings highlight the importance of considering biotic interactions alongside abiotic variables when predicting future species’ ranges under climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate exerts a fundamental influence on the global distribution of plant populations (Holdridge 1947; Box 1981), while a number of additional factors—including other abiotic conditions, dispersal capabilities and biotic interactions—can also influence the successful persistence and prevalence of plants in their existing range. Gaining an insight into the interaction between climate and biology is fundamental if we are to improve accuracy in predicting changes to species’ ranges with future climate changes (Brooker et al. 2007; van der Putten et al. 2010).
Global average temperatures have warmed by 0.7 °C between 1906 and 2005 and are expected (under the A1B scenarios) to increase by 1.7–4.4 °C by 2090–2099 relative to 1980–1999 temperatures (IPCC 2007). Predicting how species respond to global changes is a major goal for biologists and there is a wide spectrum of literature documenting the impacts of climate change on phenology (e.g. Yang and Rudolf 2010), community composition (e.g. Wahl et al. 2011), phenotypic plasticity (e.g. Matesanz et al. 2010) and species interactions (e.g. Tylianakis et al. 2008). Perhaps the response yielding the greatest attention is the shifting of species’ range margins as populations respond to moving climate envelopes with advancing latitudinal and altitudinal isotherms (Thomas and Lennon 1999; Davis and Shaw 2001; Beaugrand et al. 2002; Walther 2004; Thomas 2010). This pattern is found across a range of taxa and geographical localities, with 84 % of British vertebrate and invertebrate taxa that were tested having expanded polewards in response to a warming climate (Hickling et al. 2006). These findings are roughly equivalent to an earlier study conducted over a larger study area, which found that 81 % of populations of British birds, Swedish butterflies and Swiss herbs at poleward or high altitude range margins were expanding 6.1 km northwards or 6.1 m upwards per decade (Parmesan and Yohe 2003). This research adds to a plethora of empirical studies documenting a spatial expansion in populations at polewards range margins where abiotic conditions become increasingly favourable under climate change (Parmesan 1996; Parmesan et al. 1999, 2005; Chen et al. 2011). Species distribution models (SDMs) are being developed to track and predict these future ranges under various climate scenarios (Guisan and Thuiller 2005; Elith and Leathwick 2009). Although SDMs are seen as valuable tools for analysing the potential dynamics of species’ ranges under specific climate scenarios, many models assume that species movements are in equilibrium with shifting climate envelopes and disregard the constraints of historical and biotic factors such as barriers/facilitators to dispersal and species interactions (Morin and Lechowicz 2008; Jiménez-Valverde et al. 2008). A high degree of land use conversion in recent history has left many habitats patchy and isolated causing potential barriers to dispersal of populations at the range boundaries of distribution (Gaston 2003). In addition to this, biotic influences alter a species’ fundamental niche through competition (e.g. Connell 2011), mutualisms (e.g. van der Heijden et al. 2003), enemy pressure or bottom-up limitations (e.g. Lapointe et al. 2011). As shown by the fossil record (e.g. Davis and Shaw 2001) and empirical studies (e.g. Davis et al. 1998), species respond individually to climate change and, as a result of varying dispersal rates, interactions between those species may be eroded and new ones formulated (Urban et al. 2012). Biotic interactions shape the spatial arrangement of species within their habitats and may also limit the expansion of species distributions under climate change (Brooker et al. 2007; van der Putten et al. 2010). Competition limits the range distribution in Ulex species for example (Bullock et al. 2000), and predation on an invasive crab limits the expansion of its range in eastern North America (DeRivera et al. 2005). Stimulated by the importance of biotic interactions in empirical findings, these interactions are now being more commonly considered in SDMs. Araújo and Luoto (2007) found that incorporating Corydalis host plant occurrence alongside the distribution of climate variables improved the performance of models predicting the distribution of the clouded Apollo butterfly (Parnassius mnemosyne) at macro scales in Europe. Integrating land use patterns and biotic interactions with SDMs might therefore improve predictions of species’ ranges under climate change (Hampe 2004; Pearson and Dawson 2005; Araujo and Guisan 2006; Moore et al. 2007; Heikkinen et al. 2007; Ibáñez et al. 2009a, b; Meier et al. 2010; van der Putten et al. 2010).
