Abstract
Gossypin is a flavone that was originally isolated from Hibiscus vitifolius and has traditionally been used for the treatment of diabetes, jaundice, and inflammation. Recently, gossypin was found to have potent anticancer properties; however, its effect on human gliomas still remain unknown. To investigate the potential anticancer effects of gossypin on malignant gliomas and analyze the associated molecular mechanisms, we treated human glioma U251 cells with gossypin. Our study showed that the treatment of U251 cells with gossypin inhibited cell proliferation in a dose- and time-dependent manner and was observed to be minimally toxic to normal human astrocytes. Gossypin’s effect on cell cycle distribution was observed, and we found that it induced G2/M-phase arrest in U251 cells. An analysis of cell-cycle regulatory proteins indicated that the arresting effect of gossypin on the cell cycle at G2/M phase was involved in the phosphorylation of cell division cycle 25C (Cdc25C) tyrosine phosphatase via the activation of checkpoint kinase 1 (Chk1). These findings indicate that gossypin is a potential treatment of gliomas because of gossypin’s potential to regulate the proliferation of U251 cells via the cell-cycle regulatory proteins Chk1 and Cdc25C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are the most common malignant primary tumors of the brain and are aggressive, highly invasive, and neurologically destructive. Even with the combination of surgery, chemotherapy, and radiotherapy, the median survival rate of patients with glioblastoma, the most aggressive malignant glioma, is approximately 14 months (Sampson et al. 2010). Gossypin is a flavone isolated from hibiscus vitifolius, which has been traditionally used for the treatment of diabetes, jaundice, and inflammation. Recently, it has been demonstrated that gossypin acts as a cancer chemopreventive agent by inhibiting tumor initiation, promotion, and progression in some cell-culture systems and animal models (Kunnumakkara et al. 2007).
Although gossypin has been shown to inhibit various stages of tumor growth, the molecular mechanism of its anticancer activity has not been previously well defined, particularly in brain tumors, which are difficult to treat. In this study, we investigated the anticancer effects of gossypin on malignant human glioma U251 cells, and we demonstrated that gossypin could cause a proliferation defect in U251 cells and arrest the G2/M transition of the cell cycle.
To elucidate the mechanism causing G2/M arrest in gossypin-treated cells, we determined its effects on the expression of proteins important in the G2/M transition. The cell-cycle Chk1-Cdc25C pathway was selected for investigation. Chk1, checkpoint kinase 1, plays a pivotal role in controlling the G2/M phase of the cell cycle (Nakamizo et al. 2002). One well-defined target of Chk1 is Cdc25C (O’Neill et al. 2002). Chk1 phosphorylates the phosphatase Cdc25C on residue Ser216, which promotes the binding of Cdc25C with the molecular chaperone 14-3-3 protein. The 14-3-3 protein then sequesters Cdc25C in the cytoplasm and leads to G2/M arrest (Tyagi et al. 2005). In this study, we provide evidence that the gossypin-induced G2/M arrest provided an explanation for the activation of the Chk1-Cdc25C pathway. These findings show that gossypin is an attractive candidate for use in brain-glioma treatment. Understanding the molecular signaling mechanisms of this naturally derived compound will facilitate the development of therapeutic interventions for the prevention and treatment of brain gliomas.
Experimental Procedures
Reagents
Gossypin (Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO), stored in small aliquots at −20°C, and then thawed and diluted as needed in a cell-culture medium. The cell-proliferation reagent WST-1 was purchased from Roche Applied Science (Roche Applied Science, IN). P-Cdc25C (Ser216), p-Chk1, and total Chk1 antibodies were purchased from Cell Signaling Technology (Beverly, MA). The Cdc25C antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines and Culture Conditions
Human glioma U251 cells were purchased from the Chinese Academy of Sciences Cell Bank. The U251 cells were maintained at 37°C in a 5% CO2 incubator in DMEM medium supplemented with 10% fetal bovine serum (FBS) and were routinely passaged at 2- to 3-day intervals.