Gaining an understanding into the complex responses of species to climate change will not only require large-scale modelling approaches but crucially fine-scale investigations into how species interactions are modified by temperature, precipitation and frost gradients (Poloczanska et al. 2008; Marsico and Hellmann 2009). The response of plants to climate changes can be studied through the artificial manipulation of microclimates (Walker et al. 2006), investigation of local plants along environmental gradients (DeFrenne et al. 2009) or translocations from common garden experiments (Clausen et al. 1940; Kollmann et al. 2004). Although each have benefits, translocations allow plant ecotypes to be transferred into regions with different annual temperatures under natural conditions (Niedrist et al. 2011), whilst distinguishing between phenotypic plasticity and local adaptation across a species’ range (Macel et al. 2007; Magnani 2009). Assuming there is no dispersal limitation, a plant species will occur in a location which confers conditions which are suitable for its survival, growth and reproduction, with performance declining along a gradient of climatic severity as its reaches its range boundary (Prince and Carter 1985). If moved outside of its current range one may expect performance to rapidly decline, unless climate is not the sole or even the major determinant of range limitation. Translocation experiments therefore have the potential to identify key limiting factors on plant survival and growth, and this approach has been successfully utilised to study species at multiple trophic levels beyond their natural distributions (Marsico and Hellmann 2009; Willis et al. 2009).
Here, we use translocation experiments to test the capacity of an understory plant to persist polewards of its current geographic range and determine whether biotic interactions with its primary herbivore have a role in limiting distributions. We test the null hypotheses that: (1) biotic interactions do not exert a significant role in limiting survival and growth rates across the limits of a host plants range, and (2) biotic interactions do not exert a significant role in determining survival and growth rates at edge versus interior positions within forest fragments.
Materials and methods
The study species and system
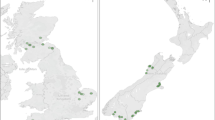
The New Zealand native Kawakawa tree (Macropiper excelsum, Piperales: Piperaceae) is a common understory plant throughout the mixed native scrub environments of New Zealand (Smith 1975). The species is confined to the coastal areas of the New Zealand mainland and a number of its offshore islands, reaching its southern limit at a latitude of ~43°5′S (Fig. 1). The battery of defences stored within M. excelsum leaves deters widespread generalist feeding activity; however, despite the tree’s range of defensive bio-compounds it still suffers herbivory. Although seven insects are known to feed on M. excelsum (Spiller and Wise 1982) it has one primary herbivore, Cleora scriptaria (Lepidoptera: Geometridae), which is effective at sequestering these compounds and feeds extensively on the plant (Hodge 1998).
Map showing survey locations of forest fragments (open squares) in relation to the present-day distribution of Macropiper excelsum on the South Island of New Zealand. Grey shading shows the extent of the species’ current distribution (Gardner 1997). Black dots represent locality records from museum specimens or direct field observations recorded by the authors
Experimental design
We conducted this experiment in lowland mixed indigenous forests on the South Island of New Zealand. We selected five experimental regions along a gradient of 5° of latitude (between 41º and 46°S) that extended across the southern range margin of the study species (Fig. 1). We focus on the poleward margin of this species’ range as—considering global poleward latitudinal shifts—this is the margin which is most likely to experience population expansion under climate change and the equatorial margin of this species on mainland New Zealand (disregarding presence on offshore islands) is inhibited by coastal geography (as opposed to an apparent climate gradient). Average annual temperatures decrease by 1 °C every 1.59º of latitude towards the poles on the South Island (Fig. 2). This suggests that for every degree rise in temperature under climate change, species will have to migrate 200 km to keep within their optimum physiological conditions. Our study locations were therefore spaced accordingly: at the southern (polewards) range limit (Banks Peninsula), up to 2° south of this latitude and beyond the current distribution of the species (polewards treatments at Timaru and Dunedin), and up to two degrees north of this latitude and within the current species’ range (the control treatments at Kaikoura and Nelson).
Variation in a air temperature and b number of frost days per month across five experimental locations between March 1992 and March 2010. Boxes are the interquartile range, whiskers are 1.5 times the interquartile range; hollow points the outliers over the previous 18 years; and filled points the average for the experimental period (March 2010–March 2011). Values represent the z-statistic and P-value from Z-tests comparing the experimental period average value to historical averages. Data were collected at the nearest airport or automatic weather station to the experimental sites (all within 50 km proximity), and were obtained from the NIWA CliFlo database
The experimental locations at the extremes of the latitudinal gradient were separated from the present-day range margin by about 300 km equating to roughly 1.5 °C temperature difference (Fig. 2). This is equivalent to a mid-range climate projection scenario of the likely increase in the average annual temperature in New Zealand by 2040 [1 °C; range of 0.2–2 °C derived from a full range of projections across emission scenarios (Ministry for the Environment 2008)]. Therefore, assuming no dispersal limitation, the location of our polewards experimental site lies within the potential climatically suitable region for M. excelsum colonisation over the next three decades.