Normal human brain tissues were obtained with the informed consent of the patients who suffered severe traumatic brain injury (TBI) and needed post-trauma surgery. Primary astrocyte cultures were performed according to the procedures described by Miller et al. (1995). The brain tissue was then treated with trypsin (0.1%), dissociated by trituration, and plated at a density of 5 × 107 cells per 75 cm2 flask in DMEM (adjusted to a pH of 7.5 with 25 mm of HEPES and 14.3 mM of NaHCO3). The tissue was supplemented with 10% FCS, 1 mM of pyruvate, 2 mM of glutamine, 50 μg/ml of streptomycin, and 50 U/ml of penicillin. The cells were maintained in DMEM containing 10% FCS for 6 days. Next, the flasks were shaken mechanically at 200 rpm overnight in a horizontal orbital shaker to remove the top layer of cells. This procedure removed the majority of the oligodendrocytes, microglia, and type-2 astrocytes and yielded mainly type-1 astrocytes with a flat shape. Within 10–14 d in culture, the astrocytes had formed a subconfluent monolayer. One day after this purification step, secondary astrocyte cultures were established by trypsinizing the primary culture and subplating it onto poly-d-lysine-precoated plastic dishes in DMEM supplemented with 10% FCS (Tokita et al. 2001). The cultures consisted of 95–99% astrocytes as determined by glial fibrillary acidic protein (GFAP) immunohistochemistry. Primary astrocytes were treated with gossypin (0 ~ 90 μM) for various incubation periods (12, 24, 48, and 72 h) before further experiments were conducted. Experiments were divided into three groups: the blank control group, the negative control (0.1% DMSO) group, and the gossypin group.
Cell Transfection
Human Chk1 siRNA and scrambled control siRNA (siRNAsc) were designed according to the recommendations in Xiao et al. (2003) and synthesized by Shanghai GenePharma Company (Shanghai, China). When the U251 cells were 70–90% confluent, they were transfected with FuGENE HD6 (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
WST-1 Assay for Cell Viability
The effect of gossypin on cell viability was determined using the cell proliferation reagent, water-soluble tetrazolium-1 (WST-1). Normal human astrocytes and malignant glioma U251 cells were plated at 5 × 103 cells per well in 96-well plates with six replicate wells at the indicated concentrations. After incubation for 12, 24, 48, or 72 h, the cell proliferation assay was performed as described in Chang et al. (2009). The absorbance was measured at 450 nm using an enzyme-linked immunosorbent assay plate reader. All data points represent the means of a minimum of six wells. The viability of the untreated cells was assumed to be 100%.
Trypan Blue dye Exclusion Test for Cell Viability
The cells were harvested with trypsin/EDTA, suspended in PBS and mixed with an equal amount of 0.4% trypan blue stain (Invitrogen) after all treatments. They were counted in four different fields, and the number of viable cells was calculated as percentage of the total cell population. The count for non-treated cells was 100%.
Soft Agar Colony Assay
Anchorage-independent glioma cell growth was determined by soft agar analysis according to a published method (Finlay et al. 1993). The U251 cells were seeded in 0.35% agar in the middle agar. The bottom of the plate contained 0.5% agar and 0.35% agar was at the top. Plates were incubated at 37°C in a 5% CO2 incubator for two weeks. Then, the U251 colonies were treated with gossypin for 72 h. After treatment, the colonies were photographed and counted under microscopic fields at 10× magnification. Groups consisting of 30 or more cells were considered colonies, whereas groups consisting of fewer cells were counted as clusters (Calin et al. 2002). Each assay was performed in triplicate in four independent experiments.