Data collection
A translocation experiment was conducted using seeds collected in March 2009 from the ripe fruit of a population at the southern range limit of M. excelsum (Stony Bay, Banks Peninsula 43°51′10″S, 173°2′26″E). Although marginal populations do not always reflect the attributes of core populations (Kawecki 2008), we selected this location for seed collection on the expectation that if this species’ range moves polewards, in line with the majority of other species’ range movements observed around the world, it is this southern population that would be expected to expand. Seeds were germinated in mixed-purpose compost covered with a layer of gravel and successively re-potted and placed under growth lamps at the University of Canterbury. Seedlings were grown to a height of ~50 cm in a glasshouse environment before being moved outside in February 2010, exposing the plants to ambient conditions before experimental translocation. Since all plants were less than 2 years old, no reproductive catkins would be produced to spread genetic material in the timeframe of this experiment. It was therefore possible to gain permission to transplant these M. excelsum plants on private land, local council reserves and trust-owned land for the purposes of this experiment.
At each of the five locations along the latitudinal gradient, we selected three lowland indigenous forest fragments for experimental translocations. We transplanted ten shrubs into each of the forest fragments (n = 150 shrubs) in February–March 2010. Five of the shrubs were planted at the edge of each forest fragment (hereafter referred to as the ‘edge’ position), and the other five were planted 50 m inside the forest (hereafter referred to as the ‘interior’ position). Shrubs were tethered to a single bamboo cane for support, watered and left in place over the austral winter of 2010.
Experimental sites were revisited in March/April 2011 to record plant survival over the one-year period. Plants were recorded as alive or dead, with individuals having a brown cambium assumed to have died. We assessed the presence or absence of non-insect damage to the plants (e.g. caused from frost, wind or pathogen damage), with presence indicated by evidence of browning or yellowing of leaves. In addition to these two health variables we recorded plant growth over the year by recording the number of leaves on each shrub on planting and on removal. To measure herbivory levels, we recorded the number of leaves showing evidence of insect attack and those unaffected by insects. Presence of the primary herbivore, C. scriptaria was determined by beating each tree for 10 s (approximately one beat per second) and counting the number of larvae collected on a 1-m2 beating tray extended beneath the tree. Upon completion of data collection, all shrubs were cut down, their root masses dug up, and all vegetative material was removed from the forest fragment to avoid any potential for establishing M. excelsum populations outside of its current distributional range.
We used Z-tests to compare the climatic conditions during the experiment with historical averages. The average temperature during the experiment (March 2010–March 2011) at each location was significantly higher than the annual temperature in the 18 years prior to the experiment (Fig. 2a). In addition, the average number of frost days per month over the experimental period was significantly lower across many of the experimental locations (Fig. 2b). This is with the exception of the polewards site, Dunedin, which had 6.5 frost days per month during the experimental period compared to an average of 5.5 frost days per year between March 1992 and March 2010.
Analysis
Generalised linear models were implemented in the R 2.11.1 statistical environment (R Development Core Team 2010). Interactions between the main effects: latitude (continuous variable) and position (discrete edge/interior variable) were analysed as it was hypothesised that there would be a difference between the response variables across the natural range of M. excelsum, and that there would also be a difference between the edge and interior of forest fragments.
Survival levels were calculated as the number of dead versus alive plants at each edge position and non-insect damage was recorded as the number of plants with evidence of damage versus un-damaged at each site (n = 30, edge and interior of each of the 15 fragments). Similarly, herbivory was recorded as the number of attacked leaves versus non-attacked on each plant (n = 145 plants) and binomial models were used on these three analyses. Caterpillar presence was measured as the number of caterpillars per plant and analysed using Poisson errors. Growth was measured as the change in the numbers of leaves for each plant (n = 145 plants) over the 2010 season and analysed using Gaussian errors. The height of plants was added as an offset to this model to account for the variation between individuals. The direct impact of herbivory (percentage of leaves attacked) on growth levels (change in the numbers of leaves for each plant) was analysed using a general linear model with Gaussian errors, again adding plant height as an offset.
Results
Of the 150 transplanted M. excelsum, five were lost due to human damage and a further 19 died over the 12-month period of the experiment. Of the surviving plants, 79 appeared to be in full health and 47 showed evidence of non-insect damage.
Survival potential and growth
Survival rate showed little variation across the range of M. excelsum (Fig. 3a; Table 1). There was little variation in non-insect plant damage and this was reflected by the lack of significant main effects of latitude and the interaction between latitude and position for this variable (Table 1). Significant interactions were detected between latitude and edge position for plant growth (Table 1), with the surprising pattern that plants transplanted outside of the species’ natural range tended to increase the number of leaves, whereas those inside the range tended to lose leaves. This pattern was stronger for plants in the forest interior than those at forest edges (Fig. 3b).