Cell Cycle Analysis
The U251 cells treated with gossypin were trypsinized and subsequently fixed with ice-cold ethanol (70%) for at least 1 h. After extensive washing, the cells were suspended in Hank’s Balanced Salt Solution (HBSS) containing 50 μg/ml Propidium Iodide (PI) (Sigma-Aldrich) and 50 μg/ml of RNase A (Boehringer Mannheim, Indianapolis, IN), incubated for 1 h at room temperature, and analyzed by FACScan (Becton–Dickinson, San Jose, CA). The cell cycle was analyzed by ModFit LT software.
Western Blots Analysis
Soluble proteins were isolated in lysis buffer (Applygen Technologies Inc., Beijing, China). The samples were subjected to SDS–PAGE on 4, 12 or 16% Tris–glycine gels. Separated proteins were transferred onto membranes by western blotting. Membranes were blocked with a blocking buffer for 1 h at room temperature and, as desired, probed with primary antibody against the desired molecule overnight at 4°C. The primary antibody was followed by a peroxidase-conjugated secondary antibody for 1 h at room temperature and then detected using ECL (Gautier et al. 1991; Jessus et al. 1995; Zeng et al. 1998).
Quantitative Real-Time PCR
For the quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) assays, the ABI 7300 HT Sequence Detection System (Applied Biosystem, Foster City, CA) was used. The primer design and real-time PCR process were performed according to the report by Ozen et al. (2005). The relative gene expression was calculated via the 2−ΔΔCt method (Livak et al. 2001).
Statistical Analysis
All tests were performed using the SPSS Graduate Pack 11.0 statistical software package (SPSS, Chicago, IL). Descriptive statistics, including the mean and SE, along with one-way ANOVAs were used to determine significant differences. In this study, P < 0.05 was considered significant.
Results
Evaluation of The Cytotoxicity of Gossypin on Human Malignant Glioma U251 Cells and Normal Astrocytes
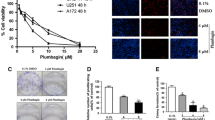
To examine the cytotoxicity of gossypin on glioma cells, U251 cells were treated with gossypin at the indicated concentrations. The effects of gossypin treatment on the survival of U251 cells were assessed by the WST-1 assay. As the growth curve in Fig. 1a indicates, the concentrations at which gossypin inhibited cell growth by 50% (IC50) were 45.0, 29.6, 16.1, and 11.2 μM at 12, 24, 48, and 72 h, respectively. To examine the toxic effect of gossypin on normal human glial cells, these cells were treated with various concentrations of gossypin (0–90 μM for 72 h), and cell viability was measured using the WST-1 assay. Cell viability slightly decreased in a dose-dependent manner in normal human glial cells. The 50% inhibition ratio was still not observed when the concentration of gossypin reached 90 μM (Fig. 1b). In addition, the exposure of normal human astrocytes to gossypin at the indicated IC50 concentrations for 12, 24, 48, and 72 h did not result in any statistically significant change in cell viability compared to the normal control (Fig. 1c).
Effect of gossypin on the cell viability of human malignant glioma U251 cells and normal astrocytes. a The cytotoxic effect of gossypin to U251 cells was measured by WST-1 assay. The concentrations at which gossypin inhibited cell growth by 50% (IC50) at 12, 24, 48, and 72 h were calculated. *P < 0.05; **P < 0.01 vs. negative control group. b The cytotoxic effect of gossypin to human normal astrocytes was measured by WST-1 assay. c The cell viability of human normal astrocytes to gossypin at the indicated IC50 concentrations for 12, 24, 48, and 72 h. d The trypan blue exclusion assay was used to assess cell viability in U251 cells. Gossypin inhibited the proliferation of U251 cells in a concentration- and time-dependent manner. e Colony formation inhibition effect of gossypin as measured by soft agar assay. After U251 colonies formation, they were treated with 29.6 μM gossypin for 72 h, and then plates were fixed and photographed (Magnification: ×100)
The antiproliferative activity of gossypin was further confirmed using a trypan blue dye exclusion assay. Trypan blue dye is the most commonly used staining reagent for testing the integrity of the biological membrane. Healthy cells exclude trypan blue, while the nonviable cells take up trypan blue (Das et al. 2004). As shown in Fig. 1d, the proliferation of U251 cells was inhibited significantly when treated with gossypin in a concentration- and time-dependent manner. The viability of the cells was reduced by 89.2% upon exposure for 120 h to 45.0 μM of gossypin. In addition, a soft agar colony assay for 12–72 h demonstrated that 29.6 μM of gossypin could inhibit colony formation in the U251 cells (Fig. 1e). The U251 cells treated with gossypin were refractory to soft agar colony growth. A treatment with 29.6 μM of gossypin for 72 h nearly abolished colony formation ability.
These observations demonstrate that gossypin reduced the viability of U251 cells, and its effect on cell proliferation was dependent on the concentration levels and treatment time. It was only slightly toxic to the primary-cultured normal human astrocytes.
Gossypin Reduces Progression via Cell-Cycle Arrest in U251 Cells
Previous studies have shown that the proliferation of cells is dependent on their progression through the cell cycle (Ogasawara et al. 2004; Chang et al. 2009; Lee et al. 2010). To gain insights into the mechanism of the antiproliferative activity of gossypin, its effect on cell-cycle distribution was determined via a flow cytometry assay. The cells were harvested at the indicated time point and analyzed to determine cell-cycle distributions. As shown in Fig. 2a, a 72-h exposure of U251 cells to 11.2 μM of gossypin resulted in an accumulation of cells in G2/M phase. Gossypin caused a 2.3-fold enrichment of cells in G2/M phase (Fig. 2b) and was accompanied by a decrease in G0/G1-phase cells compared to the blank control and the negative control groups. These results suggested that the effects of gossypin on the inhibition of cell proliferation were, at least in part, because of a reduced progression of the cell cycle, most likely because of a block or delay in the G2/M transition.
Cell cycle change in U251 cells analyzed by flow cytometry. a The population of cell cycle phase in a 72 h exposure of U251 cells to 11.2 μM gossypin was analyzed by flow cytometry analysis. Gossypin arrested the cell cycle at the G2/M phase with a decrease in G0/G1 phase. b Bar graphs represent the percentage of cell cycle phase calculated from each group. Data are the means of triplicate experiments; error bars, S.D. Significant differences are indicated by *P < 0.05
Gossypin Activates Cell-Cycle Regulated Proteins Chk1 and Cdc25C in U251 Cells
To elucidate the G2/M-arrest mechanism in the gossypin-treated cells, we determined its effect on the expression of proteins that are pivotal in the G2/M transition, including Chk1 and Cdc25C. Chk1, a checkpoint effector kinase, is responsible for cell-cycle arrest. It is theorized that Chk1 regulates the G2/M checkpoint by phosphorylating Cdc25C protein phosphatase (Sanchez et al. 1997; Yarden et al. 2002).
Our first test was to determine whether Chk1 was activated in response to gossypin. The U251 cells were exposed to 11.2 μM of gossypin for 24, 48, and 72 h, and soluble proteins were isolated at the indicated times. As shown in Fig. 3a, we observed that gossypin treatment resulted in an increase in the phosphorylation of Chk1 without any statistically significant change in the total Chk1 protein level. Chk1 phosphorylation levels were clearly higher in cells treated with gossypin compared with the negative control. Next, to insure that the signaling pathway downstream of Chk1 activation was Cdc25C in gossypin-induced cells, we examined the phosphorylation of Cdc25C at Ser216. Consistent with this hypothesis, we observed that the phosphorylation of Cdc25C at Ser216 was also considerably enhanced after the U251 cells were treated with gossypin (Fig. 3b).