Responses of M. excelsum plants transplanted across a latitudinal gradient following a 12-month transplant period. a Survival (percent of plants still alive), b growth (change in leaves number on surviving plants), c herbivory (percentage of leaves attacked on each individual). Values are means [±95 % confidence interval (CI)] for plants located at the edge (triangles) and interior (squares). Linear models are shown for each location, solid lines across latitudinal range at fragment edges and short dashed line across latitudinal range at fragment interior
Impact on species interactions
There was a highly significant interaction between latitude and edge position on herbivory levels (Table 1). Herbivory rates were highest within the species’ range and declined outside of it, but that trend was much stronger at the interior than edges of fragments where significantly more leaves were attacked. Outside of the species’ range, there was no difference in herbivory rates among edge and interior positions (Fig. 3c). There was also, predictably, a significant effect of latitude on the presence of the primary herbivore (Table 1) with C. scriptaria only being recorded inside the plants’ natural range at Kaikoura and Nelson. Enhanced growth rates appeared to be manifested through a direct impact of herbivory: the higher the percentage of leaves attacked, the lower the amount of leaf growth over the season (t 1,129 = −2.194, P < 0.05).
Discussion
Biotic interactions are fundamental in determining the ability of plants to survive and prosper in novel environments. Here, we conducted an in situ experiment, translocating a native plant species polewards of its present-day distribution. Our findings suggest that M. excelsum survives equally well—indeed exhibits accelerated growth rates—outside of its natural distribution compared with locations inside the range. These accelerated growth rates appear to be due to significantly decreased levels of herbivory from its specialised insect herbivore in polewards locations. This may indicate that M. excelsum is benefiting from suppressed insect attack at latitudes outside of the primary herbivore’s range. The pattern is probably a signal of ‘predator release’ and may initially augment the polewards range expansion of M. excelsum, adding to the increasing body of evidence that suggests biotic interactions may modulate species’ responses to climate change (Araújo and Luoto 2007; Poloczanska et al. 2008; van der Putten et al. 2010).
Contrary to results expected under a scenario of climate limitation, M. excelsum did not exhibit reduced survival rates outside of its natural range. Indeed, plants in the interior of forest fragments, where ambient microclimate conditions would theoretically be buffered from the more extreme climatic conditions outside the forest (Young and Mitchell 1994; Davies-Colley et al. 2000), appeared to have slightly elevated survival levels and enhanced growth rates at sites outside of the plants range as compared to sites within the species’ range (Fig. 3a, b). This species is particularly sensitive to frost (The Native Plant Centre 2007), yet despite frosts being more frequent than average in the southernmost location with just 2 (1992, 2003) out of the past 18 years having more frost days than 2010, survival rates at the southernmost location were still very high (Fig. 3a). If, as many models assume, climate envelopes are the predominant factor determining species’ ranges (Pearson and Dawson 2003), the high survivorship levels in regions polewards of M. excelsum’s natural distribution in this investigation indicate that the climate here is suitable for adult survival and therefore that some other factor must limit the geographical range of the species. However, it is still premature to entirely rule out the possibility of climatic controls on the geographic range of M. excelsum, as other parts of the species’ life history such as germination or propagation may limit its ability to successfully form breeding populations. In addition, due to the pragmatic limitations on introducing fertile novel species outside their range, this study was conducted over a comparatively short time for investigations into survival capabilities of transplanted organisms, with most researchers discounting the first year of measurements due to initial vigour of plant growth even in unsuitable sites (Ibáñez et al. 2008). However, studies that did undertake transplants under shorter time frames have found that initial mortalities occurred within a week of transplantation with little or no deaths occurring after this period (Silander and Klepeis 1999), suggesting that our findings may still provide insights into patterns of plant survival in polewards locations. Further investigation into the germination capacity and reproductive output of M. excelsum seeds in polewards regions, as well as an extension of the study duration to account for more extreme weather events and any potentially delayed mortalities, are necessary to confirm if, as this study would suggest, climate in this novel area is suitable for the plants’ long-term survival and persistence.