Effect of treatment of gossypin on cell cycle regulated proteins Chk1 and Cdc25C in U251 cells by Western blotting. a Effects of gossypin on the activation of Chk1. The relative expressing fold of p-Chk1/Chk1 at different time points was compared with the p-Chk1/Chk1 at 0 h which was standardized as one fold and shown in Bar graphs. b Effects of gossypin on the activation of Cdc25C. The relative expressing fold of p-Cdc25C/Cdc25C at different time points was compared with the p-Cdc25C/Cdc25C at 0 h which was standardized as one fold and shown in Bar graphs. c Effects of gossypin on the phosphorylation of Cdc25C after Chk1 siRNA pre-treatment. The relative expressing fold of p-Cdc25C/beta-actin at different time points was compared with the p-Cdc25C/beta-actin at 48 h which was standardized as one fold and shown in Bar graphs. d Effects of Chk1 siRNA on the change of the level of Cdc25c mRNA. Data are the means of triplicate experiments; error bars, S.D. Significant differences are indicated by *P < 0.05
Finally, to confirm the direct involvement of the Chk1-Cdc25C pathway, we designed siRNA that specifically eliminated Chk1 expression. The Chk1 immunoblot confirmed the effective elimination of Chk1 protein by the Chk1 siRNA at 48 and 72 h (Fig. 3c). As shown in Fig. 3c, in contrast with gossypin treatment alone, the pre-transfection of Chk1 siRNA resulted in the inhibition of Cdc25C phosphorylation which was induced by gossypin. To prove that the Chk1 siRNA did not cause an “off-target” effect on Cdc25C, the level of Cdc25c mRNA was detected by a real time RT-PCR. We found that the level of Cdc25c mRNA was not altered when cells were treated with the Chk1 siRNA (Fig. 3d).These results suggest that gossypin-induced G2-phase arrest may occur through the Chk1-mediated degradation of the endogenous Cdc25C.
Discussion
There is little knowledge of the bioactivity of gossypin on brain tumors, especially on malignant glioma cells (Babu et al. 2003). In this study, we first investigated the bioactivity of gossypin on U251 cells. Our data showed that gossypin could inhibit the growth of U251 cells in a concentration- and time-dependent manner and was confirmed by WST-1 assay. In contrast, gossypin exhibited minimal toxicity to normal astrocytes. Next, cell-cycle analyses demonstrated that gossypin arrested the cell cycle primarily at G2/M phase. To the best of our knowledge, this is the first study to demonstrate the effect of gossypin and its potential mechanisms of action on malignant glioma cells. In view of the results of this experiment, it seemed reasonable to highlight the possibility of gossypin in the clinical treatment of gliomas.
Data presented herein indicated that gossypin could cause a G2/M arrest in U251 cells. However, the mechanisms of cell-cycle arrest by gossypin have not been reported to date. Previous studies have demonstrated that the attendant delay in cell-cycle progression required the activation of DNA damage to Chk1 (Walworth et al. 1993; Dubrez et al. 1995). In higher eukaryotic organisms, the Chk1 contributes essential functions to both cell cycle and checkpoint control. Chk1 is a serine/threonine kinase that is required for both the S and G2/M-phase checkpoints (Bartek et al. 2003; Zhou and Bartek 2004). The overexpression of Chk1 can, by itself, elicit cell-cycle arrest in the G2/M phase (Walworth et al. 1993; Walworth and Bernards 1996), and the inhibition of Chk1 is known to abrogate G2/M arrest in response to DNA damage (al-Khodairy et al. 1994 and Yin et al. 2001). In addition, Chk1 is known to phosphorylate Cdc25C phosphatase at Ser216 in response to DNA damage, which is essential for the G2/M checkpoint (Graves et al. 2000 and Chen et al. 2006). The maintenance of Cdc25C phosphorylation on Ser216 after DNA damage is thought to prevent the premature progression from G2 to mitosis (Peng et al. 1997; Matsuoka et al. 1998). Therefore, Chk1 and Cdc25C are the potential targets of the G2/M checkpoint.