A number of studies have found that dispersal can be equally or even more important than climate in limiting the expansion of species’ ranges towards the poles (Grashof-Bokdam and Geertsema 1998; Norton et al. 2005; Svenning and Skov 2007; Marsico and Hellmann 2009). For those plant species that are able to expand into novel geographic areas in which their primary herbivores do not exist or are reduced in numbers, a release from high levels of herbivory may facilitate their successful colonisation, successful growth and range expansion (Keane and Crawley 2002). A similar translocation experiment on Vancouver Island found that the survivorship of Lomantium nudicaule (Apiaceae) was equal to, or improved, outside relative to inside the present-day range (Marsico and Hellmann 2009). The authors reported that the climate outside the range of Lomantium spp. had been suitable for the previous 100 years, and suggested that a more likely factor limiting the range extent of Lomantium spp. was the fragmented nature of habitat at the range margin (Marsico and Hellmann 2009). In the context of our experiment, models of potential vegetation patterns suggest that there was continuous native forest extending along the east coast of the South Island prior to human habitation (Leathwick et al. 2004). M. excelsum often grows in the understory of many of the canopy species thought to have been present, and as such there would have been the potential for M. excelsum populations to spread south of the species’ present-day range. However, in the ~730 years since human colonisation of New Zealand (Wilmshurst et al. 2008), indigenous forest cover has been reduced from 81 to 23 % of the total land area (Rutledge 2003), and the Canterbury plains—the present-day southern limit of M. excelsum—were particularly rapidly and heavily affected (Ewers et al. 2006). Dispersal limitation in this fragmented landscape may, therefore, be one mechanism responsible for the lack of naturalised M. excelsum south of Banks Peninsula.
The key influence of biotic interactions on plant growth rates is evident from this study, and these findings contribute to an increasing body of evidence that biotic limitations play a fundamental role in determining current distributions of plants and in the responses of species to climate change (Suttle et al. 2007; Araújo and Luoto 2007; van der Putten et al. 2010). C. scriptaria herbivory is positively related to M. excelsum abundance (Schnitzler et al. 2011) and this herbivore shows a preference for ‘core’ forest conditions (Schnitzler 2008). We found firstly, and at small spatial scales, herbivory is higher on trees in forest fragment interiors than at forest edges. Outside the species’ range, where no C. scriptaria larvae were recorded, herbivory still occurred at low levels yet the difference between interior and edge disappeared. The loss of this trend outside the range may be an indication of altered community interactions. With the absence of C. scriptaria, other less dominant herbivores have access to this novel food resource. We suggest that these generalist herbivores (Spiller and Wise 1982) have no clear preference for ‘core conditions’ and as such the edge/interior trend disappears. Secondly, and at larger spatial scales, we found a strong trend indicating enhanced growth (particularly inside forest fragments) at latitudes outside the species’ range (Fig. 3b). Interestingly, these trends were almost the exact opposite of the trends in herbivory levels, which were higher inside the species’ range (particularly at forest interior positions) and diminished in latitudes outside the range (Fig. 3c). These results suggest a causal link between biotic activity and plant health and as such, this experiment may emulate the erosion of trophic interactions when species become geographically separated.
Temporal phenological mismatch is frequently being recorded when the timings of vital life history events of predators and prey drift apart (Post and Forchhammer 2008), a situation that can be precipitated by climate change (Schweiger et al. 2008). Also important, yet less frequently recorded, are spatial mismatches in which species with differing levels of mobility move (or do not) to track changing isothermic distributions, resulting in geographically decoupled species interactions (Schweiger et al. 2008). Such a situation has been predicted from SDMs for lepidopteran species that exhibit a pronounced loss of range when their host plant is not able to fill its projected ecological niche [e.g. in the case of Boloria titania (Schweiger et al. 2008)], and in empirical studies where hosts experience lower levels of parasitoid attack after colonising a novel area [e.g. the butterfly Aricia agestis (Menéndez et al. 2008)]. This ‘predator release’ phenomenon is more commonly discussed in the context of the invasive potential of exotic species. The enemy release hypothesis predicts that as plant species move into novel regions, their establishment will be enhanced by the lack of natural predators that exert herbivory stress on the plant (Keane and Crawley 2002). Our data provide empirical support for this hypothesis. We recorded no C. scriptaria larvae south of the current M. excelsum distribution limit (as a result caterpillar numbers significantly reduced with increased latitude; Table 1), and we found that plants established beyond the range of the specialised herbivore appeared to gain an advantage in terms of growth (Fig. 3). Applied to the context of distribution shifts under climate change, the enemy release hypothesis may provide further insights into how plants may respond at the polewards range if they move beyond the distribution of their specialised herbivores. It is possible, therefore, that the spatial distribution of herbivores may facilitate the expansion of plant species’ ranges (Maron and Vila 2001) under climate change. However, this expectation must in turn be balanced by the expectation that, in the absence of a direct climatic control on their distribution, mobile insect herbivores such as C. scriptaria will likely expand their range to keep pace with that of their host plant. In addition, although unlikely due to the range of defensive compounds in this plant, there is the potential for herbivores in polewards locations to overcome these chemical barriers and for herbivory to gradually increase over time. Under these scenarios, predator release and the subsequent growth advantage experienced by a polewards expansion of M. excelsum will likely be short-lived.