In the present study, we tested the possibility that the G2/M-cell-cycle phase accumulation was induced by Cdc25C phosphorylation, which was activated by Chk1. Consistent with previous reports, our results showed that an increase in the phosphorylation of Cdc25C was accompanied by an increase in the activation of Chk1 after gossypin treatment. These results demonstrate a potential connection between Chk1 activation and Cdc25C phosphorylation in gossypin-induced G2/M arrest of U251 cells. To confirm the potential Chk1-Cdc25C pathway, we designed an siRNA that specifically silenced Chk1 expression. Chk1 was proposed to regulate the interactions between human Cdc25C and 14-3-3 proteins by phosphorylating Cdc25C at Ser216. Ser 216 phosphorylation mediates the binding of 14-3-3 protein to Cdc25C, and Cdc25C/14-3-3 complexes likely mediate G2/M arrest by sequestering the Cdc25C in the cytoplasm (Peng et al. 1998; Weng et al. 1998). Consistent with this hypothesis, the results prove that there is a formal link between Chk1 and Cdc25C phosphorylation in gossypin-induced G2/M arrest. We found that gossypin-induced phosphorylation of Cdc25C was successfully prevented in Chk1 siRNA pre-treatment. These results suggested that gossypin could lead to G2/M cell-cycle arrest by activating the Chk1-Cdc25C pathway.
In summary, study by our group has shown for the first time that gossypin can effectively inhibit the proliferation of malignant glioma U251 cells by causing G2/M-phase arrest. Although there was no direct evidence that gossypin could cross the blood–brain barrier in the human brain, the oral supplementation of gossypin has been reported to protect the alteration of brain oxidative stress in rats and inhibit certain biochemical processes of brain aging (Schmitt-Schillig et al. 2005; Gautam et al. 2010), demonstrates a possible effect of gossypin on the brain. Currently, the side effects of gossypin have not been reported. In summary, this suggests that gossypin may be used as a chemotherapeutic agent and could be useful for delaying the onset and/or progression of human malignant gliomas.
References
al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Lehmann AR, Carr AM (1994) Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell 5:147–160
Babu BH, Jayram HN, Nair MG, Ajaikumar KB, Padikkala J (2003) Free radical scavenging, antitumor and anticarcinogenic activity of Gossypin. J Exp Clin Cancer Res 22:581–589
Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421–429
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM (2002) Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. PNAS 99:15524–15529
Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, Zhou J, Cheng CK, Chang CA, Chiu JJ (2009) BMP-4 Induction of arrest and differentiation of osteoblast-like cells via p21CIP1 and p27KIP1 regulation. Mol Endocrinol 23:1827–1838
Chen JS, Lin SY, Tso WL, Yeh GC, Lee WS, Tseng H, Chen LC, Ho YS (2006) Checkpoint kinase 1-mediated phosphorylation of Cdc25C and bad proteins are involved in antitumor effects of loratadine-induced G2/M phase cell-cycle arrest and apoptosis. Mol Carcinog 45:461–478
Das A, Banik NL, Patel SJ, Ray SK (2004) Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and preventing proteolytic activities. Mol Cancer 3:36–46
Dubrez L, Goldwasser F, Genne P, Pommier Y, Solary E (1995) The role of cell cycle regulation and apoptosis triggering in determining the sensitivity of leukemic cells to topoisomerase I and II inhibitors. Leukemia 9:1013–1024
Finlay TH, Tamir S, Kadner SS, Cruz MR, Yavelow J, Levitz M (1993) Alpha 1-Antitrypsin- and anchorage-independent growth of MCF-7 breast cancer cells. Endocrinology 133:996–1002
Gautam P, Flora SJ (2010) Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition 26(5):563–570
Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW (1991) cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67(1):197–211
Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, Piwnica-Worms H (2000) The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem 275:5600–5605
Jessus C, Ozon R (1995) Function and regulation of cdc25 protein phosphate through mitosis and meiosis. Prog Cell Cycle Res 1:215–228
Kunnumakkara AB, Nair AS, Ahn KS, Pandey MK, Yi Z, Liu M, Aggarwal BB (2007) Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-B activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 109:5112–5121