It is thought that the largest uncertainty in predicting the impact of climate change on ecosystems is understanding how species will interact in novel conditions (Winder and Schindler 2004). Therefore, the fundamental importance of studying how species interactions are modified by climate change, must not be ignored (Suttle et al. 2007). We have shown that M. excelsum can survive polewards of its natural distribution and it is likely that the improved growth rate is due to a release from the suppressive influence of high levels of herbivory in forest outside of the range of its primary herbivore. However, this does not explain the current southern range limit of the species, which we hypothesise is caused either by historical dispersal limitation or from undetected climatic limitations to the ability of the species to germinate or reproduce. Further work would be needed to separate these hypotheses. Predator release can only benefit a plant species if it has first overcome dispersal limitation to reach a new location, and then successfully germinated and formed a reproducing population. Such work in this domain is important if we are to understand more fully how species will respond to climate change at their range margins.
References
Araujo MB, Guisan A (2006) Five (or so) challenges for species distribution modelling. J Biogeogr 33:1677–1688
Araújo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr 16:743–753
Beaugrand G, Reid PC, Ibañez F, Lindley JA, Edwards M (2002) Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694
Box EO (1981) Macroclimate and plant forms: an introduction to predictive modelling in phytogeography. Junk, The Hague
Brooker RW, Travis JMJ, Clark EJ, Dytham C (2007) Modelling species’ range shifts in a changing climate: the impacts of biotic interactions, dispersal distance and the rate of climate change. J Theor Biol 245:59–65
Bullock JM, Edwards RJ, Carey PD, Rose RJ (2000) Geographical separation of two Ulex species at three spatial scales: does competition limit species’ ranges? Ecography 23:257–271
Chen I-C, Hill JK, Ohlemuller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Clausen JD, Keck D, Hiesey WM (1940) Experimental studies on the nature of species. I. Effect of varied environments on western North American plants. Washington, DC
Connell JH (2011) The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecol Soc Am 42:710–723
Davies-Colley RJ, Payne GW, van Elswijk M, Elswijk MV (2000) Microclimate gradients across a forest edge. N Z J Ecol 24:111–121
Davis MB, Shaw RG (2001) Range shifts and adaptive responses to Quaternary climate change. Science 292:673–679
Davis AJ, Lawton JH, Shorrocks B, Jenkinson LS (1998) Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J Anim Ecol 67:600–612
DeFrenne P, Kolb A, Verheyen K, Brunet J, Chabrerie O, Decocq G, Diekmann M, Eriksson O, Heinken T, Hermy M, Jogar U, Stanton S, Quataert P, Zindel R, Zobel M, Graae BJ, Jõgar Ü (2009) Unravelling the effects of temperature, latitude and local environment on the reproduction of forest herbs. Glob Ecol Biogeogr 18:641–651
DeRivera CE, Ruiz GM, Hines AH, Jivoff P, Derivera CE (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86:3364–3376
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Ewers RM, Kliskey AD, Walker S, Rutledge D, Harding JS, Didham RK (2006) Past and future trajectories of forest loss in New Zealand. Biol Conserv 133:312–325
Gardner RO (1997) Macropiper (Piperaceae) in the south-west Pacific. N Z J Bot 35:293–307
Gaston JK (2003) The structure and dynamics of geographic ranges. Oxford University Press, USA
Grashof-Bokdam CJ, Geertsema W (1998) The effect of isolation and history on colonization patterns of plant species in secondary woodland. J Biogeogr 25:837–846
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Hampe A (2004) Bioclimate envelope models: what they detect and what they hide. Glob Ecol Biogeogr 13:469–471
Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Körber J-H, Korber J (2007) Biotic interactions improve prediction of boreal bird distributions at macro-scales. Glob Ecol Biogeogr 16:754–763
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol 12:450–455
Hodge S (1998) Herbivore damage and leaf loss in the New Zealand pepper tree (’Kawakawa’; Macopiper excelsum; Piperaceae). N Z J Ecol 22:173–180
Holdridge LR (1947) Determination of world plant formations from simple climatic data. Science 105:367–368
Ibáñez I, Clark JS, Dietze MC (2008) Evaluating the sources of potential migrant species: implications under climate change. Ecol Appl 18:1664–1678
Ibáñez I, Silander JA Jr, Allen JM, Treanor S, Wilson A (2009a) Identifying hotspots for plant invasions and forecasting focal points of further spread. J Appl Ecol 46:1219–1228
Ibáñez I, Silander JA Jr, Wilson AM, LaFleur N, Tanaka N, Tsuyama I (2009b) Multivariate forecasts of potential distributions of invasive plant species. Ecol Appl 19:359–375
IPCC (2007) Climate change synthesis report. In: Pachauri RK, Reisinger A (eds) Contribution of Working Groups I, II and III to the Fourth Assessment report of the Intergovernmental Panel on Climate Change
Jiménez-Valverde A, Lobo JM, Hortal J (2008) Not as good as they seem: the importance of concepts in species distribution modelling. Divers Distrib 14:885–890
Kawecki TJ (2008) Adaptation to marginal habitats. Annu Rev Ecol Evol Syst 39:321–342
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kollmann J, Bañuelos MJ, Banuelos MJ (2004) Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Divers Distrib 10:377–385
Lapointe BE, Pierce F, William B, Burnett B, Grant S (2011) Nutrient thresholds for bottom-up control of macroalgal Jamaica and southeast Florida. Limnol Oceanogr 42:1119–1131
Leathwick J, McGlone M, Walker S (2004) New Zealand’s potential vegetation pattern. Whenua, Lincoln
Macel M, Lawson CS, Mortimer SR, Šmilauerova M, Crémieux L, Doležal J, Edwards AR, Lanta V, Martijn T, Putten WHVD, Igual JM, Rodriguez-barrueco C, Müller- H, Steinger T, Lawson S, Edwards R, Dolezal J (2007) Climate versus soil factors in local adaptation of two common plant species. Ecology 88:424–433
Magnani F (2009) Phenotypic variability: underlying mechanisms and limits do matter. New Phytol 184:277–279
Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Marsico TD, Hellmann JJ (2009) Dispersal limitation inferred from an experimental translocation of Lomatium (Apiaceae) species outside their geographic ranges. Oikos 118:1783–1792
Matesanz S, Gianoli E, Valladares F (2010) Global change and the evolution of phenotypic plasticity in plants. In: Schlichting CD, Mousseau TA (eds) Year in evolutionary biology. Wiley-Blackwell, Malden, pp 35–55
Meier ES, Kienast F, Pearman PB, Svenning JC, Thuiller W, Araujo MB, Guisan A, Zimmermann NE (2010) Biotic and abiotic variables show little redundancy in explaining tree species distributions. Ecography 33:1038–1048
Menéndez R, González-Megías A, Lewis OT, Shaw MR, Thomas CD (2008) Escape from natural enemies during climate-driven range expansion: a case study. Ecol Entomol 33:413–421
Ministry for the Environment (2008) Climate change effects and impacts assessment: a guidance manual for local government in New Zealand, 2nd edn. Mullan B, Wratt D, Dean S, Hollis M, Allan S, Williams T, Kenny G (eds) Ministry for the Environment, Wellington
Moore P, Hawkins SJ, Thompson RC (2007) Role of biological habitat amelioration in altering the relative responses of congeneric species to climate change. Marine Ecol Prog Ser 334:11–19
Morin X, Lechowicz MJ (2008) Contemporary perspectives on the niche that can improve models of species range shifts under climate change. Biol Lett 4:573–576
Niedrist G, Bertoldi G, Della Chiesa S, Hell V, Tasser E, Tappeiner U (2011) Transplantation experiments in an inner-alpine dry valley to predict climate change effects on agriculturally used grassland ecosystem. In: Geophysical Research Abstracts, EGU General Assembly 2011, pp 10218–10218
Norton LR, Firbank LG, Scott A, Watkinson AR (2005) Characterising spatial and temporal variation in the finite rate of population increase across the northern range boundary of the annual grass Vulpia fasciculata. Oecologia 144:407–415
Parmesan C (1996) Climate and species’ range. Nature 382:765–766
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Parmesan C, Gaines S, Gonzalez L, Kaufman DM, Kingsolver J, Townsend Peterson A, Sagarin R (2005) Empirical perspectives on species borders: from traditional biogeography to global change. Oikos 108:58–75
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeogr 12:361–371
Pearson RG, Dawson TP (2005) Long-distance plant dispersal and habitat fragmentation: identifying conservation targets for spatial landscape planning under climate change. Biol Conserv 123:389–401
Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT (2008) Modelling the response of populations of competing species to climate change. Ecology 89:3138–3149
Post E, Forchhammer MC (2008) Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos Transact R Soc B-Biol Sci 363:2369–2375
Prince SD, Carter RN (1985) Topographical distribution of prickly lettuce (Lactuca serriola). III. Its performance in transplant sites beyond is distribution limit in Britain. J Ecol 73:49–64
R Development Core Team (2010) R: a language and environment for statistical computing
Rutledge D (2003) Landscape indices as measures of the effects of fragmentation: can pattern reflect process?