Lee DY, Li YSJ, Chang SF, Zhou J, Ho HM, Chiu JJ, Chien S (2010) Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by vβ3 and β1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6 K pathway. J Biol Chem 285:30–42
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression using real-time quantitiative PCR and the 2-ΔΔct method. Methods 25:402–408
Matsuoka S, Huang M, Elledge SJ (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893–1897
Miller S, Romano C, Cotman CW (1995) Growth factor upregulation of a phosphoinositide-coupled metabotropic glutamate receptor in cortical astrocytes. J Neurosci 15:6103–6109
Nakamizo A, Inamura T, Inoha S et al (2002) Suppression of Cdc2 dephosphorylation at the tyrosine 15 residue during nitrosourea-induced G2/M phase arrest in glioblastoma cell lines. J Neurooncol 59:7–13
Ogasawara T, Kawaguchi H, Jinno S, Hoshi K, Itaka K, Takato T, Nakamura K, Okayama H (2004) Bone morphogenetic protein 2-induced osteoblast differentiation requires smad-mediated down-regulation of Cdk6. Mol Cell Biol 24:6560–6568
O’Neill T, Giarratani L, Chen P, Iyer L, Lee CH, Bobiak M, Kanai F, Zhou BB, Chung JH, Rathbun GA (2002) Determination of substrate motifs for human Chk1 and hCds1/Chk2 by the oriented peptide library approach. J Biol Chem 277:16102–16115
Ozen M, Ittmann M (2005) Increased expression and activity of CDC25C phosphatase and an alternatively spliced variant in prostate cancer. Clin Cancer Res 11:4701–4706
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H (1997) Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of cdc25c on serine-216. Science 277:1501–1505
Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, Wu Z, Stephenson MT, Piwnica-Worms H (1998) C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14–3-3 protein binding. Cell Growth Differ 9:197–208
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729
Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497–1501
Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Muller WE (2005) Flavonoids and the aging brain. J Physiol Pharmacol 56:23–36
Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, Oohira A (2001) Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. J Neurosci 21:1257–1264
Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, Agarwal R (2005) Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR–Chk1/2–Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis 26:1978–1987
Walworth NC, Bernards R (1996) Rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271:353–356
Walworth N, Davey S, Beach D (1993) Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature 363:368–371
Weng Z, Fluckiger AC, Nisitani S, Wahl MI, Le LQ, Hunter CA, Fernal AA, Beau MML, Witte ON (1998) A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. PNAS 95:12334–12339
Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S, Zhang H (2003) Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem 278:21767–21773
Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC (2002) BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nature Genet 30:285–289
Yin M, Hapke G, Guo B, Azrak RG, Frank C, Rustum YM (2001) The Chk1-Cdc25C regulation is involved in sensitizing A253 cells to a novel topoisomerase I inhibitor BNP1350 by bax gene transfer. Oncogene 20:5249–5257
Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T (1998) Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395(6701):507–510
Zhou BB, Bartek J (2004) Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer 4:216–225
Acknowledgments
All authors have declared all sources of funding for the research reported in this manuscript and have no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest. This study was funded by the Chinese Natural Science Foundation (Proj. No. 81000963, 81072078 and 30872657), Jiangsu Province’s Natural Science Foundation (Grant Number: BK2010580, 2009444 and 2008475), Jiangsu Province’s Medical Major Talent Program (Grant Number: 2007061), Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, Suzhou Kunshan Social Development Foundation (KS1006 and KS1009), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lei Shi, Jian Chen,Yin-yi Wang, Guan Sun, Jing-ning Liu, Jun-xia Zhang, Wei Yan contributed equally to this study.
Rights and permissions
About this article
Cite this article
Shi, L., Chen, J., Wang, Yy. et al. Gossypin Induces G2/M Arrest in Human Malignant Glioma U251 Cells by the Activation of Chk1/Cdc25C Pathway. Cell Mol Neurobiol 32, 289–296 (2012). https://doi.org/10.1007/s10571-011-9760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9760-8