Schnitzler FR (2008) Hymenopteran parasitoid diversity and tri-trophic interactions: the effects of habitat fragmentation in Wellington. Victoria University of Wellington, New Zealand
Schnitzler F-RR, Hartley S, Lester PJ (2011) Trophic-level responses differ at plant, plot, and fragment levels in urban native forest fragments: a hierarchical analysis. Ecol Entomol 36:241–250
Schweiger O, Settele J, Kudrna O, Klotz S, Kuhn I, Kühn I (2008) Climate change can cause spatial mismatch of trophically interacting species. Ecology 89:3472–3479
Silander JA, Klepeis DM (1999) The invasion ecology of Japanese barberry (Berberis thunbergii) in the New England landscape. Biol Invasions 1:189–201
Smith AC (1975) The genus Macropiper (Piperaceae). Bot J Linn Soc 71:1–38
Spiller DM, Wise KAJ (1982) A catalogue (1860–1960) of New Zealand insects and their host plants. In: New Zealand Department of Scientific and Industrial Research, Wellington, NZ
Suttle KB, Thomsen MA, Power ME (2007) Species interactions reverse grassland responses to changing climate. Science 315:640–642
Svenning J-C, Skov F (2007) Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol Lett 10:453–460
The Native Plant Centre: New Zealand Native Plant Specialists (2007) The Native Plant Centre. 3rd December 2012. (http://www.nznativeplants.co.nz/Articles/Macropiper+excelsum.html)
Thomas CD (2010) Climate, climate change and range boundaries. Divers Distrib 16:488–495
Thomas CD, Lennon JJ (1999) Birds extend their ranges northwards. Nature 399:213
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11:1351–1363
Urban MC, Tewksbury JJ, Sheldon KS (2012) On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change. In: Proceedings biological sciences/the Royal Society. Published online 4 January 2012
van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plant. New Phytol 157:569–578
van der Putten WH, Macel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc B-Biol Sci 365:2025–2034
Wahl M, Link H, Alexandridis N, Thomason JC, Cifuentes M, Costello MJ, da Gama BAP, Hillock K, Hobday AJ, Kaufmann MJ, Keller S, Kraufvelin P, Kruger I, Lauterbach L, Antunes BL, Molis M, Nakaoka M, Nystrom J, bin Radzi Z, Stockhausen B, Thiel M, Vance T, Weseloh A, Whittle M, Wiesmann L, Wunderer L, Yamakita T, Lenz M (2011) Re-structuring of marine communities exposed to environmental change: a global study on the interactive effects of species and functional richness. PLoS ONE 6:e19514
Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, Callaghan TV, Carroll AB, Epstein HE, Jónsdóttir IS, Klein JA, Magnússon B, Molau U, Oberbauer SF, Rewa SP, Robinson CH, Shaver GR, Suding KN, Thompson CC, Tolvanen A, Totland Ø, Turner PL, Tweedie CE, Webber PJ, Wookey PA, Jonsdottir IS, Magnusson B, Totland O (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci USA 103:1342–1346
Walther GR (2004) Plants in a warmer world. Perspect Plant Ecol Evol Syst 6:169–185
Willis SG, Hill JK, Thomas CD, Roy DB, Fox R, Blakeley DS, Huntley B (2009) Assisted colonization in a changing climate: a test-study using two UK butterflies. Conserv Lett 2:46–52
Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH (2008) Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. PNAS 105:7676–7680
Winder M, Schindler DE (2004) Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85:2100–2106
Yang LH, Rudolf VHW (2010) Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett 13:1–10
Young A, Mitchell N (1994) Microclimate and vegetation edge effects in a fragmented podocarp-broadleaf forest in New Zealand. Biol Conserv 67:63–72
Acknowledgments
We would like to acknowledge the Grantham Institute for Climate Change and the Gilchrist Educational Trust for funding this research. Thanks go to the University of Canterbury for providing glasshouse facilities and vehicle use and to Dave Conder for his assistance in cultivating experimental plants. For access to experimental plots we thank the Dunedin, Timaru, Christchurch and Nelson Councils, DOC and private land owners Hugh Wilson, Sonia and Mark Armstrong. Graham Banton and Ben Rodriguez were instrumental in assisting with the experimental set up and data collection in New Zealand. The experiments comply with the current laws of New Zealand where the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by John Silander.
Rights and permissions
About this article
Cite this article
Lakeman-Fraser, P., Ewers, R.M. Enemy release promotes range expansion in a host plant. Oecologia 172, 1203–1212 (2013). https://doi.org/10.1007/s00442-012-2555-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2555-x